Abstract
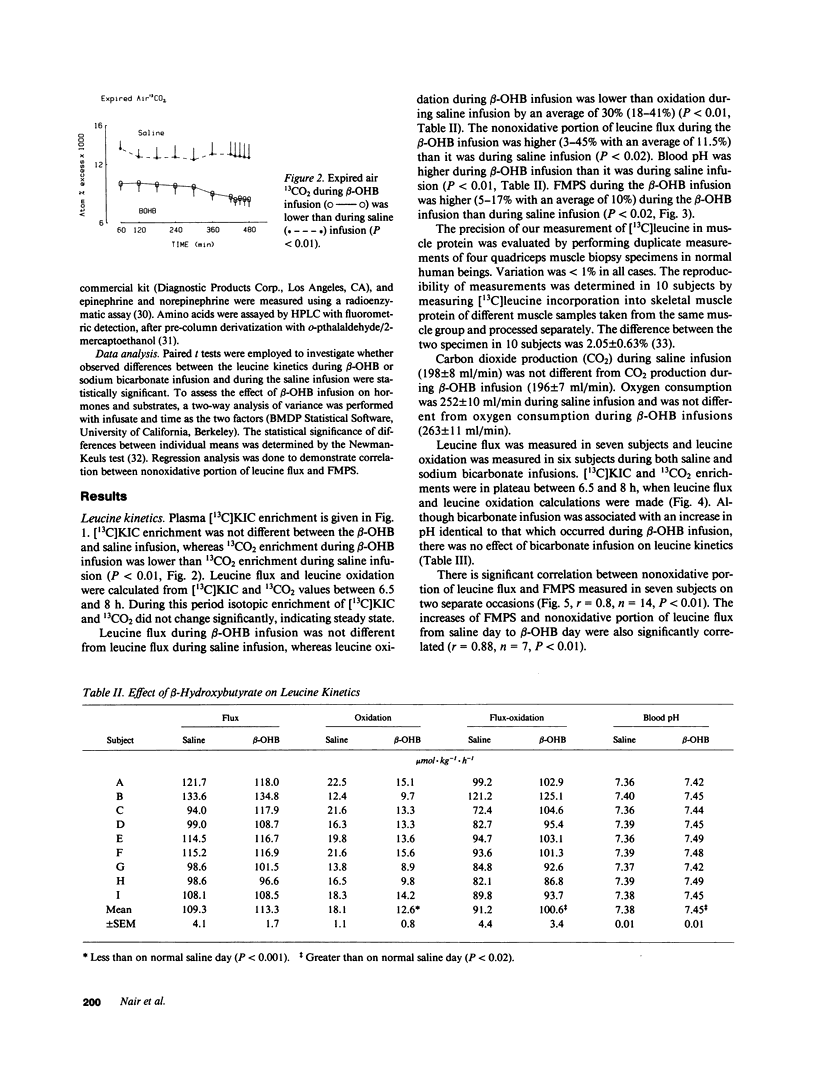
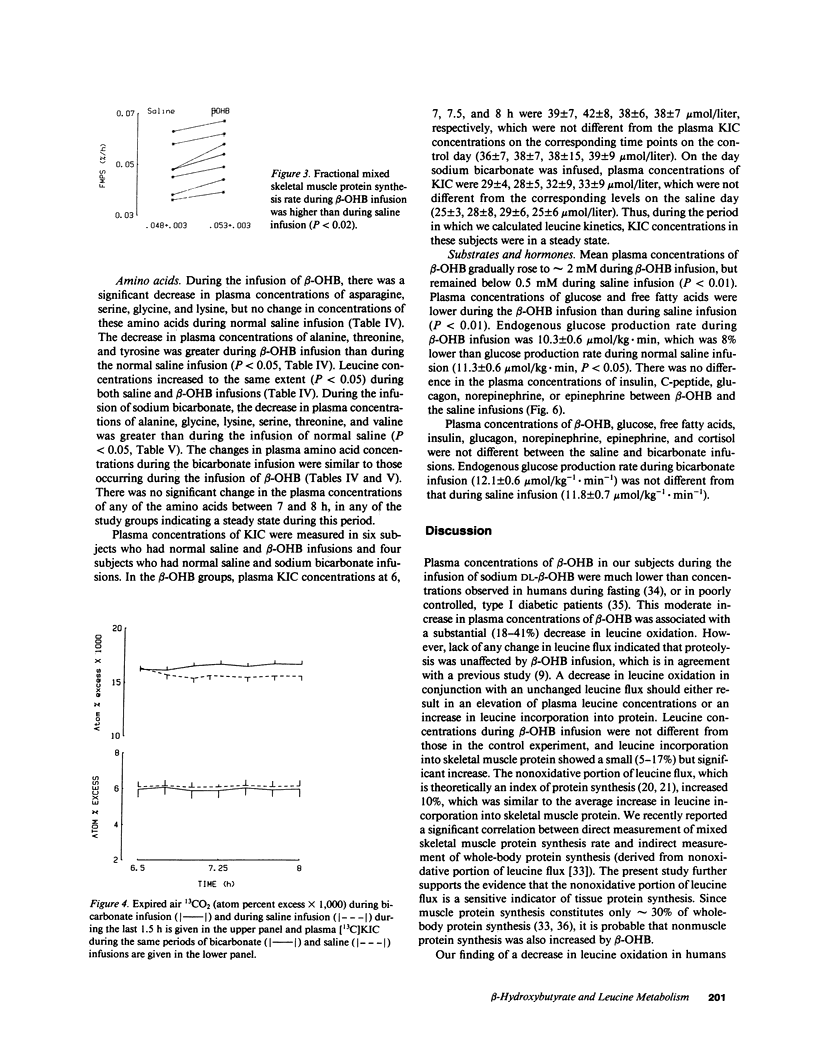
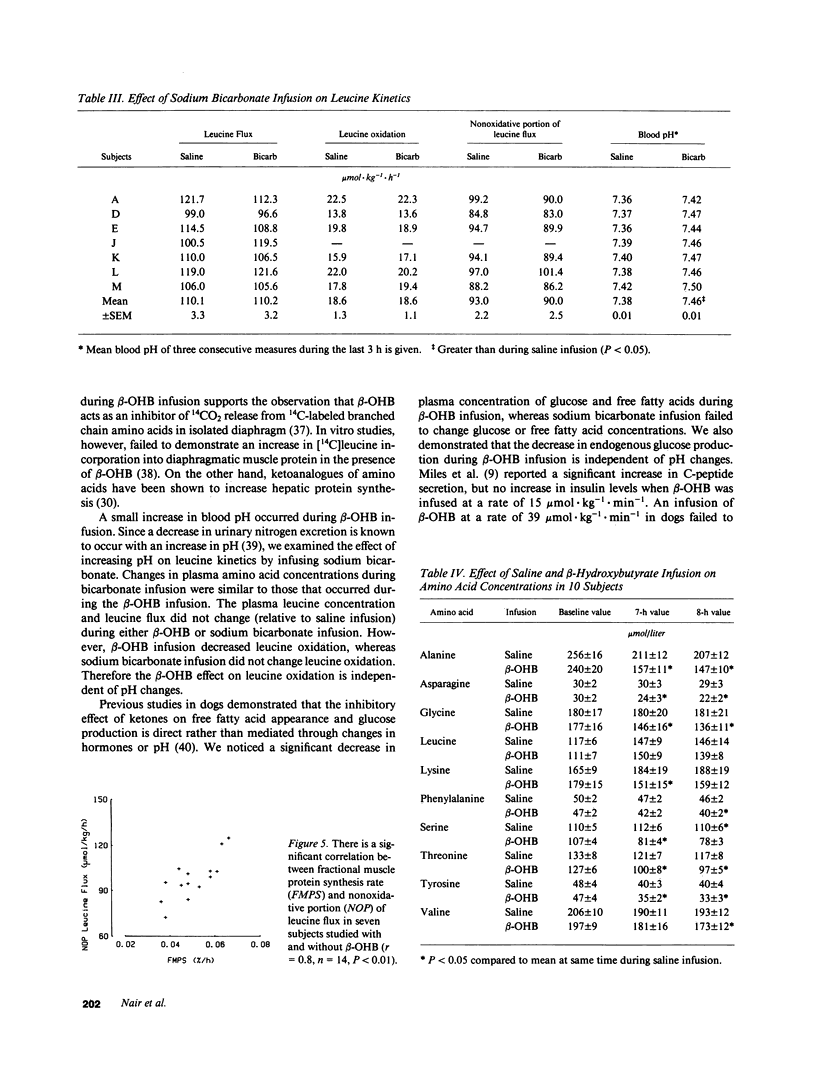
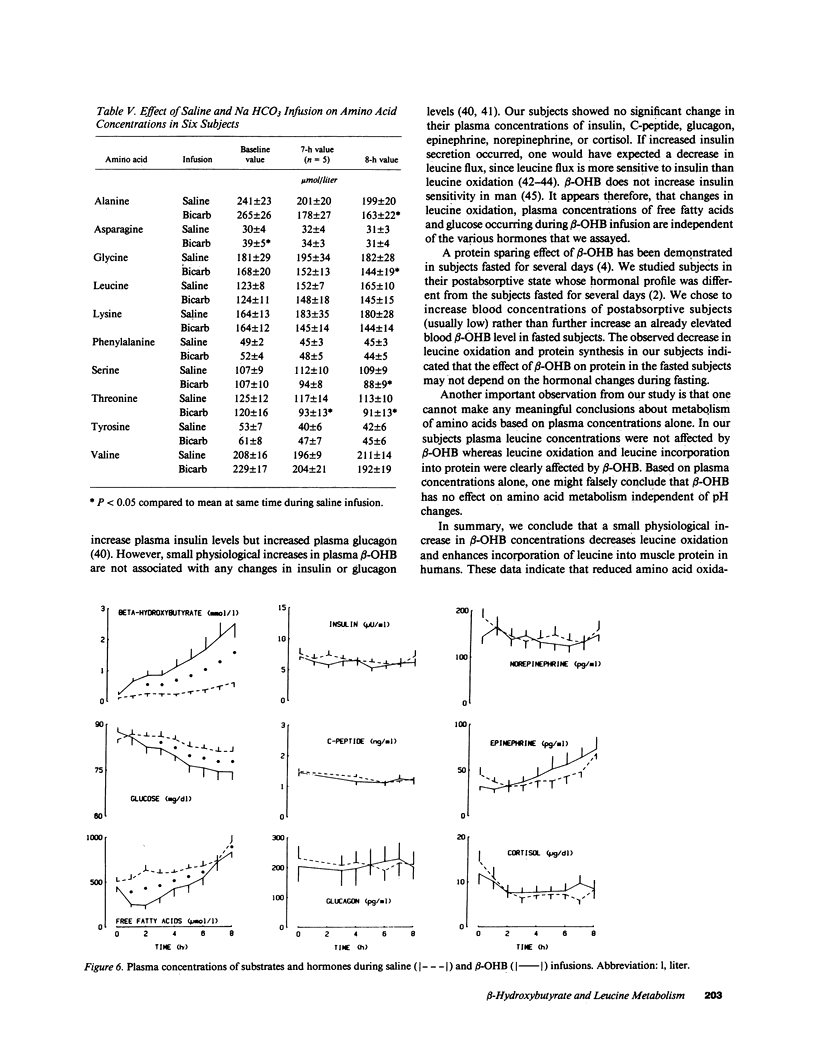
Because intravenous infusion of beta-hydroxybutyrate (beta-OHB) has been reported to decrease urinary nitrogen excretion, we investigated in vivo metabolism of leucine, an essential amino acid, using L-[1-13C]leucine as a tracer during beta-OHB infusion. Leucine flux during beta-OHB infusion did not differ from leucine flux during normal saline infusion in nine normal subjects, whereas leucine oxidation decreased 18-41% (mean = 30%) from 18.1 +/- 1.1 mumol.kg-1.h-1 (P less than 0.01), and incorporation of leucine into skeletal muscle protein increased 5-17% (mean = 10%) from 0.048 + 0.003%/h (P less than 0.02). Since blood pH during beta-OHB infusion was higher than the pH during saline infusion, we performed separate experiments to study the effect of increased blood pH on leucine kinetics by infusing sodium bicarbonate intravenously. Blood pH during sodium bicarbonate infusion was similar to that observed during the beta-OHB infusion, but bicarbonate infusion had no effect on leucine flux or leucine oxidation. We conclude that beta-OHB decreases leucine oxidation and promotes protein synthesis in human beings.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Rabin D., Diamond M. P., Lacy W. W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981 Sep;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain P. R., DeFronzo R. A. Failure of hyperketonemia to alter basal and insulin-mediated glucose metabolism in man. Horm Metab Res. 1986 Mar;18(3):185–189. doi: 10.1055/s-2007-1012266. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Friderici K. H., Buse J. F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972 Dec 25;247(24):8085–8096. [PubMed] [Google Scholar]
- Buse M. G., Buse J. Effect of free fatty acids and insulin on protein synthesis and amino acid metabolism of isolated rat diaphragms. Diabetes. 1967 Nov;16(11):753–764. doi: 10.2337/diab.16.11.753. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Edwards R., Young A., Wiles M. Needle biopsy of skeletal muscle in the diagnosis of myopathy and the clinical study of muscle function and repair. N Engl J Med. 1980 Jan 31;302(5):261–271. doi: 10.1056/NEJM198001313020504. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Cheng K. N., Halliday D. Analysis of (1-13C)leucine and (13C)KIC in plasma by capillary gas chromatography/mass spectrometry in protein turnover studies. Biomed Mass Spectrom. 1985 Aug;12(8):432–436. doi: 10.1002/bms.1200120814. [DOI] [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Rowe J. W., Goodman M. N., Matthews D. E., Bier D. M., Young V. R. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féry F., Balasse E. O. Differential effects of sodium acetoacetate and acetoacetic acid infusions on alanine and glutamine metabolism in man. J Clin Invest. 1980 Aug;66(2):323–331. doi: 10.1172/JCI109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday D., Read W. W. Mass spectrometric assay of stable isotopic enrichment for the estimation of protein turnover in man. Proc Nutr Soc. 1981 Sep;40(3):321–324. doi: 10.1079/pns19810048. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- Kelley J., Stirewalt W. S., Chrin L. Protein synthesis in rat lung. Measurements in vivo based on leucyl-tRNA and rapidly turning-over procollagen I. Biochem J. 1984 Aug 15;222(1):77–83. doi: 10.1042/bj2220077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch R. E., Frith L. O., Saunders S. J. Stimulation of albumin synthesis by keto analogues of amino acids. Biochim Biophys Acta. 1976 Sep 6;442(3):437–441. doi: 10.1016/0005-2787(76)90317-8. [DOI] [PubMed] [Google Scholar]
- Lilavivathana U., Brodows R. G., Woolf P. D., Campbell R. G. Counterregulatory hormonal responses to rapid glucose lowering in diabetic man. Diabetes. 1979 Oct;28(10):873–877. doi: 10.2337/diab.28.10.873. [DOI] [PubMed] [Google Scholar]
- Lobley G. E., Milne V., Lovie J. M., Reeds P. J., Pennie K. Whole body and tissue protein synthesis in cattle. Br J Nutr. 1980 May;43(3):491–502. doi: 10.1079/bjn19800116. [DOI] [PubMed] [Google Scholar]
- Low R. B., Stirewalt W. S., Rittling S. R., Woodworth R. C. Amino acid pools in cultured muscle cells. J Cell Biochem. 1984;25(3):123–129. doi: 10.1002/jcb.240250302. [DOI] [PubMed] [Google Scholar]
- Martin A. F., Rabinowitz M., Blough R., Prior G., Zak R. Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J Biol Chem. 1977 May 25;252(10):3422–3429. [PubMed] [Google Scholar]
- Matthews D. E., Motil K. J., Rohrbaugh D. K., Burke J. F., Young V. R., Bier D. M. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980 May;238(5):E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Haymond M. W., Gerich J. E. Suppression of glucose production and stimulation of insulin secretion by physiological concentrations of ketone bodies in man. J Clin Endocrinol Metab. 1981 Jan;52(1):34–37. doi: 10.1210/jcem-52-1-34. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Nissen S. L., Rizza R. A., Gerich J. E., Haymond M. W. Failure of infused beta-hydroxybutyrate to decrease proteolysis in man. Diabetes. 1983 Mar;32(3):197–205. doi: 10.2337/diab.32.3.197. [DOI] [PubMed] [Google Scholar]
- Müller M. J., Paschen U., Seitz H. J. Effect of ketone bodies on glucose production and utilization in the miniature pig. J Clin Invest. 1984 Jul;74(1):249–261. doi: 10.1172/JCI111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair K. S., Ford G. C., Halliday D. Effect of intravenous insulin treatment on in vivo whole body leucine kinetics and oxygen consumption in insulin-deprived type I diabetic patients. Metabolism. 1987 May;36(5):491–495. doi: 10.1016/0026-0495(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Garrow J. S., Ford C., Mahler R. F., Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983 Nov;25(5):400–403. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Halliday D., Garrow J. S. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984 Jul;27(1):13–16. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Halliday D., Griggs R. C. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol. 1988 Feb;254(2 Pt 1):E208–E213. doi: 10.1152/ajpendo.1988.254.2.E208. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawan G. L., Semple S. J. Effect of 3-hydroxybutyrate in obese subjects on very-low-energy diets and during therapeutic starvation. Lancet. 1983 Jan 1;1(8314-5):15–17. doi: 10.1016/s0140-6736(83)91560-x. [DOI] [PubMed] [Google Scholar]
- Read W. W., Read M. A., Rennie M. J., Griggs R. C., Halliday D. Preparation of CO2 from blood and protein-bound amino acid carboxyl groups for quantification and 13C-isotope measurements. Biomed Mass Spectrom. 1984 Jul;11(7):348–352. doi: 10.1002/bms.1200110706. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Edwards R. H., Halliday D., Matthews D. E., Wolman S. L., Millward D. J. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982 Dec;63(6):519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Schwenk W. F., Beaufrere B., Haymond M. W. Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol. 1985 Dec;249(6 Pt 1):E646–E650. doi: 10.1152/ajpendo.1985.249.6.E646. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Influence of beta-hydroxybutyrate infusion on glucose and free fatty acid metabolism in dogs. Am J Physiol. 1984 Dec;247(6 Pt 1):E756–E764. doi: 10.1152/ajpendo.1984.247.6.E756. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest. 1975 Jun;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- Umpleby A. M., Boroujerdi M. A., Brown P. M., Carson E. R., Sönksen P. H. The effect of metabolic control on leucine metabolism in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1986 Mar;29(3):131–141. doi: 10.1007/BF02427082. [DOI] [PubMed] [Google Scholar]
- Vazquez J. A., Paul H. S., Adibi S. A. Relation between plasma and tissue parameters of leucine metabolism in fed and starved rats. Am J Physiol. 1986 Jun;250(6 Pt 1):E615–E621. doi: 10.1152/ajpendo.1986.250.6.E615. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S. L., Campbell R. G. Decrease in resting metabolic rate during rapid weight loss is reversed by low dose thyroid hormone treatment. Metabolism. 1986 Apr;35(4):289–291. doi: 10.1016/0026-0495(86)90142-3. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Farrell R., Kerr A., Smith R. Muscle-protein catabolism after injury in man, as measured by urinary excretion of 3-methylhistidine. Clin Sci Mol Med. 1977 May;52(5):527–533. doi: 10.1042/cs0520527. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Goodenough R. D., Wolfe M. H., Royle G. T., Nadel E. R. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):458–466. doi: 10.1152/jappl.1982.52.2.458. [DOI] [PubMed] [Google Scholar]


