Abstract
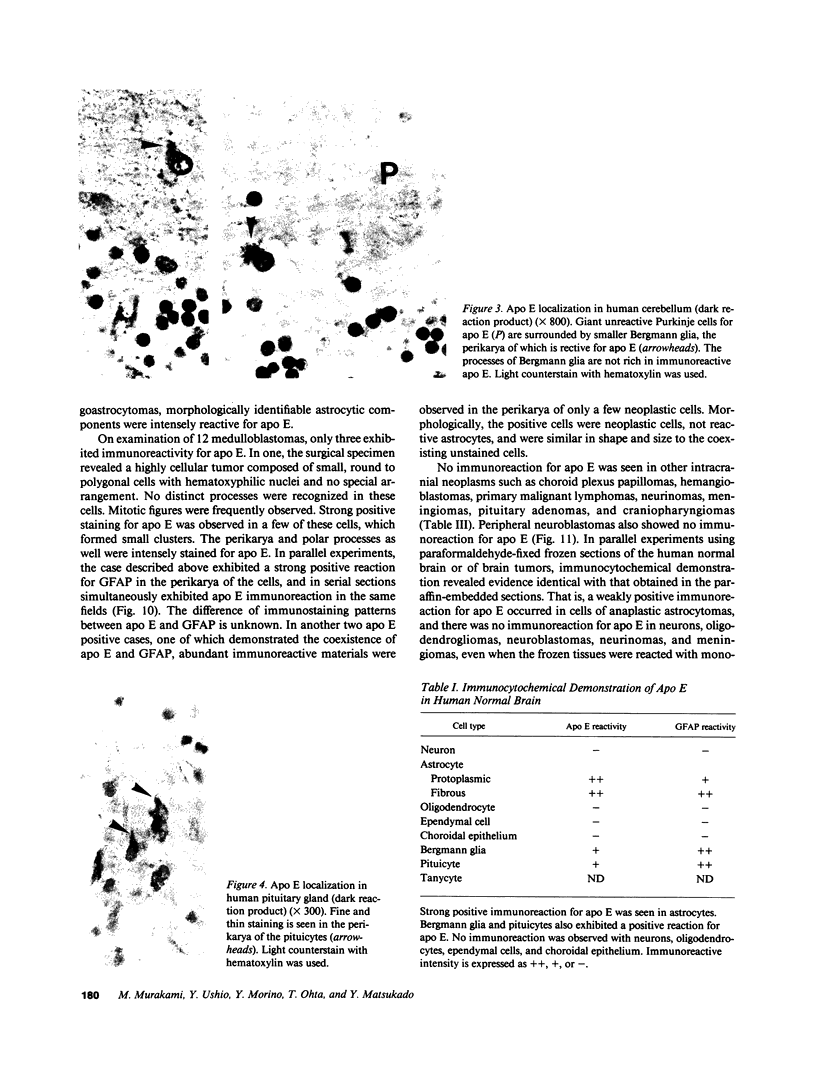
Immunocytochemical analyses revealed the presence and distribution of apolipoprotein E (apo E) in normal human brain tissue as well as in 77 human intracranial neoplasms. In normal brain tissues, the perikarya of astrocytes exhibited a strong positive reaction, whereas the Bergmann glia were stained to a moderate degree. However, no immunoreactivity was observed with neurons, oligodendrocytes, ependymal cells, and choroidal epithelium. Among the intracranial neoplasms, oligodendroglioma, choroid plexus papilloma, hemangioblastoma, primary malignant lymphoma, neurinoma, meningioma, pituitary adenoma, and craniopharyngioma were all negative. Immunoreactivity in the peripheral neuroblastoma was nil. However, the perikarya of astrocytomas and glioblastomas showed a positive reaction. Analyses on the degree of anaplasia and the amount of apo-E as an intensity of immunostaining showed a negative correlation. The astrocytic elements were stained in mixed oligoastrocytomas and medulloblastomas with glial differentiation. A few cases of ependymomas showed weak perikaryal immunostaining. Western blot analyses with anti-apo E antibody of a freshly prepared surgical specimen with astrocytomas revealed a single band with a molecular weight of approximately 37,000. The well differentiated cultured human astrocytoma cells secreted apo E into the medium. These lines of evidence suggest that apo E may serve as a potential marker specific for astrocytomas and glioblastomas, as well as an indicator of astrocytic tumor cell differentiation. The apo E localization in human brain tumors could be clinically relevant and diagnostically useful.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen M., von Gerstenberg A. C., Bock E. Mouse monoclonal antibodies reacting with human brain glial fibrillary acidic protein. J Neurochem. 1984 Jan;42(1):86–93. doi: 10.1111/j.1471-4159.1984.tb09702.x. [DOI] [PubMed] [Google Scholar]
- Bascó E., Woodhams P. L., Hajós F., Balázs R. Immunocytochemical demonstration of glial fibrillary acidic protein in mouse tanycytes. Anat Embryol (Berl) 1981;162(2):217–222. doi: 10.1007/BF00306493. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Williams D. L., Zucker S., Khan S. A., Blum C. B. Apolipoprotein E synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A. 1983 Jan;80(1):283–287. doi: 10.1073/pnas.80.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles J. K., Pitas R. E., Wilson E., Mahley R. W., Taylor J. M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985 Oct;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D. A., Zola H., McNamara P. J., Bradley J., Bradstock K. F., Hancock W. W., Atkins R. C. Membrane antigens of human cells of the monocyte/macrophage lineage studied with monoclonal antibodies. Pathology. 1983 Jan;15(1):45–52. doi: 10.3109/00313028309061401. [DOI] [PubMed] [Google Scholar]
- Carrella M., Cooper A. D. High affinity binding of chylomicron remnants to rat liver plasma membranes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):338–342. doi: 10.1073/pnas.76.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courel M. N., Girard N., Delpech B., Chauzy C. Specific monoclonal antibodies to glial fibrillary acidic protein (GFAP). J Neuroimmunol. 1986 Jun;11(4):271–276. doi: 10.1016/0165-5728(86)90080-9. [DOI] [PubMed] [Google Scholar]
- Elshourbagy N. A., Liao W. S., Mahley R. W., Taylor J. M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Horiuchi S., Tomita K., Murakami M., Morino Y., Takahashi K. Acetylated low-density lipoprotein is endocytosed through coated pits by rat peritoneal macrophages. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(1):1–13. doi: 10.1007/BF02889945. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter P. J., Darby J. K., Shooter E. M., Riccardi V. M., Weisgraber K. H., Boyles J. K., Mahley R. W. Apolipoprotein E synthesis in neurofibrosarcoma and schwannoma cell cultures from two individuals with neurofibromatosis. Exp Neurol. 1987 Feb;95(2):323–335. doi: 10.1016/0014-4886(87)90142-7. [DOI] [PubMed] [Google Scholar]
- Gullotta F., Schindler F., Schmutzler R., Weeks-Seifert A. GFAP in brain tumor diagnosis: possibilities and limitations. Pathol Res Pract. 1985 Jul;180(1):54–60. doi: 10.1016/S0344-0338(85)80075-3. [DOI] [PubMed] [Google Scholar]
- Herpers M. J., Budka H. Glial fibrillary acidic protein (GFAP) in oligodendroglial tumors: gliofibrillary oligodendroglioma and transitional oligoastrocytoma as subtypes of oligodendroglioma. Acta Neuropathol. 1984;64(4):265–272. doi: 10.1007/BF00690392. [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Murakami M., Takata K., Morino Y. Scavenger receptor for aldehyde-modified proteins. J Biol Chem. 1986 Apr 15;261(11):4962–4966. [PubMed] [Google Scholar]
- Horiuchi S., Takata K., Morino Y. Characterization of a membrane-associated receptor from rat sinusoidal liver cells that binds formaldehyde-treated serum albumin. J Biol Chem. 1985 Jan 10;260(1):475–481. [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Ignatius M. J., Gebicke-Härter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., Shooter E. M. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Ludwin S. K., Kosek J. C., Eng L. F. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976 Jan 15;165(2):197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Rall S. C., Jr, Weisgraber K. H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- Murakami M., Horiuchi S., Takata K., Morino Y. Distinction in the mode of receptor-mediated endocytosis between high density lipoprotein and acetylated high density lipoprotein: evidence for high density lipoprotein receptor-mediated cholesterol transfer. J Biochem. 1987 Mar;101(3):729–741. doi: 10.1093/jb/101.3.729. [DOI] [PubMed] [Google Scholar]
- Murakami M., Horiuchi S., Takata K., Morino Y. Scavenger receptor for malondialdehyde-modified high density lipoprotein on rat sinusoidal liver cells. Biochem Biophys Res Commun. 1986 May 29;137(1):29–35. doi: 10.1016/0006-291x(86)91171-x. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Gebicke-Härter P. J., Hangen D. H., Shooter E. M. A specific 37,000-dalton protein that accumulates in regenerating but not in nonregenerating mammalian nerves. Science. 1985 Apr 26;228(4698):499–501. doi: 10.1126/science.3983637. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Ignatius M. J., Hangen D. H., Shooter E. M. Expression of specific sheath cell proteins during peripheral nerve growth and regeneration in mammals. J Cell Biol. 1986 Feb;102(2):393–402. doi: 10.1083/jcb.102.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Fidge N. H., Nestel P. J. Studies on the in vivo and in vitro distribution of apolipoprotein A-IV in human plasma and lymph. J Clin Invest. 1985 Sep;76(3):1252–1260. doi: 10.1172/JCI112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Arnold K. S., Mahley R. W. Rate and equilibrium constants for binding of apo-E HDLc (a cholesterol-induced lipoprotein) and low density lipoproteins to human fibroblasts: evidence for multiple receptor binding of apo-E HDLc. Proc Natl Acad Sci U S A. 1979 May;76(5):2311–2315. doi: 10.1073/pnas.76.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982 Apr 25;257(8):4171–4178. [PubMed] [Google Scholar]
- Roheim P. S., Carey M., Forte T., Vega G. L. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm A. K., Hatton G. I., Nilaver G. Immunoreactive glial fibrillary acidic protein in pituicytes of the rat neurohypophysis. Brain Res. 1982 Mar 25;236(2):471–476. doi: 10.1016/0006-8993(82)90729-6. [DOI] [PubMed] [Google Scholar]
- Shelburne F. A., Quarfordt S. H. The interaction of heparin with an apoprotein of human very low density lipoprotein. J Clin Invest. 1977 Oct;60(4):944–950. doi: 10.1172/JCI108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1130–1134. doi: 10.1073/pnas.83.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco M. E., Dahl D., Roessmann U., Gambetti P. Immunohistochemical localization of glial fibrillary acidic protein in human glial neoplasms. Cancer. 1980 Feb;45(3):484–494. doi: 10.1002/1097-0142(19800201)45:3<484::aid-cncr2820450312>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson I. M., Anderson J. R., Holmes A. E. Oligodendroglioma: an analysis of 42 cases. J Neurol Neurosurg Psychiatry. 1987 Mar;50(3):304–312. doi: 10.1136/jnnp.50.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Dawson P. A., Newman T. C., Rudel L. L. Apolipoprotein E synthesis in peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2444–2451. [PubMed] [Google Scholar]
- Yoshitake S., Imagawa M., Ishikawa E., Niitsu Y., Urushizaki I., Nishiura M., Kanazawa R., Kurosaki H., Tachibana S., Nakazawa N. Mild and efficient conjugation of rabbit Fab' and horseradish peroxidase using a maleimide compound and its use for enzyme immunoassay. J Biochem. 1982 Nov;92(5):1413–1424. doi: 10.1093/oxfordjournals.jbchem.a134065. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., McPherson J., Goldberger G., Karathanasis S. K., Breslow J. L. Synthesis, intracellular processing, and signal peptide of human apolipoprotein E. J Biol Chem. 1984 May 10;259(9):5495–5499. [PubMed] [Google Scholar]
- de Armond S. J., Eng L. F., Rubinstein L. J. The application of glial fibrillary acidic (GFA) protein immunohistochemistry in neurooncology. A progress report. Pathol Res Pract. 1980;168(4):374–394. doi: 10.1016/s0344-0338(80)80273-1. [DOI] [PubMed] [Google Scholar]

























