Abstract
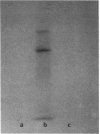
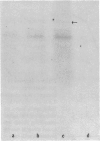
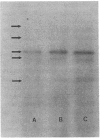
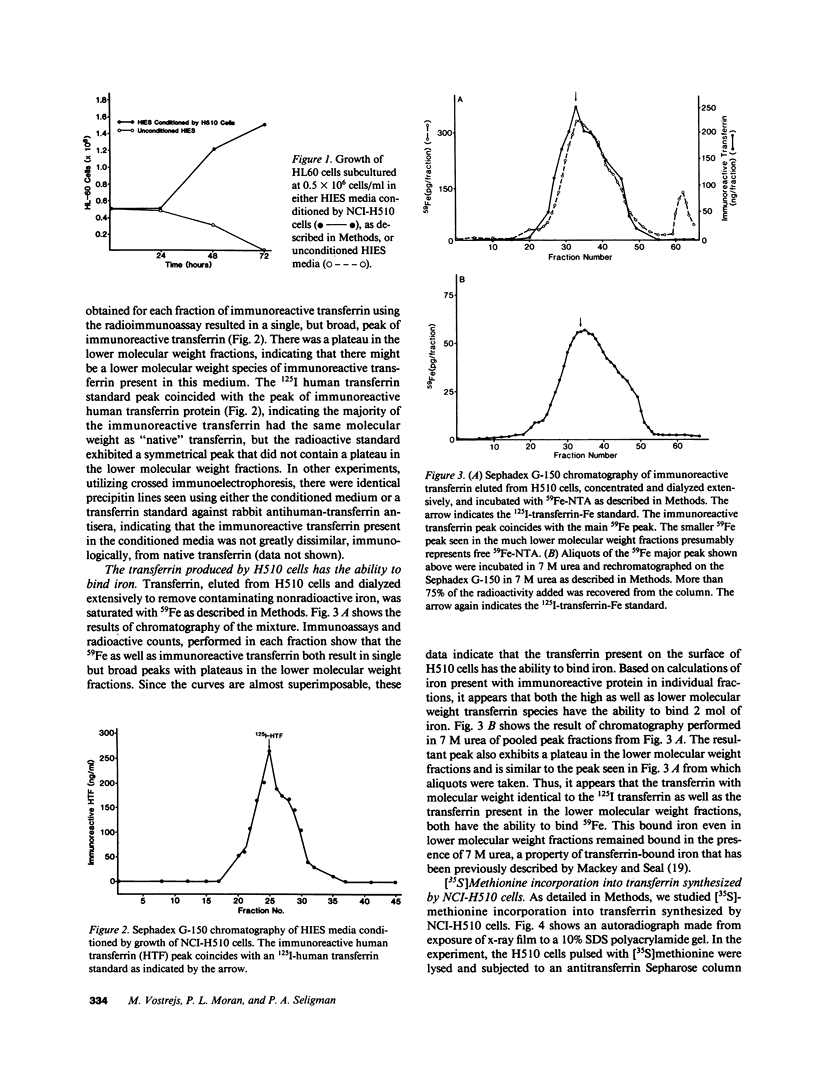
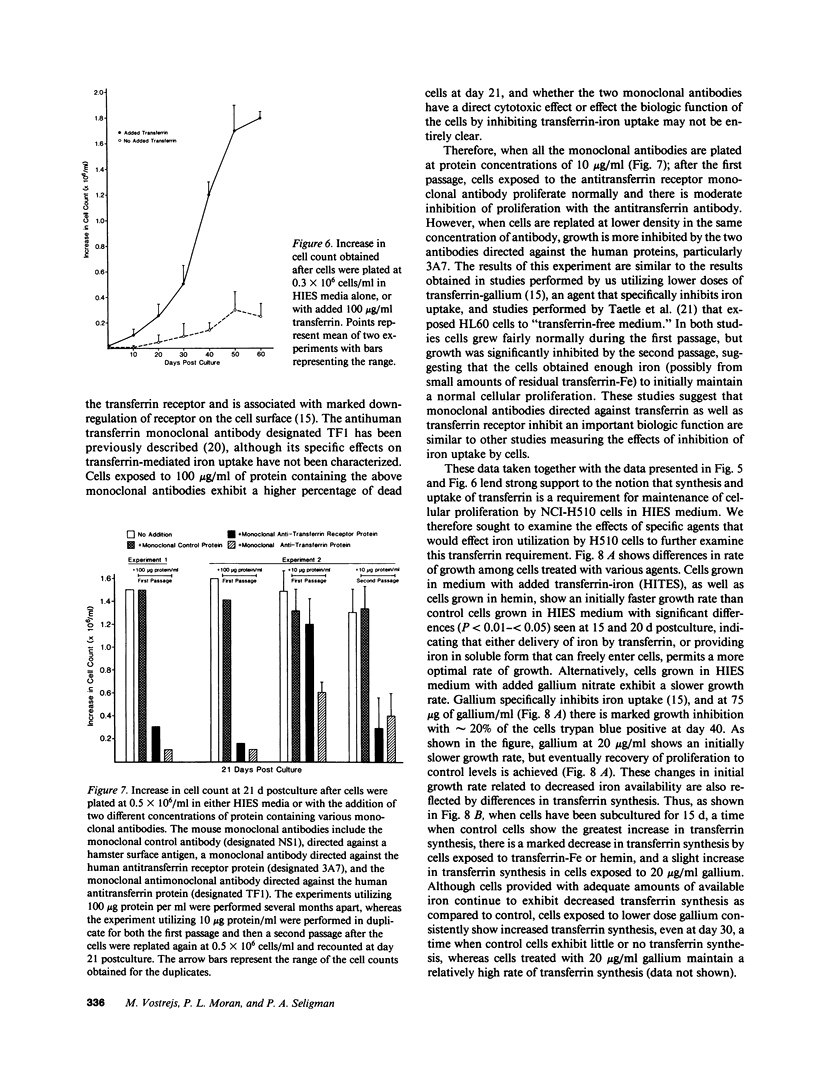
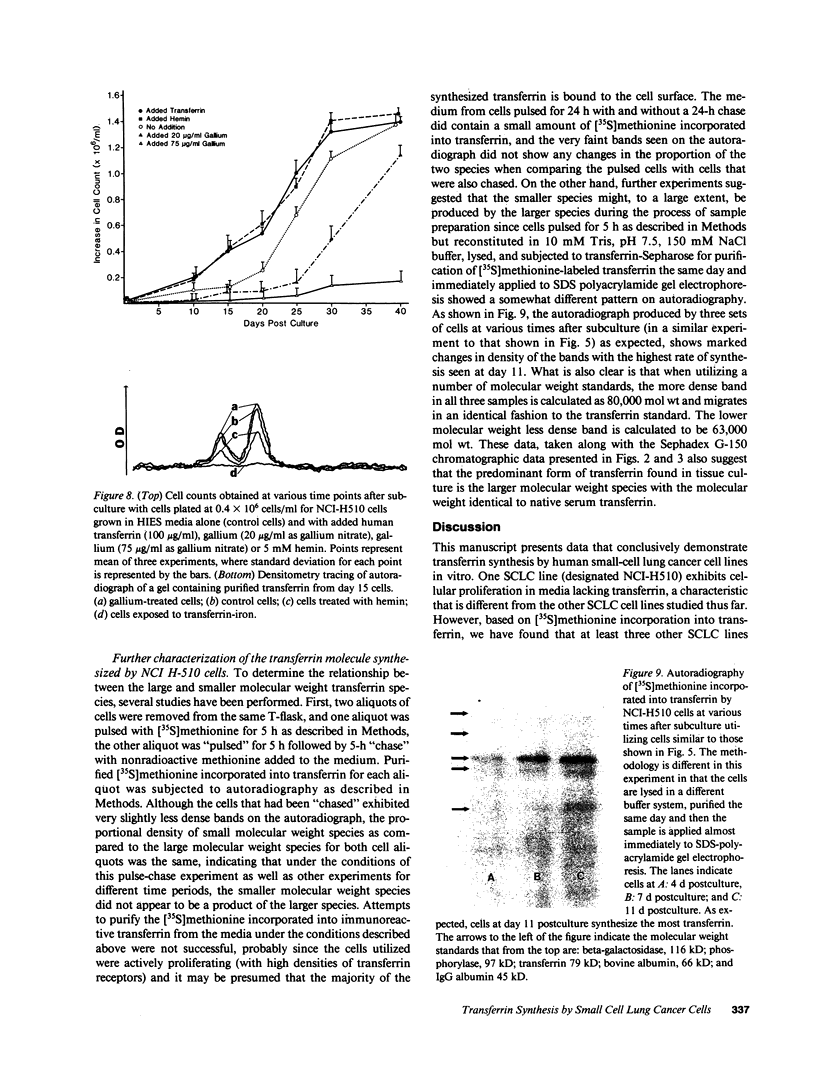
Since transferrin is required for cellular proliferation, we investigated transferrin synthesis by a small cell lung cancer line (NCI-H510) that survives in serum-free media without added transferrin. Immunoassays for human transferrin demonstrated that these cells contained immunoreactive human transferrin. Immunofluorescence studies showed that the protein is expressed on the surface of cells, presumably bound to transferrin receptor. Media conditioned by NCI-H510 cells support proliferation of human leukemic cells that would not survive in media lacking transferrin. [35S]Methionine incorporation documented transferrin synthesis by NCI-H510 cells as well as three other small cell lines. Transferrin synthesis by NCI-H510 cells increased more than 10-fold when cells entered active phases of the cell cycle, and this increase was seen before large increases in transferrin-receptor expression. Further experiments examining the effects of agents that affect iron metabolism show that the addition of transferrin-iron or hemin to the media is associated with a more rapid initial rate of proliferation and lower rates of transferrin synthesis than control cells. Gallium salts, which inhibit iron uptake, inhibited proliferation of these cells. If the cells recovered from this effect, transferrin synthesis remained greatly increased compared to control. We conclude that transferrin synthesis by these malignant cells is ultimately related to an iron requirement for cellular proliferation. It appears that this synthesized transferrin acts as part of an important autocrine mechanism permitting proliferation of these cells, and perhaps permitting tumor cell growth in vivo in areas not well vascularized.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Brown E. B. Structure and function of transferrin. Prog Hematol. 1975;9:25–56. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Cherington P. V., Smith B. L., Pardee A. B. Loss of epidermal growth factor requirement and malignant transformation. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3937–3941. doi: 10.1073/pnas.76.8.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar C. R., Massey E. J., Seligman P. A. Regulation of transferrin receptor expression on human leukemic cells during proliferation and induction of differentiation. Effects of gallium and dimethylsulfoxide. J Clin Invest. 1983 Oct;72(4):1314–1325. doi: 10.1172/JCI111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar C. R., Seligman P. A. Effects of different transferrin forms on transferrin receptor expression, iron uptake, and cellular proliferation of human leukemic HL60 cells. Mechanisms responsible for the specific cytotoxicity of transferrin-gallium. J Clin Invest. 1986 Dec;78(6):1538–1546. doi: 10.1172/JCI112746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Frazier J. L., Caskey J. H., Yoffe M., Seligman P. A. Studies of the transferrin receptor on both human reticulocytes and nucleated human cells in culture: comparison of factors regulating receptor density. J Clin Invest. 1982 Apr;69(4):853–865. doi: 10.1172/JCI110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Goubin G., Goldman D. S., Luce J., Neiman P. E., Cooper G. M. Molecular cloning and nucleotide sequence of a transforming gene detected by transfection of chicken B-cell lymphoma DNA. Nature. 1983 Mar 10;302(5904):114–119. doi: 10.1038/302114a0. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Kitada S., Hays E. F. Transferrin-like activity produced by murine malignant T-lymphoma cell lines. Cancer Res. 1985 Aug;45(8):3537–3540. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lum J. B., Infante A. J., Makker D. M., Yang F., Bowman B. H. Transferrin synthesis by inducer T lymphocytes. J Clin Invest. 1986 Mar;77(3):841–849. doi: 10.1172/JCI112381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Masaoka A., Ichimura H., Shibata K., Tanaka H., Niwa H. Comparison of actual survivorship after treatment with survivorship predicted by actual tumor-volume doubling time from tumor diameter at first observation. Cancer. 1984 Jun 15;53(12):2716–2720. doi: 10.1002/1097-0142(19840615)53:12<2716::aid-cncr2820531227>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Robbins E., Pederson T. Iron: its intracellular localization and possible role in cell division. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1244–1251. doi: 10.1073/pnas.66.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S., Durbin H., Clingan D., de Asua L. J. Iron salts and transferrin are specifically required for cell division of cultured 3T6 cells. Biochem Biophys Res Commun. 1977 Apr 11;75(3):556–562. doi: 10.1016/0006-291x(77)91508-x. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Sertoli cells synthesize and secrete transferrin-like protein. J Biol Chem. 1980 Oct 25;255(20):9523–9525. [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Taetle R., Rhyner K., Castagnola J., To D., Mendelsohn J. Role of transferrin, Fe, and transferrin receptors in myeloid leukemia cell growth. Studies with an antitransferrin receptor monoclonal antibody. J Clin Invest. 1985 Mar;75(3):1061–1067. doi: 10.1172/JCI111768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetle R., Rhyner K., Castagnola J., To D., Mendelsohn J. Role of transferrin, Fe, and transferrin receptors in myeloid leukemia cell growth. Studies with an antitransferrin receptor monoclonal antibody. J Clin Invest. 1985 Mar;75(3):1061–1067. doi: 10.1172/JCI111768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormey D. C., Mueller G. C. Biological effects of transferrin on human lymphocytes in vitro. Exp Cell Res. 1972 Sep;74(1):220–226. doi: 10.1016/0014-4827(72)90500-9. [DOI] [PubMed] [Google Scholar]
- Warrell R. P., Jr, Coonley C. J., Straus D. J., Young C. W. Treatment of patients with advanced malignant lymphoma using gallium nitrate administered as a seven-day continuous infusion. Cancer. 1983 Jun 1;51(11):1982–1987. doi: 10.1002/1097-0142(19830601)51:11<1982::aid-cncr2820511104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]