Abstract
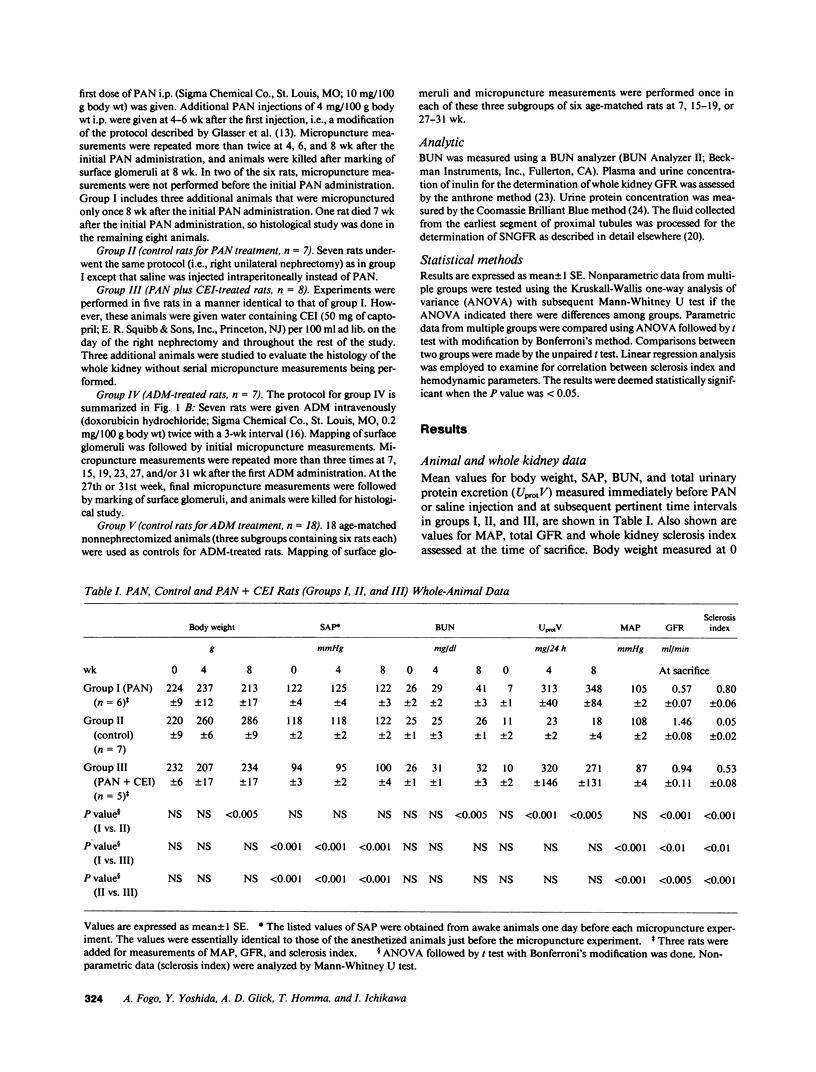
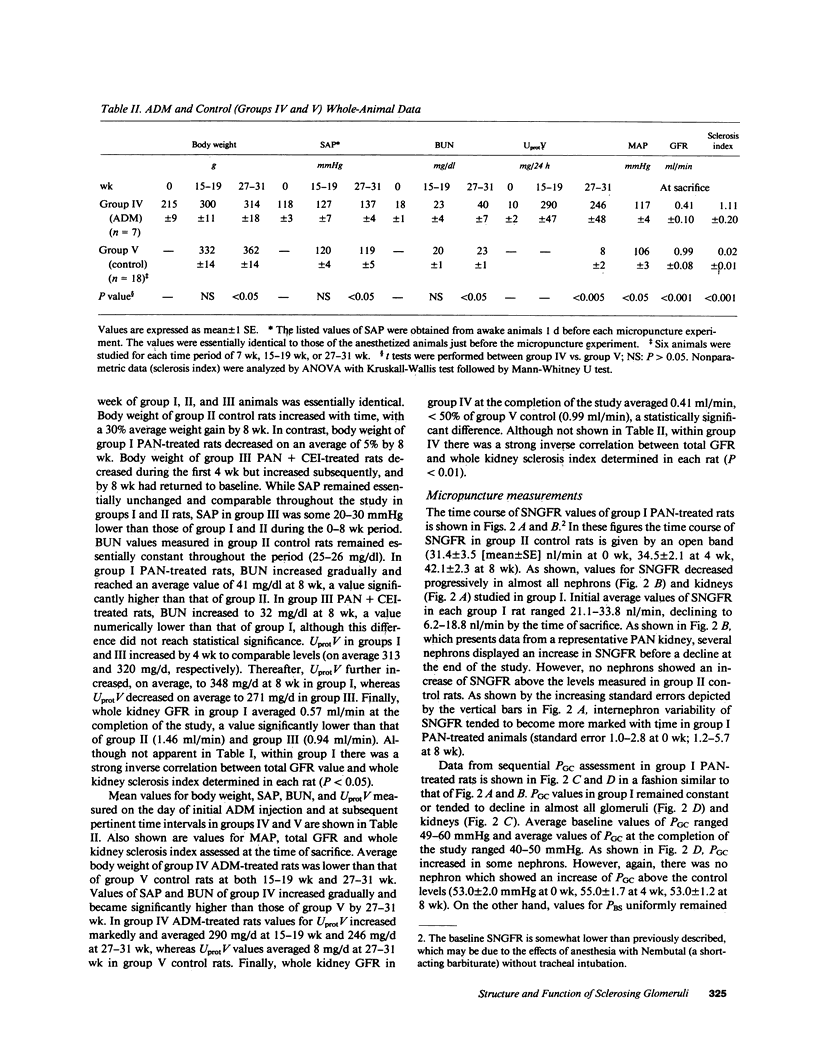
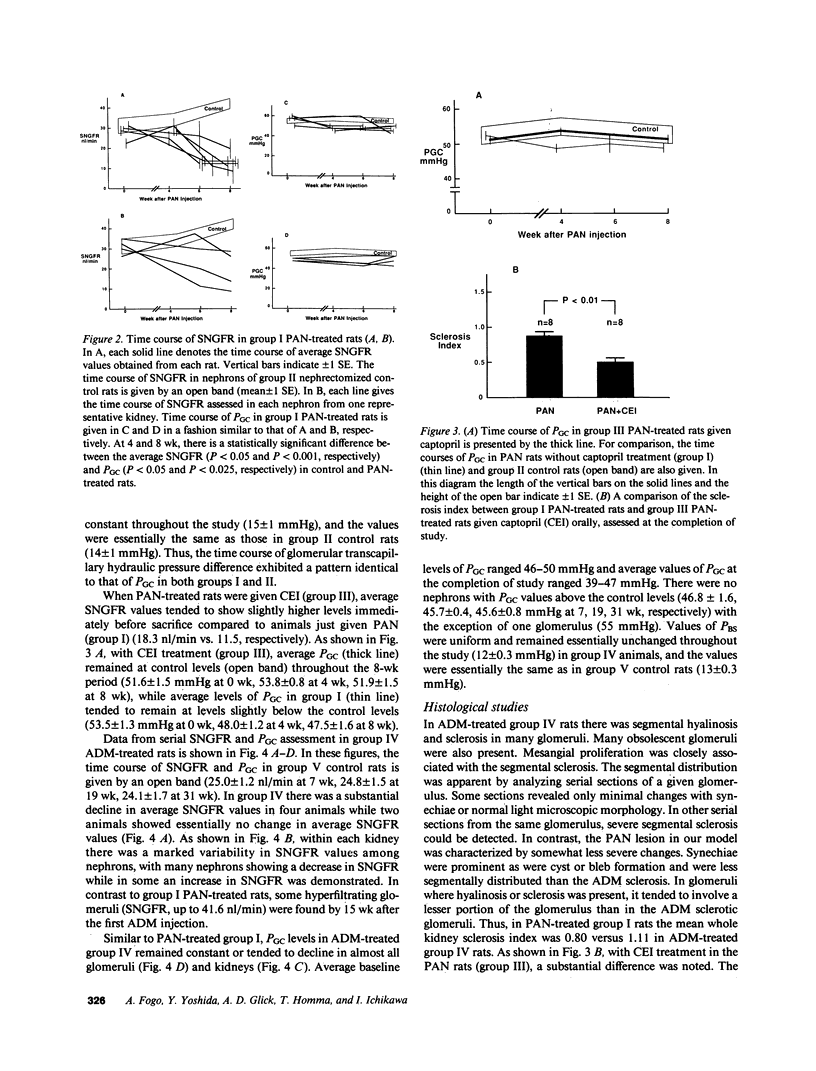
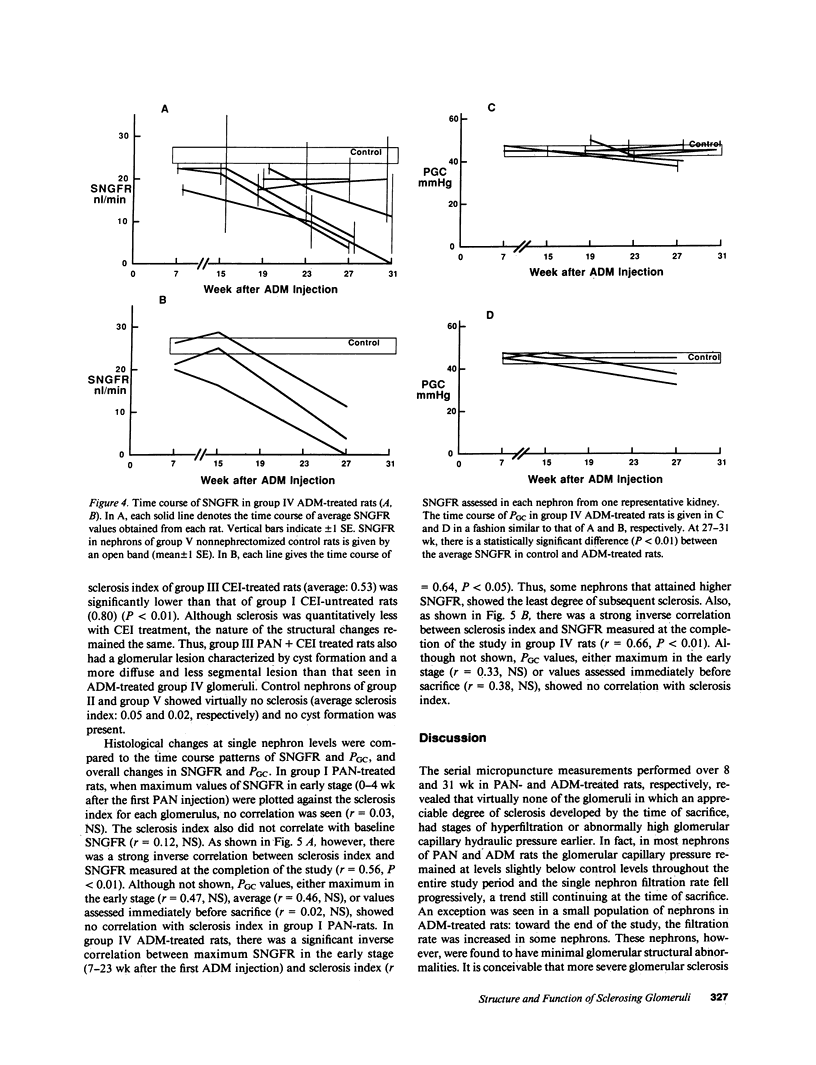
We have recently developed a micropuncture technique to assess repeatedly function of the same nephrons in chronic renal disease and subsequently examine the morphology of their glomeruli by serial thin-section histological analysis. Using this approach, a potential causal linkage between early functional patterns and late structural abnormalities was examined in glomeruli of two established rat models of glomerular sclerosis. The models are (a) puromycin aminonucleoside (PAN) administration in unilaterally nephrectomized Munich-Wistar rats and (b) adriamycin (ADM) treatment in nonnephrectomized Munich-Wistar rats. Single nephron GFR (SNGFR) and glomerular capillary hydraulic pressure (PGC) were measured repeatedly for 8 (PAN rats) or 31 wk (ADM rats). In all animals studied, values for PGC remained at, or slightly below, levels measured before PAN or ADM administration. SNGFR values declined progressively in all glomeruli in PAN rats. Although some glomeruli in ADM rats had an increase in SNGFR above levels observed in nonnephrectomized control rats, these hyperfiltering glomeruli did not have abnormally high PGC nor did they exhibit glomerular sclerosis at the completion of the study. Histological analysis revealed the existence of a significant inverse correlation between the degree of sclerosis and SNGFR assessed at the time of sacrifice in both PAN and ADM groups. Chronic administration of captopril, an angiotensin I converting enzyme inhibitor, in PAN rats substantially attenuated development of glomerular sclerosis without affecting PGC in earlier stages. The observations in these models indicate that glomerular hyperfiltration and hypertension are not required for the development of glomerular sclerosis in renal diseases, and angiotensin I converting enzyme inhibitor can exert its protective effect independently of its effect on glomerular capillary pressure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Rennke H. G., Brenner B. M. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986 Jun;77(6):1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M. A scanning electron microscopy of the glomerulus of normal and nephrotic rats. Lab Invest. 1970 Nov;23(5):489–496. [PubMed] [Google Scholar]
- Baud L., Perez J., Denis M., Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: effect of indomethacin. J Immunol. 1987 Feb 15;138(4):1190–1195. [PubMed] [Google Scholar]
- Baud L., Sraer J., Perez J., Nivez M. P., Ardaillou R. Leukotriene C4 binds to human glomerular epithelial cells and promotes their proliferation in vitro. J Clin Invest. 1985 Jul;76(1):374–377. doi: 10.1172/JCI111972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani T., Poggi A., Pozzoni R., Delaini F., Sacchi G., Thoua Y., Mecca G., Remuzzi G., Donati M. B. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest. 1982 Jan;46(1):16–23. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Meyer T. W., Hostetter T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982 Sep 9;307(11):652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- Campbell-Boswell M., Robertson A. L., Jr Effects of angiotensin II and vasopressin on human smooth muscle cells in vitro. Exp Mol Pathol. 1981 Oct;35(2):265–276. doi: 10.1016/0014-4800(81)90066-6. [DOI] [PubMed] [Google Scholar]
- Campbell D. J. Circulating and tissue angiotensin systems. J Clin Invest. 1987 Jan;79(1):1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Reid J. J., Farquhar M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976 Jan;34(1):43–59. [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Balavoine J. F., Wallon C., Dormont J. Captopril and immune regulation. Kidney Int. 1984 Jun;25(6):925–929. doi: 10.1038/ki.1984.111. [DOI] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am J Pathol. 1986 Mar;122(3):481–487. [PMC free article] [PubMed] [Google Scholar]
- Düsing R., Scherhag R., Landsberg G., Glänzer K., Kramer H. J. The converting enzyme inhibitor captopril stimulates prostacyclin synthesis by isolated rat aorta. Eur J Pharmacol. 1983 Aug 5;91(4):501–504. doi: 10.1016/0014-2999(83)90176-0. [DOI] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961 Nov 1;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENK S., ANTONOWICZ I., CRAIG J. M., METCOFF J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med. 1955 Jul;89(3):424–427. doi: 10.3181/00379727-89-21833. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Center D. M., Rounds S. Bovine and human endothelial cell production of neutrophil chemoattractant activity in response to components of the angiotensin system. Circ Res. 1985 Dec;57(6):898–902. doi: 10.1161/01.res.57.6.898. [DOI] [PubMed] [Google Scholar]
- Friedland J., Setton C., Silverstein E. Induction of angiotensin converting enzyme in human monocytes in culture. Biochem Biophys Res Commun. 1978 Aug 14;83(3):843–849. doi: 10.1016/0006-291x(78)91471-7. [DOI] [PubMed] [Google Scholar]
- Galler M., Backenroth R., Folkert V. W., Schlondorff D. Effect of converting enzyme inhibitors on prostaglandin synthesis by isolated glomeruli and aortic strips from rats. J Pharmacol Exp Ther. 1982 Jan;220(1):23–28. [PubMed] [Google Scholar]
- Gill G. N., Ill C. R., Simonian M. H. Angiotensin stimulation of bovine adrenocortical cell growth. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5569–5573. doi: 10.1073/pnas.74.12.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser R. J., Velosa J. A., Michael A. F. Experimental model of focal sclerosis. I. Relationship to protein excretion in aminonucleoside nephrosis. Lab Invest. 1977 May;36(5):519–526. [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grond J., Koudstaal J., Elema J. D. Mesangial function and glomerular sclerosis in rats with aminonucleoside nephrosis. Kidney Int. 1985 Feb;27(2):405–410. doi: 10.1038/ki.1985.24. [DOI] [PubMed] [Google Scholar]
- Gualde N., Atluru D., Goodwin J. S. Effect of lipoxygenase metabolites of arachidonic acid on proliferation of human T cells and T cell subsets. J Immunol. 1985 Feb;134(2):1125–1129. [PubMed] [Google Scholar]
- Hostetter T. H., Olson J. L., Rennke H. G., Venkatachalam M. A., Brenner B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981 Jul;241(1):F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H., Troy J. L., Brenner B. M. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981 Mar;19(3):410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- Hoyer J. R., Elema J. D., Vernier R. L. Unilateral renal disease in the rat. II. Glomerular mesangial uptake of colloidal carbon in unilateral aminonucleoside nephrosis and nephrotoxic serum nephritis. Lab Invest. 1976 Mar;34(3):250–255. [PubMed] [Google Scholar]
- Hoyer J. R., Mauer S. M., Michael A. F. Unilateral renal disease in the rat. I. Clinical, morphologic, and glomerular mesangial functional features of the experimental model produced by renal perfusion with aminonucleoside. J Lab Clin Med. 1975 May;85(5):756–768. [PubMed] [Google Scholar]
- Ichikawa I., Rennke H. G., Hoyer J. R., Badr K. F., Schor N., Troy J. L., Lechene C. P., Brenner B. M. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983 Jan;71(1):91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen S. A., Aurell M. Immunosuppressive action of captopril blocked by prostaglandin synthetase inhibitor. Lancet. 1981 May 2;1(8227):1005–1005. doi: 10.1016/s0140-6736(81)91777-3. [DOI] [PubMed] [Google Scholar]
- Kallenberg C. G., van der Laan S., de Zeeuw D. Captopril and the immune system. Lancet. 1981 Jul 11;2(8237):92–92. doi: 10.1016/s0140-6736(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Keane W. F., Raij L. Relationship among altered glomerular barrier permselectivity, angiotensin II, and mesangial uptake of macromolecules. Lab Invest. 1985 Jun;52(6):599–604. [PubMed] [Google Scholar]
- Kelly C. J., Zurier R. B., Krakauer K. A., Blanchard N., Neilson E. G. Prostaglandin E1 inhibits effector T cell induction and tissue damage in experimental murine interstitial nephritis. J Clin Invest. 1987 Mar;79(3):782–789. doi: 10.1172/JCI112885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Vernillo A. T., Farquhar M. G. Reduced sialylation of podocalyxin--the major sialoprotein of the rat kidney glomerulus--in aminonucleoside nephrosis. Am J Pathol. 1985 Mar;118(3):343–349. [PMC free article] [PubMed] [Google Scholar]
- Lowe J. R., Dixon J. S., Guthrie J. A., McWhinney P. Serum and synovial fluid levels of angiotensin converting enzyme in polyarthritis. Ann Rheum Dis. 1986 Nov;45(11):921–924. doi: 10.1136/ard.45.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinides G. N., Groggel G. C., Cohen A. H., Cook T., Baranowski R. L., Westenfelder C., Border W. A. Failure of angiotensin converting enzyme inhibition to affect the course of chronic puromycin aminonucleoside nephropathy. Am J Pathol. 1987 Nov;129(2):394–401. [PMC free article] [PubMed] [Google Scholar]
- Mauer S. M., Fish A. J., Blau E. B., Michael A. F. The glomerular mesangium. I. Kinetic studies of macromolecular uptake in normal and nephrotic rats. J Clin Invest. 1972 May;51(5):1092–1101. doi: 10.1172/JCI106901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken D. E., Flamenbaum W. Micropuncture studies of proximal tubule albumin concentrations in normal and nephrotic rats. J Clin Invest. 1971 Jul;50(7):1498–1505. doi: 10.1172/JCI106635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S., Oh Y., Tsuruda H., Onoyama K., Fujimi S., Fujishima M. Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int. 1986 Feb;29(2):502–510. doi: 10.1038/ki.1986.28. [DOI] [PubMed] [Google Scholar]
- Oliver J. A., Sciacca R. R. Local generation of angiotensin II as a mechanism of regulation of peripheral vascular tone in the rat. J Clin Invest. 1984 Oct;74(4):1247–1251. doi: 10.1172/JCI111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan D. G., Missirian-Bastian A., Goetzl E. J. Human T-lymphocyte subset specificity of the regulatory effects of leukotriene B4. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3501–3505. doi: 10.1073/pnas.81.11.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977 Apr;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Pfeffer J. M., Pfeffer M. A., Frohlich E. D. Validity of an indirect tail-cuff method for determining systolic arterial pressure in unanesthetized normotensive and spontaneously hypertensive rats. J Lab Clin Med. 1971 Dec;78(6):957–962. [PubMed] [Google Scholar]
- Raij L., Azar S., Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984 Aug;26(2):137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- Simkin N. J., Jelinek D. F., Lipsky P. E. Inhibition of human B cell responsiveness by prostaglandin E2. J Immunol. 1987 Feb 15;138(4):1074–1081. [PubMed] [Google Scholar]
- Swartz S. L., Williams G. H., Hollenberg N. K., Levine L., Dluhy R. G., Moore T. J. Captopril-induced changes in prostaglandin production: relationship to vascular responses in normal man. J Clin Invest. 1980 Jun;65(6):1257–1264. doi: 10.1172/JCI109788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNIER R. L., PAPERMASTER B. W., GOOD R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959 Jan 1;109(1):115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Cotran R. S., Karnovsky M. J. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J Exp Med. 1970 Dec 1;132(6):1168–1180. doi: 10.1084/jem.132.6.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening J. J., Rennke H. G. Glomerular permeability and polyanion in adriamycin nephrosis in the rat. Kidney Int. 1983 Aug;24(2):152–159. doi: 10.1038/ki.1983.139. [DOI] [PubMed] [Google Scholar]
- Weinstock J. V., Blum A. M. Isolated liver granulomas of murine Schistosoma mansoni contain components of the angiotensin system. J Immunol. 1983 Nov;131(5):2529–2532. [PubMed] [Google Scholar]
- Yokosawa H., Ogura Y., Ishii S. Purification and inhibition by neuropeptides of angiotensin-converting enzyme from rat brain. J Neurochem. 1983 Aug;41(2):403–410. doi: 10.1111/j.1471-4159.1983.tb04756.x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Fogo A., Shiraga H., Glick A. D., Ichikawa I. Serial micropuncture analysis of single nephron function in subtotal renal ablation. Kidney Int. 1988 Apr;33(4):855–867. doi: 10.1038/ki.1988.77. [DOI] [PubMed] [Google Scholar]
- Zatz R., Dunn B. R., Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986 Jun;77(6):1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]


