Abstract
The regulation of blood vessel formation is of fundamental importance to many physiological processes, and angiogenesis is a major area for novel therapeutic approaches to diseases from ischemia to cancer. A poorly understood clinical manifestation of pathological angiogenesis is angiodysplasia, vascular malformations that cause severe gastrointestinal bleeding. Angiodysplasia can be associated with von Willebrand disease (VWD), the most common bleeding disorder in man. VWD is caused by a defect or deficiency in von Willebrand factor (VWF), a glycoprotein essential for normal hemostasis that is involved in inflammation. We hypothesized that VWF regulates angiogenesis. Inhibition of VWF expression by short interfering RNA (siRNA) in endothelial cells (ECs) caused increased in vitro angiogenesis and increased vascular endothelial growth factor (VEGF) receptor-2 (VEGFR-2)–dependent proliferation and migration, coupled to decreased integrin αvβ3 levels and increased angiopoietin (Ang)–2 release. ECs expanded from blood-derived endothelial progenitor cells of VWD patients confirmed these results. Finally, 2 different approaches, in situ and in vivo, showed increased vascularization in VWF-deficient mice. We therefore identify a new function of VWF in ECs, which confirms VWF as a protein with multiple vascular roles and defines a novel link between hemostasis and angiogenesis. These results may have important consequences for the management of VWD, with potential therapeutic implications for vascular diseases.
Introduction
Angiogenesis, the formation of new vessels from pre-existing ones, occurs physiologically in specific circumstances such as wound healing and the menstrual cycle. Dysregulated angiogenesis contributes to the pathogenesis of many disorders, including diabetes, cancer, and macular degeneration (reviewed in Carmeliet1). Angiogenic factors such as vascular endothelial growth factor (VEGF) and the angiopoietins (Ang) orchestrate signaling pathways that promote endothelial cell (EC) migration, proliferation, and ultimately the formation of a new vessel. VEGF-A is a major regulator of angiogenesis (reviewed in Grothey and Galanis2) and acts on ECs mainly through VEGF receptor-2 (VEGFR-2), a tyrosine kinase receptor (reviewed in Olsson3), to promote endothelial proliferation, migration, and sprouting of tip cells in the early stages of angiogenesis (reviewed in Gerhardt4). Ang-1 and Ang-2, which bind to the endothelial Tie-2 receptor, act in the later stages of blood vessel formation and are essential for the maturation of a stable vascular network and for the maintenance of endothelial integrity (reviewed in Thomas and Augustin5). Ang-1 and Ang-2 were originally identified as agonist and antagonist of Tie-2 signaling, respectively, with Ang-1 supporting EC survival and endothelial integrity6 and Ang-2 promoting blood vessel destabilization and regression.7 However, recent data suggest a more complex picture that includes cross-talk between the VEGF and the Ang pathways.8
Growth factor signaling pathways are influenced by surface adhesion molecules that mediate cell-cell or cell-matrix interactions, particularly by members of the integrin superfamily. The integrin that has received most attention in ECs is αvβ3 (reviewed in Hodivala-Dilke9), which mediates binding to several extracellular matrix proteins and growth factor receptors including VEGFR-2, thus influencing VEGFR-2 signaling (reviewed in Somanath et al10). αvβ3 plays a complex role in angiogenesis. Although the original data pointed to a uniquely pro-angiogenic role, more recent studies have highlighted the function of αvβ3 as both pro- and anti-angiogenic, possibly depending on the local extracellular environment and the specific ligand(s) (reviewed in Hodivala-Dilke9 and Somanath et al10).
One such ligand is von Willebrand factor (VWF),11 a multimeric plasma glycoprotein that mediates platelet adhesion to both the subendothelial matrix and endothelial surfaces and acts as a carrier for coagulation factor VIII in the circulation.12 VWF plays an essential role in hemostasis: its deficiency or dysfunction causes von Willebrand disease (VWD), the most common congenital bleeding disorder in humans,13 and increased levels of VWF are involved in acute coronary thrombosis and are a clinical marker of risk associated with atherosclerosis.14 Endothelial VWF is also involved in the regulation of inflammation by modulating leukocyte adhesion through direct and indirect mechanisms.15,16 VWF is synthesized by ECs, where it is both constitutively released into the circulation and stored within Weibel-Palade bodies (WPBs), from where it can be rapidly released upon stimulation.17 VWF drives the formation of WPBs,18 which also store regulators of angiogenesis and inflammation, including Ang-2 (reviewed in Metcalf et al19). In up to 10% of cases, VWD is associated with angiodysplasia,20 the most common vascular lesion of the gastrointestinal tract that can be responsible for intractable bleeding. Angiodysplastic lesions are characterized by a thin-walled, fragile vascular network with a disrupted architecture, increased permeability, and susceptibility to rupture. Recent studies have suggested that angiogenesis is involved in the pathogenesis of vascular malformations.21 Increased VEGF expression has been reported in human colonic angiodysplasia22 and, more recently, anti-angiogenic treatments have been cautiously tested to control bleeding from vascular malformations, with promising results (reviewed by Bauditz and Lochs23).
Because of its relationship with regulators of angiogenesis and the clinical observations of angiodysplasia in VWD patients, we postulated that VWF may regulate angiogenesis. Using human umbilical vein ECs (HUVECs), we found that inhibition of VWF expression by short interfering RNA (siRNA) in vitro resulted in increased stability of the capillary network in a 3D tube-formation assay, increased VEGFR-2-dependent proliferation and migration, which was coupled to a decrease in αvβ3 levels and activity and increased Ang-2 release. In VWF-deficient mice, we observed increased angiogenesis and a larger vascular network. Finally, we validated these findings in VWD patients using ECs derived from circulating progenitor cells. We conclude that endothelial VWF regulates angiogenesis.
Methods
Reagents
Unless otherwise specified, all reagents were purchased from Sigma-Aldrich.
Cells
HUVECs (Lonza) were cultured in endothelial cell growth medium-2 (EGM-2; Lonza). Blood outgrowth ECs (BOECs) were isolated from healthy volunteers and VWD patients as described in Ingram et al.24 This study was approved by the ethics committees of the Hammersmith, Queen Charlotte's, and Royal Marsden hospitals; informed consent was obtained from all individuals in accordance with the Declaration of Helsinki.
Design and delivery of VWF-specific siRNA
RNA interference of VWF expression was induced with siRNA (siVWF) using the target sequences 5′-AAGGGCTCGAGTGTACCAAAA-3′ or 5′-ACGGCTTGCACCATTCAGCTA-3′ (Qiagen) or 5′-GGGATCTGTGATGAGAACGGA-3′. AllStars Negative Control siRNA (siCTL; (Qiagen) was used as a control. Transfection of HUVECs was by nucleofection or as described in Birdsey et al.25
Polymerase chain reaction and immunoblots
RNA and protein were prepared from siCTL- or siVWF-treated HUVECs after 24-48 hours. Levels of VWF and β3 integrin mRNA, normalized to GAPDH, were measured by quantitative real-time polymerase chain reaction (RT-PCR) using the following primers to VWF: (5′-GCAGTGGAGAACAGTGGTG-3′, 5′-GTGGCAGCGGGCAAAC-3′), β3 integrin (5′-TGACGAAAATACCTGCAACCG-3′, 5′-GCATCCTTGCCAGTGTCCTTAA-3′), and GAPDH (5′-CAAGGTCATCCATGACAACTTTG-3′, 5′-GGGCCATCCACAGTCTTCTG-3′).
Immunoblotting of whole-cell lysates was performed with antibodies to: VWF (polyclonal: A0082; Dako); αV (polyclonal: Ab1930; Millipore); β3 (polyclonal: 4702; Cell Signaling Technology); and β1 (clone: P5D2; Millipore). Quantification was performed by densitometry and normalized against α-tubulin (clone: 1A4).
Immunofluorescence of HUVECs
Immunofluorescence was performed as described in Birdsey et al25 with antibodies to VWF, vascular endothelial (VE)–cadherin (clone: 55-7H1; BD Biosciences), CD45 (clones: 2B11+PD7/26/16; Serotec), or intercellular adhesion molecule-2 (ICAM-2; clone: BT-1; Serotec). Images were captured using an LSM510 META confocal microscope (Carl Zeiss) using a 63× objective lens and running Version 3.2 of the LSM acquisition software.
In vivo angiogenesis Matrigel assay
The assays were performed as described in Birdsey et al25 on VWF-deficient mice (KO)26 (The Jackson Laboratory) or littermate controls. Experiments were performed according to the Animals (Scientific Procedures) Act 1986.
Staining of Matrigel plugs
Sections (4 μm) of paraffin-embedded Matrigel plugs were stained with hematoxylin and eosin or with anti-CD31 antibody (polyclonal: Ab28364), anti–VEGFR-2 antibody (polyclonal: Ab2349; Abcam), anti-CD45 antibody (clone: 30-F11; BD Biosciences), or immunoglobulin G control (Insight Biotechnology), as described in Birdsey et al.25 Volocity software (Improvision) was used to quantify nucleated cells. Images were taken with a BX50 camera (Olympus) with Viewfinder software Version 3.0.1 (Pixera) using a 4× objective lens. The whole section was photographed using between 2 and 7 fields of view.
Integrin trafficking
Measurement of β3 integrin internalization was performed as described in Roberts et al.27 Briefly, 48-hours after transfection, siCTL- or siVWF-treated cells were serum starved for 2 hours in M199 10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–NaOH, pH 7.5, and bovine serum albumin (BSA) 0.1%, then surface labeled on ice with 0.2 mg/mL succinimidyl 2-(biotinamido)-ethyl-1,3′-dithiopropionate (NHS-SS-biotin; Pierce/Thermo Fisher Scientific). Cells were transferred to serum-free medium containing 0.6mM primaquine to prevent recycling, and incubated at 37°C for 7.5 minutes. Cells were then incubated on ice and stripped of their residual surface-bound biotin by treatment with the reducing agent sodium 2-mercaptoethane sulfonate. Cells were lysed in 50mM Tris-HCl (pH 7.5), 150mM NaCl, 5mM Na2-EDTA (ethylenediaminetetraacetic acid), 0.5% deoxycholate, 1% Triton X-100, 0.05% sodium dodecyl sulfate, 15mM NaF, and 1.5mM Na3VO4 supplemented with protease inhibitor cocktail (Sigma P8340). Lysates were subjected to capture enzyme-linked immunosorbent assay (ELISA) in microtiter plates coated with β3 antibody (clone: VI-PL2; BD Pharmingen) and detected by streptavidin-conjugated peroxidase using o-phenylenediamine dihydrochloride as a substrate.
Ang-2 release
Ang-2 was detected in the cell supernatant of siCTL- or siVWF-treated HUVECs for 48 hours using the Human Angiopoietin-2 DuoSet ELISA kit (R&D Systems Europe).
Proliferation of HUVECs and BOECs
siCTL- or siVWF-treated HUVECs or BOECs were incubated with M199 medium containing 1% fetal calf serum (FCS) for 4 hours. Cells were seeded onto gelatin-coated 24-well plates at 3684 cells/cm2 in M199/10% FCS ± 100 ng/mL VEGF165 (PeproTech) and ± 10 μg/mL VWF (purified from plasma concentrate in-house). Cells were counted using a hemo-cytometer 96 hours after seeding.
Migration of HUVECs and BOECs
siCTL- or siVWF-treated HUVECs were incubated with M199 medium/1% FCS for 4 hours. Migration was measured ± 50 ng/mL of VEGF165 and 10 μg/mL of purified VWF as described in Huang et al28 using a Zeiss LSM510 META microscope. Individual cell migration was quantified from 3 separate experiments measuring 10 cells per treatment using ImageJ software Version 1.43 (National Institutes of Health) with tracking (Institut Curie) and chemotaxis (Ibidi) plug-ins. The migration of cells at the periphery of BOEC colonies was measured with the same procedure as for the migration of HUVECs.
In vitro Matrigel assay
siCTL- or siVWF-treated HUVECs or BOECs were incubated with M199 medium/1% FCS for 4 hours. Cells were resuspended in M199/1% FCS ± 10 μg/mL of purified VWF and seeded onto growth factor–depleted Matrigel (BD Biosciences)–coated 48-well plates at 50 000 cells/cm2. Capillary tubes were photographed with a CKX41 microscope (Olympus) using a 4× objective lens and a DP12-2 camera (Olympus). Total capillary tube length at 24 hours after seeding was quantified using ImageJ software.
Staining of mouse ears
VWF-deficient mice or littermate controls were culled, the ears removed, fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) for 60 minutes, washed in 50mM NH4Cl PBS, and the cartilage layer removed. The vascular layer was permeabilized with 0.5% Triton X-100 PBS containing 20% goat serum overnight. Samples were washed 3 times in PBS and incubated with 14 μg/mL anti–α-SMA-Cy3 (clone: 1A4) overnight. Mounted samples were imaged as described in Birdsey et al25 using a Zeiss LSM510 META confocal microscope with a 10× objective. A tile scan was performed to obtain a composite image of the whole ear. Digitized images (2040 × 2047 pixels) were segmented using an automated multiscale and region growing technique (RISA-J29), and additional analyses were performed using ImageJ software. Vascular density was calculated as a percentage area of vessels/ear area and fractal dimension, a measure of space filling, was calculated using the box-counting method.30
Adhesion assay
Plates (96-well) were coated with 1% gelatin, 100 μg/mL type 1 collagen (BD Biosciences), 30 μg/mL VWF (purified in-house), or 1% BSA overnight at 4°C, washed 2 times with PBS, and blocked with 1% BSA for 1 hour. siCTL- or siVWF-treated HUVECs were labeled with 6.25μM 5-chloromethylfluorescein diacetate (Invitrogen) following the manufacturer's protocol, and reseeded at 20 000 cells per well onto the coated plates. After 40 minutes, the total cellular fluorescence was measured with a Synergy plate reader (BioTek Instruments) with absorption and emission settings at 492 and 517 nm, respectively. Wells were then washed gently 3 times with PBS containing Ca2+ and Mg2+ at 37°C to remove nonadherent cells. The fluorescence of bound cells was then measured with the Synergy plate reader using the same settings. The percentage of bound cells relative to the total number seeded was then calculated.
Flow cytometry
siCTL- or siVWF-treated HUVECs were harvested, washed 2 times with Hanks balanced salt solution (with Ca2+ and Mg2+) containing 1% FCS, and resuspended in 20 μg/mL primary antibody solution for 30 minutes at 4°C. Cells were washed 2 times, resuspended in secondary antibody solution, and incubated for 30 minutes at 4°C. Cells were then washed 4 times and resuspended in PBS containing 1% paraformaldehyde. Flow cytometry was performed using an Epics XL flow cytometer (Beckman Coulter).
Statistical analysis
All numerical data are expressed as means ± SEM. We analyzed datasets for significance with the Student t test. P values < .05 were considered statistically significant.
Results
Endothelial VWF regulates in vitro angiogenesis
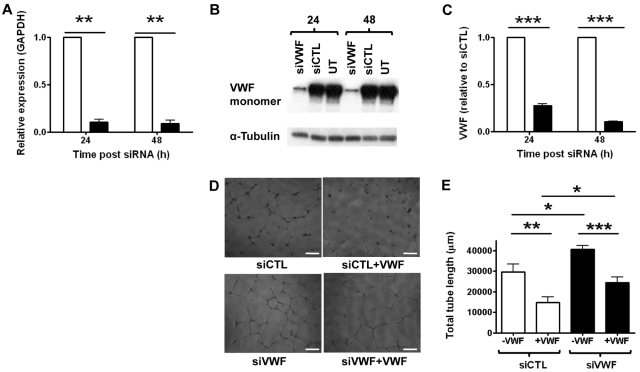
To study the role of endothelial VWF in angiogenesis, we inhibited its expression in HUVECs using specific siRNA (siVWF). siVWF inhibited VWF expression by ≥ 90% compared with control siRNA (siCTL), both at the mRNA and the protein level (Figure 1A-C and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We investigated the angiogenic properties of siVWF-treated HUVECs in an in vitro tube formation assay on growth factor–depleted Matrigel. Inhibition of VWF expression resulted in increased capillary tube network formation compared with control cells (Figure 1D left panels and 1E), suggesting that VWF may repress constitutive proangiogenic signaling pathways in ECs. To test whether extracellular VWF could reverse this phenotype, purified human plasma-derived VWF was added in solution to the Matrigel assay. The addition of soluble VWF to the medium inhibited capillary network formation to a similar extent in both control and VWF-deficient cells (Figure 1D right panels and 1E), suggesting that extracellular VWF is involved in regulating the vascular network. However, even after the addition of extracellular VWF, VWF-deficient cells still displayed a significantly increased capillary network compared with controls, suggesting that other mechanisms independent of extracellular VWF are also involved in the proangiogenic phenotype in VWF-deficient cells.
Figure 1.
VWF-deficient cells display enhanced angiogenesis in vitro. VWF-specific (20nM; siVWF, closed bars), or control siRNA (20nM; siCTL, open bars) was transfected into HUVECs for 24 or 48 hours. VWF expression was measured in these cells by (A) RT-PCR, (B) Western blotting (representative blot shown), or (C) VWF ELISA. (D) HUVECs treated with control or VWF-specific siRNA for 24 hours were seeded onto Matrigel. Capillary network formation was observed in the presence (right panels) or absence (left panels) of 10 μg/mL VWF after 24 hours and quantified in panel E by measuring total tube length in micrometers. (Bar = 200 μm). Data for siVWF in panels A and C have been normalized to siCTL. siCTL, nonspecific siRNA-treated cells; siVWF, VWF siRNA-treated cells; UT, untreated cells (n = 3). Error bars = mean ± SEM. *P < .05; **P < .01; ***P < .001 (Student t test).
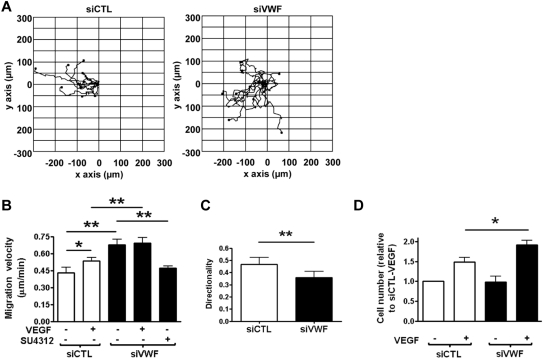
The in vitro tube-formation assay in Matrigel encompasses many of the steps involved in the angiogenic process, including adhesion, alignment, tube formation, and migration.31 We therefore investigated the role of VWF in EC migration using a scratch-wound assay and time-lapse microscopy. As shown in Figure 2A and B, migration velocity was increased in siVWF-treated ECs compared with control ECs, and this was associated with a loss of directionality (Figure 2A-C). The phenotype was confirmed using a second, independent siRNA sequence to inhibit VWF expression (data not shown). The increased migration of siVWF-treated ECs was dependent on VEGFR-2 signaling, because it was inhibited by a specific VEGFR-2 tyrosine kinase inhibitor, SU4312 (Figure 2B). Therefore, these results indicate that VWF inhibits a constitutive VEGFR-2–dependent pathway(s) that promotes EC migration. Interestingly, the addition of soluble VWF did not affect the migration of siCTL- or siVWF-treated cells (data not shown), suggesting that this phenotype is mediated by intracellular VWF.
Figure 2.
VWF-deficient cells display increased migration and proliferation in vitro due to increased VEGFR-2 signaling. (A) Representative trajectory plots of siCTL- or siVWF-treated HUVECs migrating into a wounded area for 16 hours, starting at 30 hours after siRNA treatment. The starting point of each cell trajectory is plotted at the center of the graph and the wounded area is to the left of zero on the x-axis. (B) Migration velocity (in micrometers per minute) with or without 50 ng/mL VEGF or with 4μM VEGFR-2 inhibitor SU4312. (C) Directionality of cell migration (Euclidean distance/accumulated distance). (D) Proliferation (in cells per square centimeter) of HUVEC 24 hours after control or siVWF treatment, cultured with or without 100 ng/mL VEGF for 96 hours. Data for siVWF in panel D has been normalized to siCTL-VEGF. siVWF was still effective at 120 hours after siRNA treatment (data not shown). siCTL, nonspecific siRNA-treated cells; siVWF, VWF siRNA-treated cells (n = 3). Error bars = mean ± SEM. *P < .05; **P < .01 (Student t test).
VEGF also induces the proliferation of ECs, another essential process in the formation of new vessels. We therefore tested the role of VWF in an in vitro EC proliferation assay. No difference in basal proliferation was observed in siVWF- compared with siCTL-treated cells; however, VEGF-induced proliferation was higher in siVWF-treated ECs than in siCTL-treated cells (Figure 2D). The phenotype was confirmed using a second, independent siRNA sequence to inhibit VWF expression (data not shown). Again, the addition of soluble VWF did not affect proliferation of siCTL- or siVWF-treated cells (data not shown), indicating that intracellular VWF may be involved in regulating EC proliferation. These data indicate that endothelial VWF inhibits tube formation, proliferation, and migration of ECs in vitro through pathways that involve VEGFR-2 signaling and that are regulated by both intracellular and extracellular VWF.
Endothelial VWF regulates αvβ3 integrin levels and activity in ECs
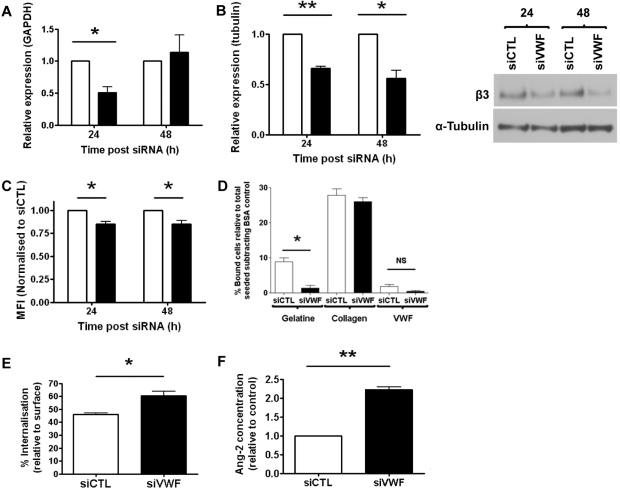
The best-characterized endothelial receptor for VWF is the integrin αvβ3, which plays multiple roles in angiogenesis and has been associated with both increased and decreased neovascularization (reviewed in Hodivala-Dilke9). We investigated whether the phenotype of VWF-deficient cells might be linked to αvβ3. Levels of β3 mRNA were decreased after 24 hours of siRNA treatment, and returned to normal after 48 hours (Figure 3A). Total β3 protein levels were also significantly decreased at 24 and 48 hours after treatment (Figure 3B), and surface levels of αvβ3 were significantly reduced in siVWF-treated ECs (24-48 hours; Figure 3C). Treatment with a second, independent VWF siRNA sequence confirmed the decrease in β3 mRNA and protein levels (data not shown). Protein levels of αv and β1 integrin subunit were unaffected (supplemental Figure 2). Adhesion assays to extracellular matrix proteins confirmed the functional defect of αvβ3 integrin in VWF-deficient HUVECs, because binding of ECs to the αvβ3-dependent substrate gelatin32 was decreased compared with controls (Figure 3D). ECs binding to VWF, which is also αvβ3 dependent, was very low and, although lower in VWF-depleted cells, it did not achieve significance (Figure 3D). Binding to the β1 integrin ligand collagen type I was unaffected (Figure 3D).
Figure 3.
VWF-deficient cells display reduced β3 integrin expression and αvβ3-dependent adhesion, increased rate of β3 integrin internalization, and elevated release of Ang-2. (A) β3 mRNA expression was measured by RT-PCR. (B) Total β3 integrin protein expression was measured by Western blotting (representative blot shown) and quantified by densitometry relative to tubulin. (C) Surface levels of total αvβ3 (antibody: LM609) were measured by flow cytometry at 24 and 48 hours after siRNA treatment. (D) Control or siVWF-treated cells, 48 hours after transfection, were seeded onto different extracellular matrix substrates. After 40 minutes, nonadherent cells were removed by gentle washing and the number of bound cells was quantified relative to the total number seeded. (E) Internalization of β3 integrin from the cell surface after incubation at 37°C for 7.5 minutes. (F) Levels of Ang-2 in the supernatant of control or siVWF-treated cells were measured 48 hours after transfection by ELISA. Data (A-C, F) were normalized to control siRNA-treated cells at each time point. Open bars, siCTL; closed bars, siVWF. Error bars indicate mean ± SEM (n = 3). *P < .05; **P < .01 (Student t test).
VWF binds αvβ3 via its C-terminal RGD site.11 Intracellular trafficking of αvβ3 was recently shown to be affected by RGD mimetics,33 suggesting that interaction with RGD-containing proteins such as VWF could modulate αvβ3 trafficking. Consistent with this hypothesis and with the reduced levels of surface β3, Figure 3E shows that internalization rates of αvβ3 are increased in VWF-deficient HUVECs. Therefore, VWF appears to regulate both the levels of αvβ3 in ECs and its internalization.
Endothelial VWF regulates Ang-2 release in ECs
VWF is essential for the formation of WPBs in ECs, which store a number of proteins including Ang-2.19 We speculated that the loss of VWF may result in the inability of ECs to store Ang-2 and thus increase its dysregulated release. Ang-2 is known to promote VEGF-dependent stimulation of EC sprouting and migration,8 and has recently been found to induce degradation of integrin β3.34 Therefore, dysregulated Ang-2 release from VWF-deficient ECs could be involved in the proangiogenic effects of VWF. In support of this hypothesis, we found that Ang-2 levels in the supernatant of VWF siRNA-treated cells were increased compared with control cells (Figure 3F); these data were confirmed using a second, independent VWF siRNA sequence (data not shown). These results suggest that VWF regulates VEGF-dependent angiogenesis via multiple pathways involving the integrin αvβ3 and the growth factor Ang-2, and that these pathways may cross-talk and converge to influence VEGFR-2 signaling.
VWF-deficient mice show increased angiogenesis and blood vessel density
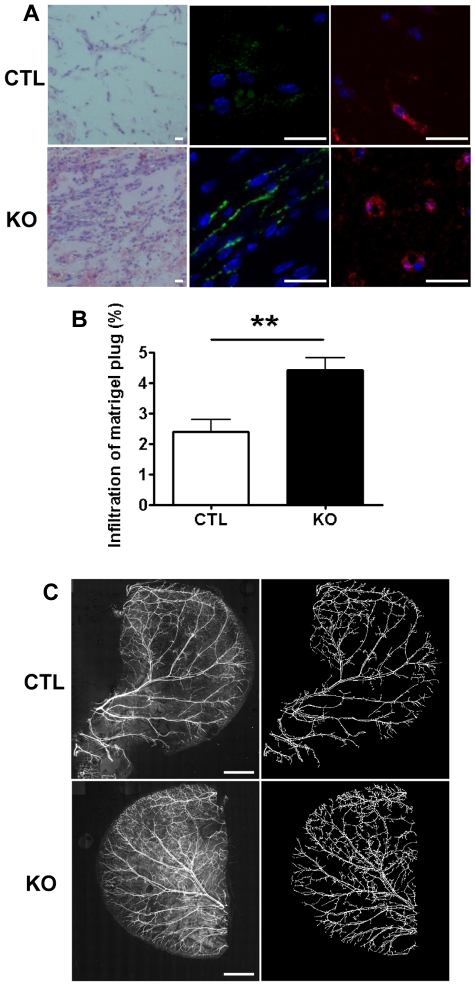
Results from the in vitro studies suggest that VWF is required to control vessel formation and that a lack of VWF results in increased angiogenesis. To confirm this in vivo, we studied angiogenesis in VWF-deficient mice using the Matrigel plug model.25 VWF-deficient mice (KO) or littermate controls (CTL) were injected subcutaneously with Matrigel, and plugs were excised after 7 days. A significantly higher cellular infiltrate, previously shown to be correlated with new vessel formation,35,36 was found in Matrigel plugs from VWF-deficient mice compared with CTL mice (Figure 4A left panels and B). Immunofluorescent staining for CD31 (Figure 4A middle panels) and VEGFR-2 (Figure 4A right panels) confirmed the presence of vessels in Matrigel plugs from VWF-deficient mice. These data suggest that lack of VWF promotes angiogenesis in vivo.
Figure 4.
VWF-deficient mice display increased angiogenesis and mature blood vessel density. (A) Matrigel was injected subcutaneously into littermate control (CTL; top panels) or VWF-deficient mice (KO; bottom panels). Seven-day-old plugs were excised, sectioned, and stained with hematoxylin and eosin (left panels), or for immunofluorescence microscopy, with anti-CD31 (middle panels) or VEGFR-2 (right panels). Size bar = 20μm. (B) Cellular infiltrate was quantified in hematoxylin and eosin sections from CTL (open bars) or KO (closed bars). n = 5 KO mice and n = 6 CTL mice. (C) CTL or KO mice were culled and whole ears were removed. Ears were stained with anti–α-SMA-Cy3 and a tile scan was performed to obtain a composite image of the whole ear (left panels). Size bar = 2 mm. Blood vessels were identified, segmented, and converted to binary images (right panels). Total ear area, blood vessel area (relative to total ear area), and fractal dimensions were calculated. n = 5 KO mice and n = 7 CTL mice, Error bars indicate mean ± SEM. **P < .01 (Student t test).
Because we observed an increase in angiogenesis, we speculated that the constitutive vascular network in the VWF-deficient mice might also be increased. The mouse ear is highly vascularized and is a useful tissue for the study of blood vessel density. Whole-mount staining of ears was performed with antibodies specific for α-smooth muscle actin (Figure 4C) and CD31 (not shown). The entire vasculature of each ear was quantified after tile scanning. In comparison with controls, the density of blood vessels in the ear (relative to total ear area) was significantly higher in VWF-deficient mice (CTL = 12.9 ± 1.1; VWF-deficient = 19.0 ± 1.1; P < .01); the fractal dimension of the vasculature, a measure of space filling,30 was also significantly higher (CTL = 1.48 ± 0.01; VWF-deficient = 1.56 ± 0.02; P < .01). Total ear area was not significantly different (CTL = 23.9 ± 0.9; VWF-deficient = 21.4 ± 1.3; P = .14). These data suggest that VWF is involved in the control of vascular development.
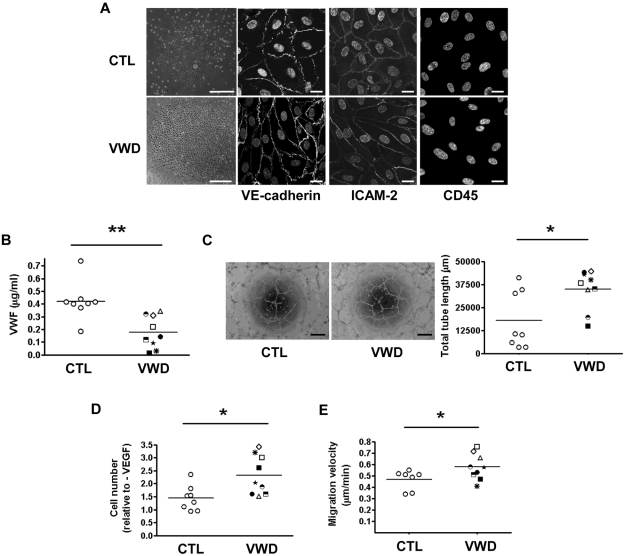
Endothelial cells from VWD patients show enhanced angiogenesis
To determine the relevance of our experimental findings to VWD, we isolated circulating progenitor cells from patients with VWD and cultured them to expand BOECs.37 BOECs were isolated from 9 VWD patients (4 with type 1, 3 with type 2M, and 2 with type 2A), all with plasma VWF activity and antigen levels below the normal range, and 8 healthy controls. The data on the levels and functional activity of VWF in the patients' plasma is provided in supplemental Table 1. After Ficoll Hypaque separation of peripheral blood mononuclear cells and culture on collagen type I, BOEC colonies began to appear between days 7 and 22 (see representative colonies, Figure 5A); these were expanded to obtain confluent monolayers of ECs with typical endothelial cobblestone morphology. The cells expressed the endothelial markers VE-cadherin and ICAM-2, and were negative for the leukocyte marker CD45 (Figure 5A). Experiments were carried out with BOECs at passage 4. As expected, BOECs from VWD patients produced lower levels of VWF in culture compared with healthy controls (Figure 5B), confirming that in these patients, BOECs recapitulate the VWD phenotype. In agreement with what we observed in siVWF-treated HUVECs, BOECs from VWD patients exhibited a significant increase in tube formation on Matrigel compared with healthy donors (Figure 5C), as well as increased VEGF-dependent proliferation (Figure 5D) and basal migration compared with healthy controls (Figure 5E). Thus, ECs isolated from VWD patients with decreased VWF levels show enhanced in vitro angiogenesis. These results, together with our previous observations on siRNA-treated HUVECs and VWF-deficient mice, confirm that endothelial VWF regulates angiogenesis.
Figure 5.
BOECs from patients with VWD show increased in vitro angiogenesis, proliferation, and migration. (A) Representative images of BOECs from a healthy control (top, far left) and a VWD patient (bottom, far left). Colonies appeared after 7-22 days in culture (size bar = 200 μm). BOECs were stained for VE-cadherin, ICAM-2, and CD45. Nuclei were stained with TO-PRO 3 (Invitrogen). Size bar = 20 μm. (B) Secretion of VWF (μg/mL) from cultured BOECs after 48 hours. (C) BOEC were seeded onto Matrigel for 24 hours and capillary network formation was quantified by measuring total tube length in micrometers. Size bar = 200 μm. (D) Proliferation of BOECs cultured with 100 ng/mL VEGF for 96 hours relative to unstimulated controls. (E) Migration velocity of cells at the periphery of endothelial colony prior to the first passage. Each patient is identified with a unique symbol (see supplemental Table 1 for code and information on VWD patients). Error bars indicate mean ± SEM. *P < .05; **P < .01 (Student t test).
Discussion
In this study we identified a novel function for VWF in the control of angiogenesis. Using 3 different model systems, we showed that (1) loss of endothelial VWF results in increased in vitro angiogenesis; (2) neo-angiogenesis and vascularization are increased in the VWF-deficient mouse; and (3) the phenotypes can be recapitulated in ECs from patients with VWD.
In vitro, VWF deficiency caused increased VEGFR-2-dependent migration and proliferation and increased capillary network formation. The addition of extracellular VWF inhibited tube formation in Matrigel in both control and VWF-deficient cells. However, even in the presence of exogenous VWF, the capillary tube network was significantly greater in VWF-deficient cells, suggesting that both extracellular and intracellular VWF regulates angiogenesis. Interestingly, exogenous VWF did not inhibit VEGFR-2– dependent endothelial migration or proliferation in either control or VWF-deficient cells, suggesting that in these models VWF acts as an intracellular regulator of angiogenesis.
Our results suggest that VWF regulates angiogenesis through multiple cross-talking pathways involving αvβ3, VEGFR-2 signaling and Ang-2. The best-characterized receptor for VWF on ECs is the integrin αvβ3, which exerts both proangiogenic and anti-angiogenic functions (reviewed in Hodivala-Dilke et al38). A large body of literature shows that αvβ3 has proangiogenic properties (reviewed in Desgrosellier and Cheresh39). In contrast, in vitro and in vivo studies using β3-deficient mice have shown that the loss of β3 integrin results in increased angiogenesis via a VEGFR-2–dependent pathway,40,41 and perturbation of αvβ3 trafficking was shown to affect VEGFR-2 activity.33 In our study, the lack of VWF in ECs in vitro caused a decrease in β3 levels and activity and increased angiogenesis. These were accompanied by an increase in VEGFR-2–dependent endothelial proliferation and migration in vitro and VEGFR-2 expression in vivo. VWF regulation of β3 integrin levels and activity could indirectly influence angiogenesis via VEGFR-2 signaling. Our data also show a reduction in the directionality of migration in siVWF-treated cells compared with controls. Integrin αvβ3 is involved in the regulation of directionality of cellular migration: White et al42 have shown that inhibition of αvβ3 trafficking, reduction in β3 protein expression, or treatment with a cyclic RGD peptide all reduced cellular directionality in fibroblasts. Because VWF regulates αvβ3 trafficking and β3 levels and interacts with αvβ3 via an RGD site, it is possible that the loss in directionality observed in the VWF-deficient cells may be directly linked to the reduction in αvβ3 levels and activity. The mechanism through which VWF regulates β3-integrin levels and function is unknown. In our study, VWF deficiency in HUVECs resulted in an increase in β3-integrin protein internalization, which can lead to increased degradation.34,43 This could be influenced by the increased levels of Ang-2 (see the next paragraph). Whether VWF also affects β3 levels through other mechanisms remains to be established.
Our results also implicate the growth factor Ang-2 in VWF-dependent regulation of angiogenesis. VWF is essential for the formation of WPBs, which contain Ang-2 and other angiogenesis mediators,19 as well as other, as-yet-unidentified proteins. Decreased or dysfunctional VWF can cause a loss or decrease in WPB production and, as a consequence, cause defective packaging of these proteins, resulting in constitutive release of WPB components such as Ang-2. In our in vitro model, loss of VWF resulted in increased Ang-2 levels in the supernatant of the cells. Ang-2 destabilizes blood vessels and acts synergistically with VEGF-A to promote angiogenesis8,44,45; overexpression of Ang-2 in vivo results in unstable, dysplastic vessels.46 Therefore, the increased angiogenesis observed in VWF-deficient cells and mice, as well as the angiodysplasia in patients with VWD, may be in part attributed to an increase in Ang-2 release. Indeed, increased levels of Ang-2 have been reported in patients with arteriovenous malformations.47 Increased Ang-2 levels could also explain, at least in part, the reduced levels of integrin αvβ3 in VWF-deficient cells: in a recent paper by Thomas et al, Ang-2 was shown to induce αvβ3 internalization and degradation in ECs.34 Based on these results, we propose that VWF modulates angiogenesis by regulating Ang-2 storage in ECs, thus indirectly influencing VEGFR-2 signaling and β3-integrin trafficking. Other mechanisms involving the modulation of αvβ3 function by direct VWF binding are also possible. Studies are ongoing to test this hypothesis.
In VWF-deficient mice, we observed both enhanced angiogenesis in the Matrigel plug assay and increased vessel density in the ear. Whether the latter is due to enhanced vascularization (de novo vessel formation in absence of preexisting ones) during embryonic development or if it represents an increase in sprouting angiogenesis in the adult is unknown. No developmental defects have been described in VWF-deficient mice. To our knowledge, this is the first report that investigates angiogenesis in these animals. Future studies will be required to determine the role of VWF in pathological and therapeutic angiogenesis.
No major developmental defects have been observed in patients with VWD, but vascular abnormalities of both the macro- and microcirculation have been reported, and the presence of angiodysplasia in the large intestinal vessels of some VWD patients is well documented.20 Abnormalities in the nail-fold capillary bed of VWD patients include morphological changes with increased dilatation, microscopic bleeding, and torquing (dysplasia).48–50 In this study, we present a plausible hypothesis for the link between increased angiogenesis, vascular malformations and VWD. We show that a lack of VWF results in increased vascularization, suggesting that VWF is required to control the formation of the vascular network. We also show that VEGF-dependent angiogenesis is enhanced in VWF-deficient cells in vitro. Increased VEGF expression and signaling have been linked to vascular malformations.51,52 Finally, we show that VWF regulates Ang-2 release from ECs. Because of the destabilizing effect Ang-2 has on blood vessels, an increase in Ang-2 plasma levels in patients with VWD might contribute to promoting blood vessel plasticity and thus exacerbate angiodysplasia. Studies of Ang-2 levels in patients with VWD are under way.
A barrier to the study of the molecular basis of diseases affecting the vascular endothelium is the difficulty in obtaining ECs from patients. We applied a novel approach to the study of the cellular mechanisms of an endothelial-based disease such as VWD. Our study clearly shows that isolation of blood cells with stem-like properties, which are able to expand in culture and acquire key features of ECs, is a feasible and valid approach to the study of the molecular basis of VWD and other diseases. In this study, no functional difference with respect to in vitro angiogenesis was observed in BOECs from patients with type 1, type 2A, or type 2M disease; clearly, the size of the patient population was too small to detect any possible differences between VWD subtypes. All patients had reduced VWF levels, and this study focused on the effects of decreased VWF on angiogenesis. However, because of its many binding partners, it is plausible that dysfunctional VWF may also affect angiogenesis through different mechanisms. For example, interaction of VWF with endothelial glycoprotein Ib has been reported to affect cell migration.53 Further studies in a larger patient population are ongoing to address this question.
In conclusion, this study defines a novel function for VWF, proposing a mechanism for VWF-mediated regulation of angiogenesis and confirming VWF as a multifunctional vascular protein. These results extend our understanding of the pathogenesis of angiodysplasia in VWD. Moreover, they raise the possibility that elevated VWF levels, as observed in many diseases, may inhibit angiogenesis. Because of the wide variation in VWF plasma levels13 and the key role of angiogenesis in many diseases from cancer to atherosclerosis, the implications of our results are potentially far reaching.
Supplementary Material
Acknowledgments
We thank Dr Graeme Birdsey, Mr Andrea Sperone, and Prof Justin Mason (National Heart and Lung Institute, Imperial College London) for their constructive criticism and support of this study; Prof Peter Barnes (National Heart and Lung Institute, Imperial College London) for support and encouragement; Dr David Ingram (Indianapolis, IN) for advice on BOEC culture; Dr Mathieu-Benoit Voisin (William Harvey Research Institute, Queen Mary College, London, United Kingdom) for advice on staining murine blood vessels; and Dr Clare Wykes (National Heart and Lung Institute, Imperial College London) for preliminary work in support of this study.
This work was supported by a UK Medical Research Council project grant (G0600868). R.E.S. was supported by an unrestricted educational grant from Baxter. D.F.C. and F.F. were supported by UK Medical Research Council funding of the Cell Biology Unit. T.A.J.M. was supported by a BHF project grant (PG/06/111/21534). R.D.S. was partly supported by the Wellcome Trust and a BHF intermediate fellowship (FS/10/047/28393).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.D.S. performed research, data analysis, and interpretation, generated reagents, expanded the original research design, and co-wrote the manuscript; F.F. and K.E.P. developed tools and assays to perform specific aspects of the research and contributed to data analysis and interpretation; N.H.D., T.A.J.M., and R.E.S. performed specific aspects of research; E.M.P. contributed to research, to the original idea, and to manuscript writing; D.O.H. contributed to data interpretation and to manuscript writing; A.D.H. contributed expertise and tools to carry out data analysis and interpretation; D.F.C. provided reagents and expertise, contributed to the experimental plan, the design of selected experiments, data analysis, and to manuscript writing; M.A.L. conceived ideas, contributed to the experimental plan and design of selected experiments, to data analysis, and to manuscript writing; and A.M.R. conceived ideas, designed the experimental plan, supervised research, carried out data analysis and interpretation, and co-wrote the manuscript.
Conflict of interest disclosure: R.E.S. was supported by an unrestricted educational grant from Baxter. The remaining authors declare no competing financial interests.
The current affiliation for E.M.P. is Department of Pediatric Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA.
Correspondence: Anna M. Randi, MD, PhD, Imperial College London, NHLI Vascular Sciences, Hammersmith Hospital, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: a.randi@imperial.ac.uk.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6(9):507–518. doi: 10.1038/nrclinonc.2009.110. [DOI] [PubMed] [Google Scholar]
- 3.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4(4):241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12(2):125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 6.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 7.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18(38):5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 8.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99(17):11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodivala-Dilke K. alphavbeta3 integrin and angiogenesis: a moody integrin in a changing environment. Curr Opin Cell Biol. 2008;20(5):514–519. doi: 10.1016/j.ceb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Roth R, Heuser JE, Sadler JE. Integrin alpha(v)beta(3) on human endothelial cells binds von Willebrand factor strings under fluid shear stress. Blood. 2009;113(7):1589–1597. doi: 10.1182/blood-2008-05-158584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadler JE. New concepts in von Willebrand disease. Annu Rev Med. 2005;56:173–191. doi: 10.1146/annurev.med.56.082103.104713. [DOI] [PubMed] [Google Scholar]
- 14.Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation. 2008;117(11):1449–1459. doi: 10.1161/CIRCULATIONAHA.107.722827. [DOI] [PubMed] [Google Scholar]
- 15.Denis CV, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98(7):4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendu R, Terraube V, Christophe OD, et al. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood. 2006;108(12):3746–3752. doi: 10.1182/blood-2006-03-010322. [DOI] [PubMed] [Google Scholar]
- 17.Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- 18.Wagner DD, Saffaripour S, Bonfanti R, et al. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991;64(2):403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- 19.Metcalf DJ, Nightingale TD, Zenner HL, Lui-Roberts WW, Cutler DF. Formation and function of Weibel-Palade bodies. J. Cell Sci. 2008;121(Pt 1):19–27. doi: 10.1242/jcs.03494. [DOI] [PubMed] [Google Scholar]
- 20.Fressinaud E, Meyer D. International survey of patients with von Willebrand disease and angiodysplasia. Thromb Haemost. 1993;70(3):546. [PubMed] [Google Scholar]
- 21.Park SO, Wankhede M, Lee YJ, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119(11):3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junquera F, Saperas E, de T I, Vidal MT, Malagelada JR. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94(4):1070–1076. doi: 10.1111/j.1572-0241.1999.01017.x. [DOI] [PubMed] [Google Scholar]
- 23.Bauditz J, Lochs H. Angiogenesis and vascular malformations: antiangiogenic drugs for treatment of gastrointestinal bleeding. World J Gastroenterol. 2007;13(45):5979–5984. doi: 10.3748/wjg.v13.45.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 25.Birdsey GM, Dryden NH, Amsellem V, et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111(7):3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denis C, Methia N, Frenette PS, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95(16):9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11(18):1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 28.Huang MT, Mason JC, Birdsey GM, et al. Endothelial intercellular adhesion molecule (ICAM)-2 regulates angiogenesis. Blood. 2005;106(5):1636–1643. doi: 10.1182/blood-2004-12-4716. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Perez M, Hughes AD, Thom SA, Parker KH. Improvement of a retinal blood vessel segmentation method using the Insight Segmentation and Registration Toolkit (ITK). Conf Proc IEEE Eng Med Biol Soc. 2007;2007:892–895. doi: 10.1109/IEMBS.2007.4352434. [DOI] [PubMed] [Google Scholar]
- 30.Smith TG, Jr, Lange GD, Marks WB. Fractal methods and results in cellular morphology–dimensions, lacunarity and multifractals. J Neurosci Methods. 1996;69(2):123–136. doi: 10.1016/S0165-0270(96)00080-5. [DOI] [PubMed] [Google Scholar]
- 31.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12(3):267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 32.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182(3):1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds AR, Hart IR, Watson AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15(4):392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 34.Thomas M, Felcht M, Kruse K, et al. Angiopoietin-2 stimulation of endothelial cells induces alphaVbeta3 integrin internalization and degradation. J Biol Chem. 2010;285(31):23842–23849. doi: 10.1074/jbc.M109.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13(6):665–676. doi: 10.1089/scd.2004.13.665. [DOI] [PubMed] [Google Scholar]
- 36.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135(3):523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 37.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodivala-Dilke KM, Reynolds AR, Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 2003;314(1):131–144. doi: 10.1007/s00441-003-0774-5. [DOI] [PubMed] [Google Scholar]
- 39.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8(1):27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds AR, Reynolds LE, Nagel TE, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64(23):8643–8650. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 42.White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177(3):515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalton SL, Scharf E, Briesewitz R, Marcantonio EE, Assoian RK. Cell adhesion to extracellular matrix regulates the life cycle of integrins. Mol Biol Cell. 1995;6(12):1781–1791. doi: 10.1091/mbc.6.12.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Q, Stamenkovic I. Angiopoietin-2 is implicated in the regulation of tumor angiogenesis. Am J Pathol. 2001;158(2):563–570. doi: 10.1016/S0002-9440(10)63998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashizume H, Falcon BL, Kuroda T, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70(6):2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng Y, Vom HF, Pfister F, et al. Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost. 2007;97(1):99–108. [PubMed] [Google Scholar]
- 47.Redondo P, Martinez-Cuesta A, Quetglas EG, Idoate M. Active angiogenesis in an extensive arteriovenous vascular malformation: a possible therapeutic target? Arch Dermatol. 2007;143(8):1043–1045. doi: 10.1001/archderm.143.8.1043. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien JR. Familial capillary fragility (diffuse capillary teleangiectasia). Proc Int Soc Hematol. 1953;20:546–548. [Google Scholar]
- 49.Blackburn EK. Primary capillary haemorrhage (including von Willebrand's disease). Br J Haematol. 1961;7:239–249. [Google Scholar]
- 50.Koscielny JK, Latza R, Mursdorf S, et al. Capillary microscopic and rheological dimensions for the diagnosis of von Willebrand disease in comparison to other haemorrhagic diatheses. Thromb Haemost. 2000;84(6):981–988. [PubMed] [Google Scholar]
- 51.Ozawa CR, Banfi A, Glazer NL, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 53.Lian J, Guoping C, Shapiro SS, Tran LP, Beacham DA. Glycoprotein Ibα can mediate endothelial cell migration on von Willebrand Factor-containing substrata. Exp Cell Res. 1999;252(1):114–122. doi: 10.1006/excr.1999.4612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.