Abstract
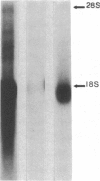
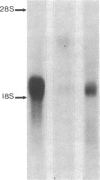
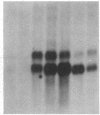
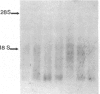
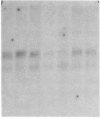
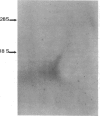
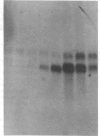
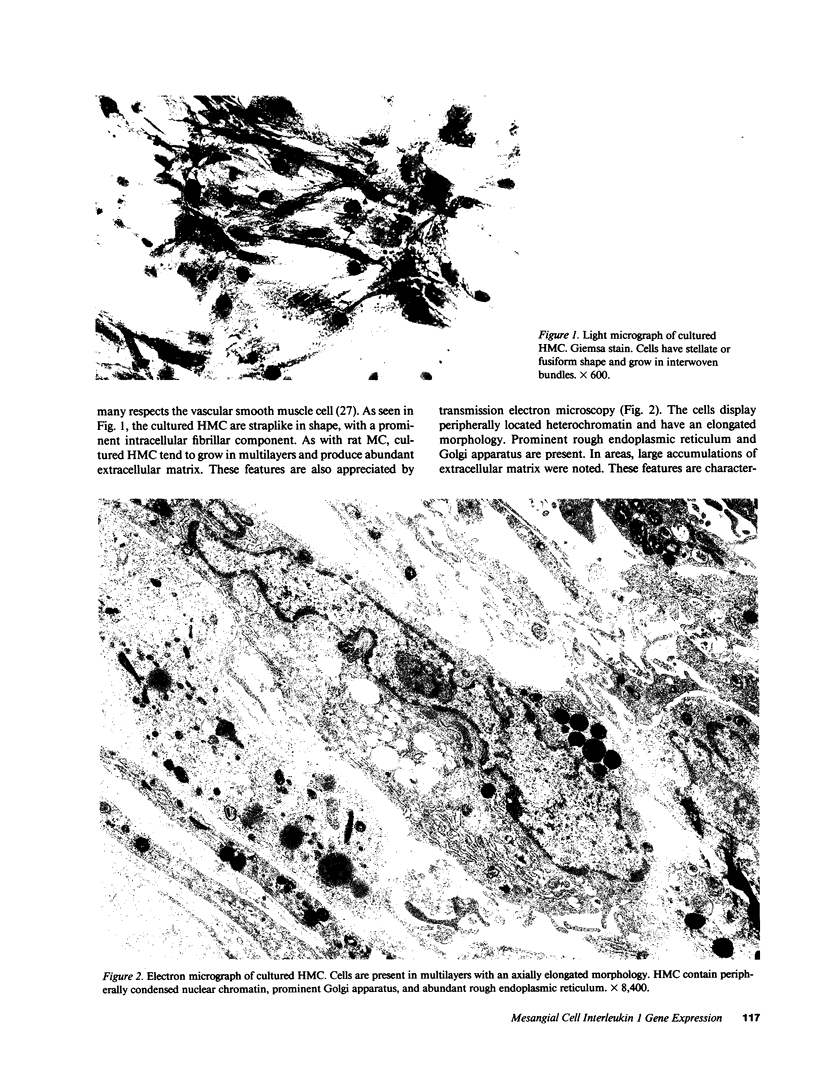
Glomerular mesangial cell (MC)--derived IL-1 may be an important factor in the development of the hypercellularity and sclerosis characteristic of many forms of glomerulonephritis. To define the regulation of IL-1 synthesis by human MC, Northern blot analyses were performed using specific probes for monocytic IL-1 alpha and beta mRNA. Proliferating MC expressed mRNA for both IL-1 alpha and beta, whereas nonproliferating MC contained no detectable IL-1 mRNA. Synchronized MC expressed IL-1 alpha and beta mRNA within 2 h of stimulation with serum. This serum effect could be reproduced with platelet-derived growth factor and epidermal growth factor. Immune precipitations of 35S-methionine-labeled cells indicate that the mesangial IL-1 is synthesized as a 33-kD precursor protein with a pI of 7.2. Extracellular mesangial IL-1 has a pI of 7.0 and molecular weight of 17 kD, consistent with its identification as IL-1 beta. Cellular proliferation in glomerular disease may be driven in part by peptide growth factor-mediated induction of mesangial IL-1 gene expression and protein synthesis.
Full text
PDF



Images in this article
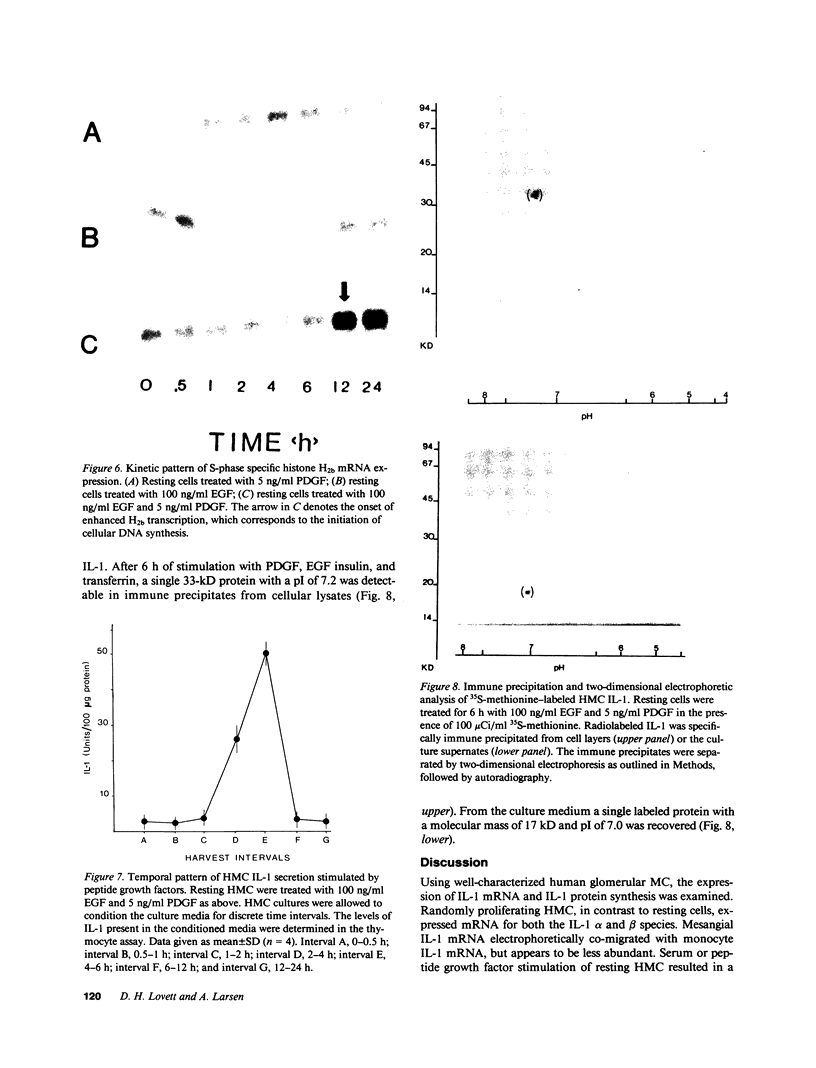
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E., Poptic E., DiCorleto P. Production of platelet-derived growth factorlike protein by rat mesangial cells in culture. J Clin Invest. 1987 Sep;80(3):675–683. doi: 10.1172/JCI113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auron P. E., Warner S. J., Webb A. C., Cannon J. G., Bernheim H. A., McAdam K. J., Rosenwasser L. J., LoPreste G., Mucci S. F., Dinarello C. A. Studies on the molecular nature of human interleukin 1. J Immunol. 1987 Mar 1;138(5):1447–1456. [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Is epidermal growth factor present in human blood? Interference with the radioreceptor assay for epidermal growth factor. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1036–1041. doi: 10.1016/0006-291x(83)90666-6. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. Clin Endocrinol Metab. 1984 Mar;13(1):191–205. doi: 10.1016/s0300-595x(84)80013-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cameron P., Limjuco G., Rodkey J., Bennett C., Schmidt J. A. Amino acid sequence analysis of human interleukin 1 (IL-1). Evidence for biochemically distinct forms of IL-1. J Exp Med. 1985 Sep 1;162(3):790–801. doi: 10.1084/jem.162.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Giri J. G., Lomedico P. T., Mizel S. B. Studies on the synthesis and secretion of interleukin 1. I. A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985 Jan;134(1):343–349. [PubMed] [Google Scholar]
- Goldsmith M. R., Rattner E. C., Koehler M. M., Balikov S. R., Bock S. C. Two-dimensional electrophoresis of small-molecular-weight proteins. Anal Biochem. 1979 Oct 15;99(1):33–40. doi: 10.1016/0003-2697(79)90041-1. [DOI] [PubMed] [Google Scholar]
- Kashgarian M. Mesangium and glomerular disease. Lab Invest. 1985 Jun;52(6):569–571. [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Formation of the 3' end of histone mRNA by post-transcriptional processing. Nature. 1984 Mar 8;308(5955):203–206. doi: 10.1038/308203a0. [DOI] [PubMed] [Google Scholar]
- Kronheim S. R., March C. J., Erb S. K., Conlon P. J., Mochizuki D. Y., Hopp T. P. Human interleukin 1. Purification to homogeneity. J Exp Med. 1985 Mar 1;161(3):490–502. doi: 10.1084/jem.161.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Ballard D. W., Chua A. O., McGuire J. S., Flood P. M., Horowitz M. C., Langdon R., Lightfoot L., Gubler U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986 Dec 1;164(6):2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. The role of protein breakdown in growth, quiescence, and starvation of vascular smooth muscle cells. J Cell Physiol. 1984 Mar;118(3):317–323. doi: 10.1002/jcp.1041180315. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Haensch G. M., Goppelt M., Resch K., Gemsa D. Activation of glomerular mesangial cells by the terminal membrane attack complex of complement. J Immunol. 1987 Apr 15;138(8):2473–2480. [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. A thymocyte-activating factor derived from glomerular mesangial cells. J Immunol. 1983 Apr;130(4):1796–1801. [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. Stimulation of rat mesangial cell proliferation by macrophage interleukin 1. J Immunol. 1983 Dec;131(6):2830–2836. [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B., Kashgarian M., Ryan J. L. Neutral proteinase activity produced in vitro by cells of the glomerular mesangium. Kidney Int. 1983 Feb;23(2):342–349. doi: 10.1038/ki.1983.25. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B., Ryan J. L., Atkins E. Production of an endogenous pyrogen by glomerular mesangial cells. J Immunol. 1985 Feb;134(2):670–672. [PubMed] [Google Scholar]
- Lovett D. H., Szamel M., Ryan J. L., Sterzel R. B., Gemsa D., Resch K. Interleukin 1 and the glomerular mesangium. I. Purification and characterization of a mesangial cell-derived autogrowth factor. J Immunol. 1986 May 15;136(10):3700–3705. [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Mathieson B. J., Mage M., Schmidt J. A., Oppenheim J. J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982 May;128(5):2147–2152. [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raugi G. J., Lovett D. H. Thrombospondin secretion by cultured human glomerular mesangial cells. Am J Pathol. 1987 Nov;129(2):364–372. [PMC free article] [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Foellmer H. G., Perfetto M., Biemesderfer D., Kashgarian M. Mesangial cell hillocks. Nodular foci of exaggerated growth of cells and matrix in prolonged culture. Am J Pathol. 1986 Oct;125(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Stein H. D., Kashgarian M. The mesangium and glomerulonephritis. Klin Wochenschr. 1982 Sep 15;60(18):1077–1094. doi: 10.1007/BF01715838. [DOI] [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Windle J. J., Shin H. S., Morrow J. F. Induction of interleukin 1 messenger RNA and translation in oocytes. J Immunol. 1984 Mar;132(3):1317–1322. [PubMed] [Google Scholar]