Abstract
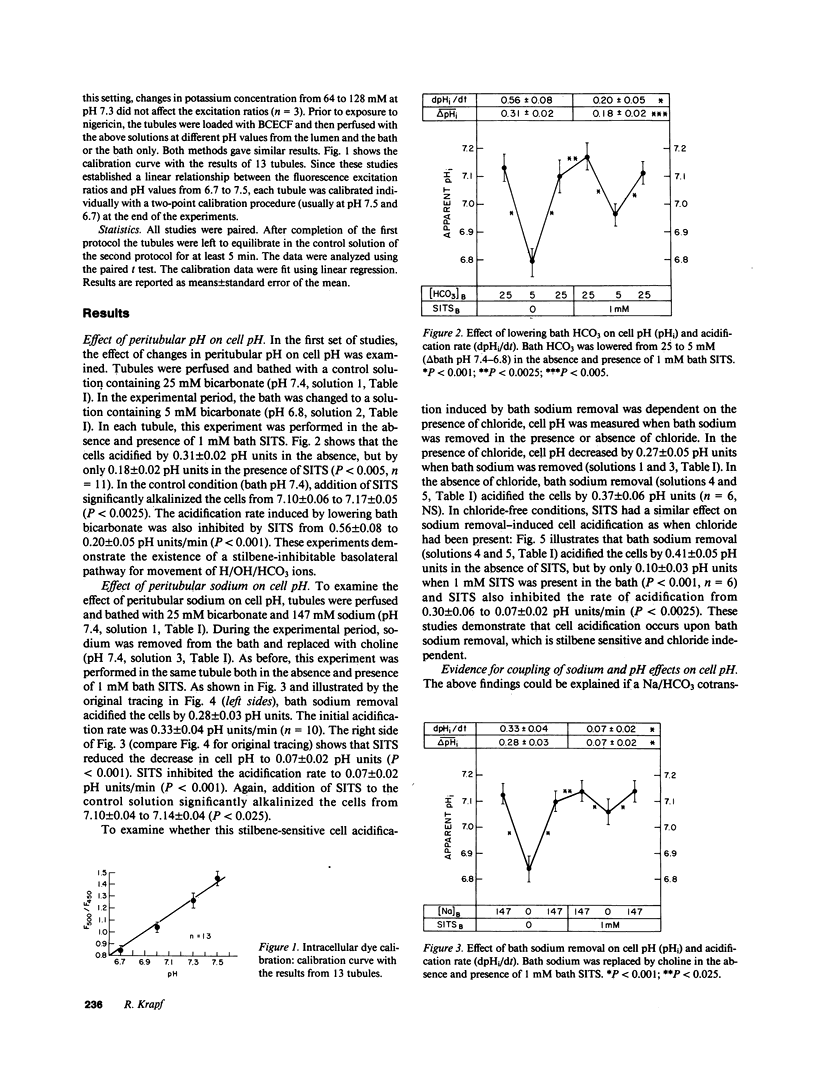
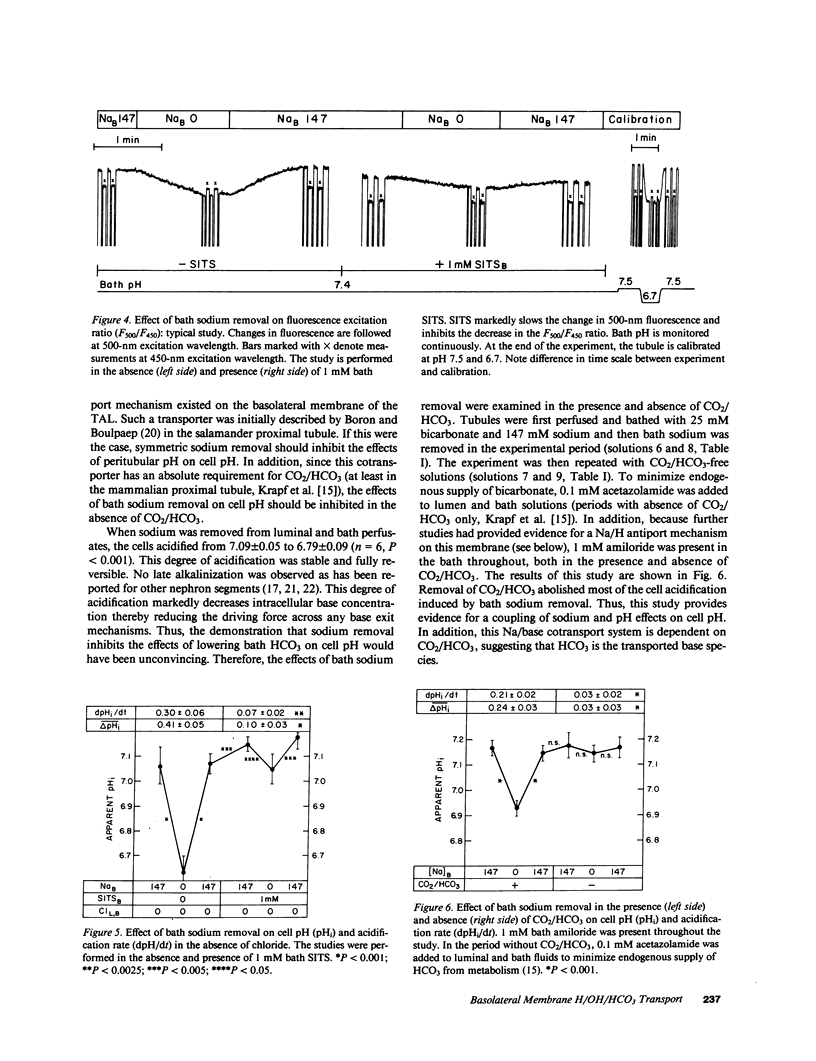
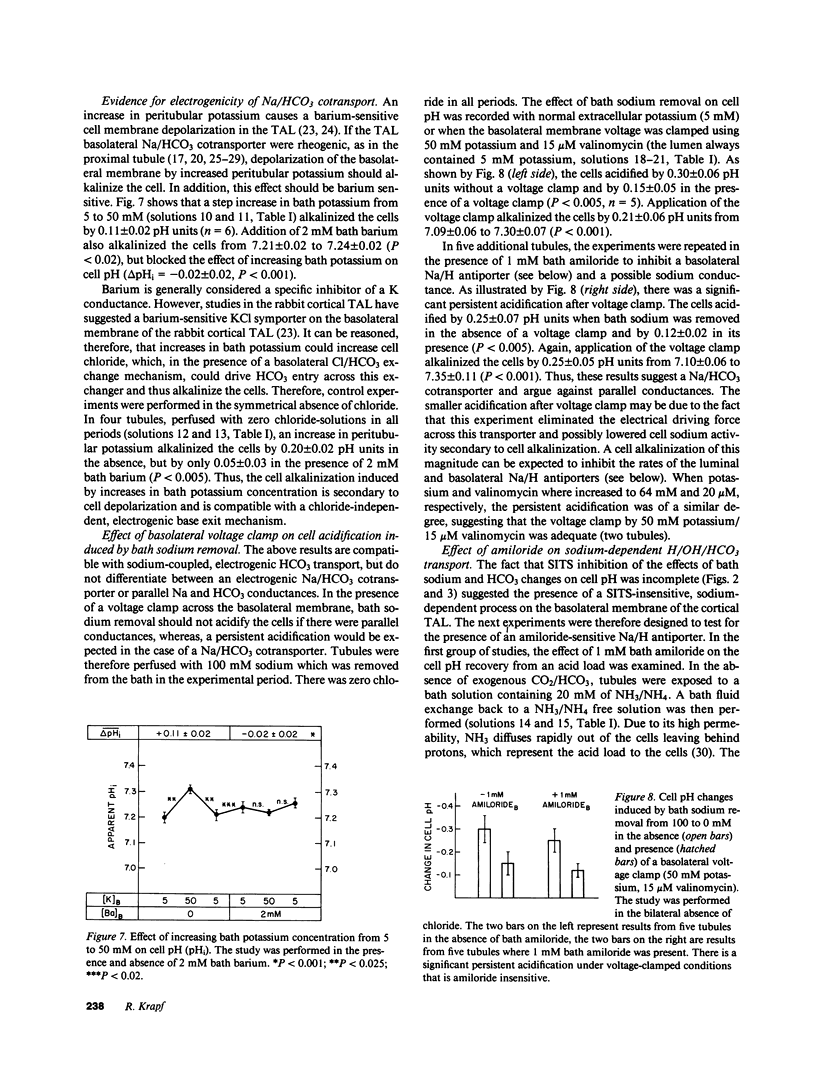
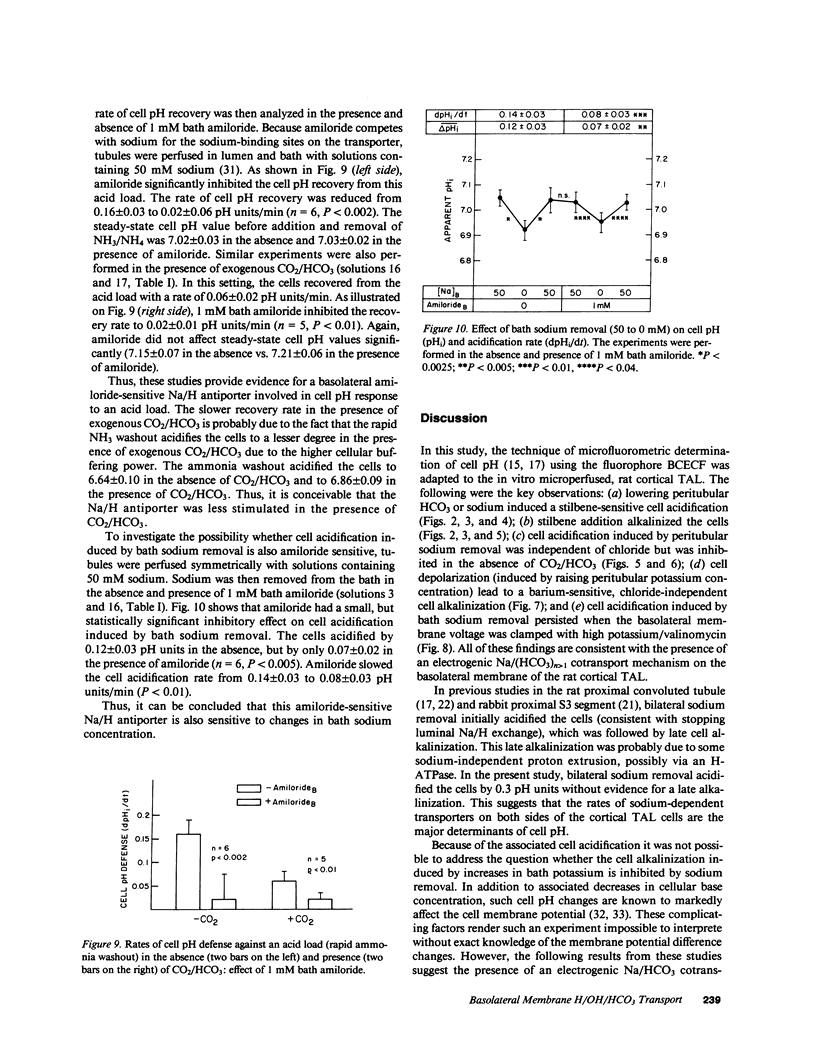
Mechanisms involved in basolateral H/OH/HCO3 transport in the in vitro microperfused rat cortical thick ascending limb were examined by the microfluorometric determination of cell pH using (2',7')-bis-(carboxyethyl)-(5,6)-carboxyfluorescein. The mean cell pH in this segment perfused with 147 mM sodium and 25 mM HCO3 at pH 7.4 was 7.13 +/- 0.02 (n = 30). Lowering bath HCO3 from 25 to 5 mM (constant PCO2 of 40 mmHg) acidified the cells by 0.31 +/- 0.02 pH units at a rate of 0.56 +/- 0.08 pH units/min. Removal of bath sodium acidified the cells by 0.28 +/- 0.03 pH units at a rate of 0.33 +/- 0.04 pH units/min. The cell acidification was stilbene inhibitable and independent of chloride. There was no effect of bath sodium removal on cell pH in the absence of CO2/HCO3. Depolarization of the basolateral membrane (step increase in bath potassium) independent of the presence of chloride. Cell acidification induced by bath sodium removal persisted when the basolateral membrane was voltage clamped by high potassium/valinomycin. Although these results are consistent with a Na/(HCO3)n greater than 1 cotransporter, a Na/H antiporter was also suggested: 1 mM bath amiloride inhibited the cell pH defense against an acid load (rapid ammonia washout), both in the presence and absence of CO2/HCO3, and inhibited the cell acidification induced by bath sodium reduction from 50 to 0 mM. In conclusion, an electrogenic Na/(HCO3)n greater than 1 cotransporter in parallel with a Na/H antiporter exist on the basolateral membrane of the rat cortical thick ascending limb.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Chambers M. Basolateral membrane Cl/HCO3 exchange in the rat proximal convoluted tubule. Na-dependent and -independent modes. J Gen Physiol. 1987 Apr;89(4):581–598. doi: 10.1085/jgp.89.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Biagi B. A., Sohtell M. Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule. Am J Physiol. 1986 Feb;250(2 Pt 2):F267–F272. doi: 10.1152/ajprenal.1986.250.2.F267. [DOI] [PubMed] [Google Scholar]
- Biagi B. A., Sohtell M. pH sensitivity of the basolateral membrane of the rabbit proximal tubule. Am J Physiol. 1986 Feb;250(2 Pt 2):F261–F266. doi: 10.1152/ajprenal.1986.250.2.F261. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983 Apr;244(4):F442–F454. doi: 10.1152/ajprenal.1983.244.4.F442. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Dobyan D. C., Magill L. S., Friedman P. A., Hebert S. C., Bulger R. E. Carbonic anhydrase histochemistry in rabbit and mouse kidneys. Anat Rec. 1982 Nov;204(3):185–197. doi: 10.1002/ar.1092040303. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Lucci M. S., Carter N. W. Micropuncture determination of pH, PCO2, and total CO2 concentration in accessible structures of the rat renal cortex. J Clin Invest. 1979 Aug;64(2):476–482. doi: 10.1172/JCI109485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Hamilton K. L., Johnson K. E. Intracellular acidosis blocks the basolateral Na-K pump in rabbit urinary bladder. Am J Physiol. 1984 Dec;247(6 Pt 2):F946–F954. doi: 10.1152/ajprenal.1984.247.6.F946. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Andreoli T. E. CO2-stimulated NaCl absorption in the mouse renal cortical thick ascending limb of Henle. Evidence for synchronous Na +/H+ and Cl-/HCO3- exchange in apical plasma membranes. J Gen Physiol. 1982 Nov;80(5):683–711. doi: 10.1085/jgp.80.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. W., Knepper M. A., Burg M. B. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol. 1984 Jul;247(1 Pt 2):F35–F44. doi: 10.1152/ajprenal.1984.247.1.F35. [DOI] [PubMed] [Google Scholar]
- Good D. W. Sodium-dependent bicarbonate absorption by cortical thick ascending limb of rat kidney. Am J Physiol. 1985 Jun;248(6 Pt 2):F821–F829. doi: 10.1152/ajprenal.1985.248.6.F821. [DOI] [PubMed] [Google Scholar]
- Grassl S. M., Aronson P. S. Na+/HCO3-co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J Biol Chem. 1986 Jul 5;261(19):8778–8783. [PubMed] [Google Scholar]
- Grassl S. M., Holohan P. D., Ross C. R. HCO3- transport in basolateral membrane vesicles isolated from rat renal cortex. J Biol Chem. 1987 Feb 25;262(6):2682–2687. [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Properties of the basolateral membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch. 1983 Mar;396(4):325–334. doi: 10.1007/BF01063938. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., London R., Boulpaep E. L., Giebisch G. Chloride transport across the basolateral cell membrane of the Necturus proximal tubule: dependence on bicarbonate and sodium. J Membr Biol. 1983;71(3):227–240. doi: 10.1007/BF01875464. [DOI] [PubMed] [Google Scholar]
- Hebert S. C. Hypertonic cell volume regulation in mouse thick limbs. II. Na+-H+ and Cl(-)-HCO3- exchange in basolateral membranes. Am J Physiol. 1986 Jun;250(6 Pt 1):C920–C931. doi: 10.1152/ajpcell.1986.250.6.C920. [DOI] [PubMed] [Google Scholar]
- Iino Y., Burg M. B. Effect of acid-base status in vivo on bicarbonate transport by rabbit renal tubules in vitro. Jpn J Physiol. 1981;31(1):99–107. doi: 10.2170/jjphysiol.31.99. [DOI] [PubMed] [Google Scholar]
- Khuri R. N., Agulian S. K., Kalloghlian A. Intracellular potassium in cells of the distal tubule. Pflugers Arch. 1972;335(4):297–308. doi: 10.1007/BF00586220. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Krapf R., Alpern R. J., Rector F. C., Jr, Berry C. A. Basolateral membrane Na/base cotransport is dependent on CO2/HCO3 in the proximal convoluted tubule. J Gen Physiol. 1987 Dec;90(6):833–853. doi: 10.1085/jgp.90.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf R., Berry C. A., Alpern R. J., Rector F. C., Jr Regulation of cell pH by ambient bicarbonate, carbon dioxide tension, and pH in the rabbit proximal convoluted tubule. J Clin Invest. 1988 Feb;81(2):381–389. doi: 10.1172/JCI113330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnerholm G., Ridderstråle Y. Intracellular distribution of carbonic anhydrase in the rat kidney. Kidney Int. 1980 Feb;17(2):162–174. doi: 10.1038/ki.1980.20. [DOI] [PubMed] [Google Scholar]
- Lönnerholm G., Wistrand P. J. Carbonic anhydrase in the human kidney: a histochemical and immunocytochemical study. Kidney Int. 1984 Jun;25(6):886–898. doi: 10.1038/ki.1984.106. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Giebisch G., Lang F., Wang W. Cellular Mechanism of the furosemide sensitive transport system in the kidney. Klin Wochenschr. 1982 Oct 1;60(19):1173–1179. doi: 10.1007/BF01716719. [DOI] [PubMed] [Google Scholar]
- Oberleithner H. Intracellular pH in diluting segment of frog kidney. Pflugers Arch. 1985 Jul;404(3):244–251. doi: 10.1007/BF00581246. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Grassi S. M., Aronson P. S. Stoichiometry of Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987 Apr;79(4):1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Wang W., Dietl P., Oberleithner H. Evidence for Na+ dependent rheogenic HCO3- transport in fused cells of frog distal tubules. Pflugers Arch. 1987 Mar;408(3):291–299. doi: 10.1007/BF02181472. [DOI] [PubMed] [Google Scholar]
- Wang W., Dietl P., Silbernagl S., Oberleithner H. Cell membrane potential: a signal to control intracellular pH and transepithelial hydrogen ion secretion in frog kidney. Pflugers Arch. 1987 Jul;409(3):289–295. doi: 10.1007/BF00583478. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Koseki C., Taniguchi J., Imai M. Functional heterogeneity in the hamster medullary thick ascending limb of Henle's loop. Pflugers Arch. 1987 May;408(6):600–608. doi: 10.1007/BF00581162. [DOI] [PubMed] [Google Scholar]


