Abstract
With the increasing worldwide prevalence of antibiotic resistant bacteria, bacteriophage endolysins (lysins) represent a very promising novel alternative class of antibacterial in the fight against infectious disease. Lysins are phage-encoded peptidoglycan hydrolases which, when applied exogenously (as purified recombinant proteins) to Gram-positive bacteria, bring about rapid lysis and death of the bacterial cell. A number of studies have recently demonstrated the strong potential of these enzymes in human and veterinary medicine to control and treat pathogens on mucosal surfaces and in systemic infections. They also have potential in diagnostics and detection, bio-defence, elimination of food pathogens and control of phytopathogens. This review discusses the extensive research on recombinant bacteriophage lysins in the context of antibacterials, and looks forward to future development and potential.
Key words: lysin, endolysin, bacteriophage, pathogen, antibacterial, infection, lytic, enzyme
Introduction
Bacteriophage (phage) are considered ubiquitous and estimated to be the most abundant biological entities on earth.1,2 Phages are viruses that specifically infect bacteria. They were first discovered in the pre-antibiotic era by d'Herelle in 1917,3 although their antibacterial behavior was previously described by Hankin in 1896,4 and Twort in 1915.5,6 Since the commercialization of antibiotics in the 1940s phage therapy has been largely neglected in the west until recently with growing concerns for the increasing prevalence of multi-antibiotic resistant so called “superbugs” including methicillin-resistant Staphylococcus aureus (MRSA) and the scarcity of new antibiotics.7,8 Over millions of years phage have evolved to develop two methods to release their progeny from host bacterial cells. Filamentous phage are released through bacterial cell walls without causing cell death9 whereas non-filamentous phage use specific lysins to either inhibit the synthesis of peptidoglycan (single stranded RNA or DNA phage encoded enzymes) or hydrolyze the peptidoglycan using a holin-endolysin system (double stranded DNA phage encoded enzymes).10 Lysins accumulate in the cytosol during the late stage of the lytic cycle and hydrolyse the peptidoglycan in the bacterial cell wall thus releasing mature phage progeny.11 Lysins usually don't have signal sequences and so are dependent on a second protein called a holin to reach their substrate.12 At a genetically determined time in the terminal stage of the lytic cycle, holins form pores in the inner membrane of the infected cell resulting in access of lysin to the peptidoglycan causing rapid cell lysis.13 For phage, both the holin and lysin are essential for host cell lysis using this system. However, when lysins are used as recombinant enzymes and applied exogenously to Gram-positive bacteria they cause rapid lysis as no membrane is present to inhibit their access to the cell wall.14,15 It is this potent ability to rapidly lyse pathogenic Gram-positive cells upon direct contact with peptidoglycan “lysis from without” that has laid the foundation for exploiting lysins as powerful novel antibacterials. In the case of Gram-negative bacteria, the outer membrane prevents access of exogenous lysins to the cell wall peptidoglycan and therefore, their exploitation as antibacterials is limited. It was in 2001 that a phage lysin was first shown to successfully prevent and eliminate a bacterial infection in vivo.16 Unlike antibiotics, phage lysins can be used to selectively target specific pathogenic bacteria without effecting surrounding commensal microflora: they are reported to have a narrow host range similar to that of their phage rendering them generally either species17–19 or genus specific,20,21 although, in at least one case there is evidence that lysins can target more than a single genus.22
There is currently an ever-growing concern over the global spread of antibiotic resistance among human and animal pathogens and the need for novel effective antibacterials (like phage lysins) to combat them is well recognised.23,24 MRSA is now the most commonly reported antibiotic resistant bacterium in clinical settings25 and there is also a significant level of resistance emerging within the genus of Enterococcus, Pneumococcus and Streptococcus.23 This review will focus on the nature of phage lysins and their diverse applications including: the control and treatment of pathogens on mucosal surfaces and in systemic infection, there potential in diagnostics and detection, biodefense, elimination of food pathogens and control of phytopathogens.
Lysin Structure
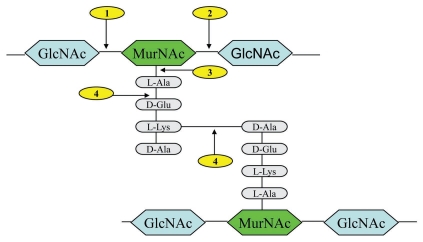
In general, lysins studied to date display a two domain modular structure.26,27 This comprises an N-terminal catalytic domain and a C-terminal cell wall binding domain.14,28,29 The former is categorized into four different groups depending on cleavage sites (Fig. 1). The four are (a) N-acetylmuramidases (lysozymes) and (b) N-acetyl-β-D-glucosaminidases (glycosidases), which hydrolyze the β-1-4 glycosidic bond in the sugar moiety of the cell wall (c) N-acetylmuramoyl-L-alanine amidases, which cleave the amide bond connecting the sugar and peptide moieties of the bacterial cell wall (d) L-alanoyl-D-glutamate endopeptidases and interpeptide bridge-specific endopeptidases, which attack the peptide moiety of the cell wall peptidoglycan (See Fig. 1).14,30,31 Lysins typically comprise of one of these four muralytic abilities in addition to a cell wall binding domain.10 However a number of lysins have been reported comprising of two catalytic domains including those of Staphylococcus aureus (LysK, Phi11 and MV-L) where endopeptidase activity is a common feature.20,32,33 Of all lysins reported to date, the streptococcal phage lysin PlyC is particularly unique as it displays a multimeric modular structure consisting of two distinct gene products designated PlyCA (50-kDa heavy chain) and PlyCB (8-kDa light chain).34
Figure 1.
Typical peptidoglycan structure of Gram-positive bacteria, showing lysin cleavage sites. The cleavage sites are indicated: (1) N-acetylmuramidases; (2) N-acetyl-β-D-glucosaminidases; (3) N-acetylmuramoyl-L-alanine amidases; (4) L-alanoyl-D-glutamate endopeptidases and interpeptide bridge-specific endopeptidases. Abbreviations: GlcNAc (N-acetyl glucosamine), MurNAc (N-acetyl muramic acid).
The C-terminal binding domain of the majority of lysins is responsible for attaching the enzyme to its specific substrate in the bacterial cell wall via non-covalent binding of carbohydrate ligands.29 A recent study on the crystal structure of the pneumococcal phage lysin Cpl-1 in free and choline-bound states suggested that the choline-binding domain assists in the correct positioning of the N-terminal catalytic domain.35 While it appears that the C-terminal domain is necessary for lytic activity of some endolysins,19,29,36 this is not always the case. A number of enzymes have shown increased lytic activity upon removal of the binding domain.37–39 For example, when LysK was truncated to its N-terminal endopeptidase domain, CHAP (cysteine/histidine-dependant amidohyrolase/peptidase), it had a two-fold higher lytic activity than the native enzyme.39 It is possible that the C-terminal binding domain in the native enzyme may be limiting the potential activity of the N-terminal lytic domain by only allowing it to configure and function when bound to its target in the cell wall.39,40 In contrast to lysins against Gram-positive pathogens, the enzymes associated with Gram-negative phages are often globular single module enzymes10,41 as in the T7 lysin (lysozyme).42 In a recently-reported two-domain lysin KZ144 from a Pseudomonas phage, the substrate-binding activity was located at the N-terminus.41 In recent years, research on a number of lysins has led to the elucidation of their crystal structure as in the case of T7 lysin,42 T4 lysin,43 CpL-1,35 PlyL,40 PlyPSA44 and PlyB.45 The diversity of enzymztic activities with phage lysins and their association with distinct modules make it possible to engineer novel lysins with various combinations of binding and catalytic domains,46–49 increasing their antibacterial and therapeutic potential.
Critical Properties of Lysins as Potential Therapeutics
If these recombinant enzymes are to fulfill their potential as antibacterials a number of important factors have to be investigated such as drug toxicity, immunogenicity, efficacy, resistance and synergy. To date a number of in vitro and in vivo trials have been carried out on various lysins to assess these parameters.
Toxicity.
Bacteriophages are the most abundant life forms on the planet and have co-evolved with bacteria over millions of years, as they do not infect mammalian cells,50 lysins should not present a potential toxic threat to humans and animals. To date this theory has been supported by successful preclinical treatment of systemic infections with lysin in mouse models, where no signs of toxicity have been noted.16,51–53 Therapeutic lysin treatment, be it topical, systemic or intravenous is thus far observed to have no harmful, abnormal or irritant side-effects in pre-clinical trials in vivo.14
Immunogenicity.
As lysins are proteins, they are capable of stimulating an immune response when administered mucosally or systemically.31 This response could potentially decrease lysin activity. In vitro and in vivo trials have been conducted to explore this. When rabbit hyperimmune serum was raised against the pneumococccal lysin Cpl-1, it was found that lytic activity in vitro was slowed but not blocked.52 Similar results were seen with B. anthracis and S. pyogenes lysins.54 In vivo analysis showed that in five out of six cases, mice that received three intravenous doses of Cpl-1 tested positive for Immunoglobulin G (IgG) against the enzyme but this only had a moderate inhibitory effect on activity.52
In a study with the Listeria-specific lysins, Ply118 and Ply500, it was demonstrated that the affinity of the C-terminal binding domain for its target in the cell wall is in the nanomolar range, similar to the binding affinity of an IgG molecule for its antigen.29 Fischetti's group showed that increased cytokine production could also result from using phage lysin in the treatment of systemic infection.55 This inflammatory effect is likely dependant on the amount of lysin delivered in the treatment and may be reduced with regulated smaller doses of enzyme.31,55 Immunogenicity of lysins in systemic infection treatment can further be reduced by PEGylation where the protein is conjugated to polyethylene glycol (PEG), reducing antibody binding, as shown with lysostaphin.56 This also causes a slight reduction in lysin activity but it is compensated for by greatly improved pharmacokinetics.56 While it is clear that lysins can illicit an immune response, this does not neutralize their activity or prevent their use as antibacterials in the treatment of systemic infections.
Resistance.
The occurrence of lysin-resistant bacteria is unlikely since phage have naturally evolved with their bacterial hosts over millions of years to produce these enzymes that are essential for the release of progeny phage. It has been suggested that lysins evolved to target specific molecules in the host peptidoglycan that are essential for bacterial viability.14,31 This is supported by the fact that choline, the cell wall receptor for pneumococcal lysin is essential for cell viability.30,57 Similarly polyrhamnose, the cell wall receptor for Group A streptococcal lysin is also important for cell viability.58,59 Repeated exposure of Streptococcus pneumoniae and Bacillus cereus to low concentrations of lysin Pal and PlyG respectively on agar plates and in liquid culture did not result in the emergence of resistant mutants even after numerous cycles.17,18 The polysaccharide capsule of S. pneumoniae associated with increased virulence of the bacterial cell did not inhibit lysin activity.17 A susceptible B. cereus isolate was demonstrated to develop up to 1,000 to 10,000-fold increases in antibiotic resistance upon mutagenesis with methanesulfonic acid ethyl ester while remaining sensitive to PlyG lysin, where no resistant mutants were found.18 While this approach has not been tested on all the available lysins, it is unlikely that the outcome would be different. In addition the use of lysins with two catalytic domains, each with different peptidoglycan specificity, may further reduce the likelihood of resistant strains emerging.31,60
Synergy.
Studies have demonstrated that some lysins can work in synergy with others or with certain antibiotics both in vitro and in vivo.51,60,61 When Cpl-1 (lysozyme/muramidase) and Pal (amidase) were used in combination, the bacteremic titre was reduced to a greater extent than by either lysin alone in a murine sepsis model.51,61 In another recent in vitro study, the staphylococcal bacteriophage lysin LysK and lysostaphin demonstrated antibacteria synergy.60 Synergy has also been demonstrated between lysins and antibiotics in vitro.33,62,63 Two pneumococcal enzymes, Cpl-1 and LytA have been used in synergy with a number of antibiotics including penicillin, gentamicin, cefotaxime and moxifloxacin. In the majority of cases, there was enhanced activity where an antibiotic was combined with one or other of the enzymes.62,63 The staphylococcal lysin MV-L showed enhanced activity against the VISA strain Mu50 when used in combination with glycopeptide antibiotics vancomycin or teicoplanin.33 While these studies have only been done in vitro, it is clear that the approach of combining lysins with antibiotics generally gives rise to increased antibacterial activity.
Applications of Lysins
Numerous successful pre-clinical trials have revealed the ability of lysins to prevent pathogenic colonization of the mucosa,16,17,52,53,55,64 the first being in 2001.16 The potential use of lysins as antibacterials against systemic diseases has also been demonstrated since then by a number of other studies.20,51,52 While antibiotics often kill bacteria indiscriminately, lysins possess high specificity permitting the normal commensal microflora to be left undisturbed. Moreover, antibiotics such as penicillin and cephalosporin function by inhibiting peptidoglycan synthesis, thereby lysing only dividing cells. Lysins on the other hand destroy the peptidoglycan directly, killing both growing and non-growing cells.65
Lysins against Different Pathogenic Bacteria
Streptococci.
The pneumococcal lysins Cpl-1 and Pal have been used successfully in pre-clinical trials in the elimination of antibiotic-resistant S. pneumoniae, the causative agent of pneumonia, acute otitis media (AOM), septicemia, bronchitis and meningitis.51,52,66 A 2,000 µg dose of Cpl-1 when used intravenously in a mouse model one hour after infection reduced pneumococcal titres from a median of log10 4.70 CFU/ml to undetectable levels (<log10 2.00 CFU/ml) after 15 minutes. Compared to the lysin-treated mice, only a 20% survival rate was seen in untreated mice.52 The Cpl-1 lysin has also been introduced by intraperitoneal injection to mice.67 100% of Cpl-1-treated mice survived fatal pneumonia and showed rapid recovery when treatment had been initiated at 24 hours post infection. Cpl-1 prevented bacteremia, systemic hypertension and reduced pulmonary bacterial counts.67 In murine models of infection, Cpl-1 was shown to eliminate and prevent acquired otitis media, endocarditis and bacterial meningitis.55,57,68
The Pal lysin was also used successfully to eliminate pneumococcal colonization of the nasopharynx in mice. This was achieved with a single dose of the enzyme and no recolonisation was observed.17 These studies show that Cpl-1 and Pal lysins have excellent potential in the prevention, control and treatment of mucosal and systemic pneumococcal infection.
PlyC lysin from the streptococcal bacteriophage C1 has been found to be active against groups A, C and E streptococci. Group A streptococci (GAS) such as S. pyogenes is a common cause of pharyngitis and rheumatic fever.16 In one time-course assay, where cell death of streptococci equated to a spectrophotometric loss in turbidity, 10 ng of PlyC completely eliminated a culture of ∼107 GAS in 5 seconds. This enzyme was also able to successfully prevent and eliminate upper respiratory colonisation of mice by GAS.16 Mice were orally and nasally administered GAS premixed with either buffer or lysin. 24 hours post treatment, none of the lysin treated mice were colonised compared to 100% of the control mice. Up to one week later, only a single colony of GAS from 20 swabs was detected in the lysin-treated mice.16
PlyGBS is another well-studied recombinant streptococcal lysin and is active against group A, B, C, G and L streptococci.64 It has been developed as a prophylactic for Group B streptococcal (GBS) vaginal colonization in pregnant women before infant delivery and also for use as a decontaminant to eliminate GBS from new-borns. This would potentially reduce the rate of neonatal meningitis and sepsis.64 Mice models were successfully used to demonstrate that a single dose of PlyGBS could cause a 3 log10 reduction in cell titre in mice that had been vaginally challenged with GBS. Optimum pH of PlyGBS (∼pH 5.0) is within the range normally found in the human vaginal tract, and the enzyme did not show activity against vaginal commensal flora such as Lactobacillus acidophilus which play a role in defence against pathogens.64 In another study, DNA mutagenesis techniques were used to produce PlyGBS mutants with up to 28-fold better activity against GBS than the wild-type enzyme.69
Staphylococcus aureus and MRSA.
S. aureus is responsible for a number of infections ranging from skin infections to fatal sepsis, endocarditis, septicemia, meningitis and bovine mastitis in dairy herds.7,70 MRSA is the most important cause of antibiotic resistant healthcare-associated infection worldwide which may result in prolonged hospital stay and higher mortality rates.25 With the increasing prevalence of MRSA and increasing incidence of Community-Acquired MRSA, there is an urgent need for an effective anti-staphylococcal agent.71,72 LysK lysin from staphylococcal phage K has been reported to be active against nine species of Staphylococcus from both human and bovine sources, including MRSA and VRSA.20,39,60 Another staphylococcal lysin, LysH5, was reported to eliminate S. aureus growing in milk, which had been present at 106 CFU/ml.73 A third lysin, Phi11, was active against live mastitis-causing Coagulase-negative Staphylococcus.74 This enzyme was also shown to eliminate S. aureus biofilms, which is very clinically relevant given the infection problems associated with biofilm formation on medical devices.75
The first staphylococcal lysin to be tested in vivo in a mouse infection model is MV-L from the phage MR11.33 In one experiment, this was administered intranasally 60 hours post infection with 2 × 109 MRSA cells in each nasal cavity. Six hours after lysin treatment, complete elimination of the bacteria was achieved in one of nine mice treated. The remaining mice had much lower bacterial titres in their nasal cavities compared to control mice. In a second experiment, mice treated intraperitoneally with MV-L at 0 minutes and 30 minutes after being challenged with MRSA survived up to 60 days post infection.33 Although an immune response to this lysin was evident, it did not block lytic activity.33
Enterococci.
Enterococci are commonly part of the resident microflora of the lower intestinal tract in mammals. However, under circumstances where the natural flora is disturbed, the bacteria can become invasive and cause nosocomial infections such as endocarditis, bacteremia and urinary tract infections.25 The majority of these infections are caused by E. faecalis and E. faecium, both of which tend to be resistant to antibiotics including vancomycin, making these infections extremely difficult to eradicate.25 The E. faecalis phage lysin PlyV12 showed strong lytic activity in vitro against a variety of clinical and laboratory strains including VRE (Vancomycin-Resistant Enterococcus). The lysin also showed activity against Staphylococcus and Groups A, B and C streptococci making it one of the first lysins showing a broad lytic spectrum outside the host range of its parent phage.76 This is likely due to a peptidoglycan feature which is common to the genera examined.76
Bacillus anthracis.
B. anthracis is associated with bio-warfare and is thus classed as a category A biological weapon.77,78 Two recombinant lysins, PlyG and PlyPH are active against this pathogen.18,78 Since B. anthracis is highly toxic to humans, a B. cereus strain lacking the B. anthracis virulence plasmid was utilised for in vivo studies. In one experiment, PlyG was injected into mice 15 minutes after they were infected with 1 × 106 CFU of an antibiotic resistant B. cereus strain resulting in a 76.9% survival rate compared to the 100% death rate of infected control mice within 5 hours.18 PlyPH, is a putative lysin which was shown to be effective against B. anthracis in; in vitro and in vivo studies.78 These lysins have the potential for exploitation for detection and treatment of B. anthracis infections.
Clostridium.
C. difficile is a major cause of nosocomial-associated diarrhoea and colitis. It usually presents itself as a secondary infection where it proliferates in the gastro-intestinal (GI) tract after the normal commensal flora has become unbalanced due to antibiotic therapy.79,80 CD27L is a lysin identified from the C. difficile phage CD27,80 and was capable of lysing diverse strains of C. difficile. Importantly the lysin did not negatively effect other commensal gut flora normally present in the GI tract, including non pathogenic Clostridium-like Firmicutes. The lysin demonstrated a broad pH range (pH 4.5–pH 8.3) and was expressed in a Lactococcus lactis strain which could potentially be used as a delivery system to the site of infection in the GI tract where the lysin should remain functional.80
Ply3626 is a lysin with activity against C. perfringens which can cause necrotic enteritis, gas gangrene and food poisoning.81–83 This enzyme may be exploited as an anti-bacterial for the treatment of C. perfringens infection in humans. In addition it has been proposed as a bio-control agent in poultry and in food as discussed below.82
Further Applications of Phage Lysins
Veterinary and food applications.
Lysins may not only be applied and developed to treat human infections. They also have an application in the veterinary sector. This would benefit the animal and in some cases also prevent the spread of zoonotic disease or transmission of a pathogen into food. Bovine mastitis is the most common cause of death in adult dairy cows and the cause of significant annual economic losses worldwide.84 Its presence can also lead to food contamination particularly in the context of raw milk cheeses.73,74,85 The Ply700 enzyme has shown lytic activity against mastitis causing streptococci in cows' milk, and another lysin, LysH5, has shown lytic activity against prevalent mastitis causing staphylococci, albeit in pasteurised milk.73,85
A recent study revealed that a recombinant phage lysin LySMP has a broad lytic spectrum against the increasingly antibiotic-resistant swine pathogen Streptococcus suis, which can cause conditions such as endocarditis and septicemia in pigs and is also an important zoonotic agent for humans.86,87 Mortality can approach 20% in pigs carrying S. suis, in the absence of treatment.88
The recombinant phage lysin PlyC has been developed as an enzyme disinfectant against Streptococcus equi, the causative agent of equine strangles in horses.89 Serious complications occur in 20% of infected horses, and an overall mortality rate as high as 8% occurs on farms where infection is endemic.89,90 The lysin was assessed for its ability to help control S. equi spread and transmission in horse stalls and barns and was shown to be superior to chemical disinfectants which can be toxic, easily inactivated, environmentally unfriendly and have a broad bacteriocidal range. In one experiment, PlyC remained fully active in a horse stable environment. It was active against >20 clinical isolates of S. equi, and on a per-weight basis was 1,000 times more active than the common oxidising chemical Virkon-S, a widely used disinfectant in the livestock industry for disease prevention and control.89 Typically 1 g of PlyC was able to sterilize a 108 CFU/ml culture of S. equi in 30 mins.89
Lysins also have a potential role to play in the food industry as can be seen with Ply3626. This enzyme has shown lytic activity against several strains of C. perfringens, which is a common cause of food poisoning and leads to economic losses in poultry production.82 It has been proposed that Ply3626 may be exploited as a control for this pathogen in poultry intestines, as a bio-preservative in raw poultry products and as a bio-control agent to be added directly to food or feed.82
Ply511 is a Listeria bacteriophage lysin; cloned in, produced and secreted by L. lactis to create dairy starter cultures with biopreservation properties against pathogenic Listeria monocytogenes. The lysin caused rapid lysis of this pathogen when secreted from a lactose-using L. lactis strain that could be employed for fermentation of milk.91 A truncated version of Ply511 lysin showed increased lytic activity over the native enzyme.91 Recombinant starter cultures have also been developed to specifically inhibit L. monocytogenes contamination during the ripening of soft cheese.91
In cheese ripening research a phage holin-lysin system was cloned into a lactococcal starter strain for the controlled release of host intracellular enzymes which are known to be involved in flavour formation during cheese ripening.14,92
Plant protection against phytopathogens.
Lysins have also been demonstrated to have potential for the control and elimination of phytopathogenic bacteria. In one study, a transgenic potato plant expressing the coliphage T4 lysin (lysozyme) was constructed to protect potatoes against Gram-negative Erwinia carotovora which causes soft rot. The lysin was secreted into the intracellular spaces of the transgenic plant and shown to kill the invading pathogen upon contact.10,14,93 In another study, a lysin from bacteriophage ϕEa1h was cloned into an expression vector and expressed in E. coli. When crude preparations of the enzyme were applied to the surface of pears inoculated with the Gram-negative plant pathogen Erwinia amylovora, infection was inhibited.94 Although both enzymes possess muramidase catalytic activity there is evidence, that the antibacterial effect is due to binding rather than peptidoglycan hydrolysis.95 Further studies may lead to the development of lysins as a safe alternative to chemical bactericides.
Diagnostics.
Lysins can also have applications in pathogen detection. Currently detection methods for human B. anthracis infection are slow and make successful treatment extremely difficult, given that the window to treat spore-exposed individuals is only 48 hours.78 Improved detection methods are being developed but to date are unfeasible for use outside a lab environment.96 A novel detection method using PlyG lysin has been developed which generates a result within 15 minutes.18 This system is incorporated into a hand-held illuminator, which detects ATP release from lysed bacilli after addition of lysin.18 In another study, magnetic beads coated with the cell wall-binding domain of lysins Ply118 and Ply500 from Listeria phage, were used for immobilization and separation of bacterial cells from contaminated food. This novel immobilisation and separation technique enabled the recovery of more than 90% of the L. monocytogenes cells present.97
The lysin, Ply118, has also been developed as a molecular biology tool. Loessner et al.15 demonstrated that it could be used for fast, efficient and gentle recovery of DNA, RNA, or native intracellular proteins from small scale Listeria cultures. Similarly, the multimeric phage lysin PlyC has proven to be superior to mutanolysin in efficiently digesting the cell wall of S. pyogenes for proteome-based studies of cell wall-anchored proteins in GAS.98
Additional Lysins
Along with the lysins targeting the Gram-positive pathogens discussed above, a number of similar enzymes99–104 which also have potential to eliminate Gram-positive pathogens including Streptococcus, Staphylococcus, Actinomyces, Micrococcus and Enterococcus are under investigation in different laboratories. These are also included in Table 1. In the case of the Av-1 lysin against the oral bacteria Actinomyces naeslundii, its precise catalytic activity is yet to be published.103
Table 1.
Recombinant phage lysins targeting pathogenic bacteria
| Lysin, Origin | Activity | Murine sepsis challenge* | Reference |
| Ply118, L. monocytogenes Φ A118 | amidase | − | 91 |
| Ply511, L. monocytogenes Φ A511 | amidase | − | 91 |
| Ply500, L. monocytogenes Φ A500 | endopeptidase | − | 29 |
| Pal, S. pneumoniae Φ Dp-1 | amidase, endopeptidase | + (nasopharynx, oropharynx. intraperitoneal) | 17, 51 |
| Cpl-1, S. pneumoniae Φ Cpl-1 | muramidase | + (intraperitoneal, nasopharynx, mucosa, bloodstream, aortic valve) | 51–53, 55 |
| PlyGBS, GBS Φ NCTC 11261 | muramidase, endopeptidase | + (vagina, oropharynx) | 64 |
| PlyC, S. pyogenes Φ C1 | amidase | + (oral mucosa, nasal mucosa) | 16, 89 |
| GBS lysin, S. agalactiae Φ B30 | muramidase, endopeptidase | − | 99 |
| LambdaSa1 prophage lysin, S. agalactiae LambdaSa1 prophage, | glycosidase | − | 100 |
| LambdaSa2 prophage lysin, S. agalactiae LambdaSa2 prophage | glycosidase, endopeptidase | − | 100 |
| Ply700, S. uberis (ATCC 700407) prophage | amidase | − | 85 |
| LySMP, S. suis Φ SMP | glycosidase, endopeptidase | − | 87 |
| PlyG, B. anthracis Φ γ | amidase | + (intraperitoneal) | 18 |
| PlyPH, B. anthracis Ames prophage | amidase | + (intraperitoneal) | 18, 78 |
| PlyB, B. anthracis Φ Bcp1 | muramidase | − | 45 |
| Lys16, S. aureus Φ P68 | endopeptidase | − | 101 |
| LysK, S. aureus Φ K | amidase, endopeptidase | − | 20 |
| Phi11 lysin, S. aureus Φ11 | amidase, endopeptidase | − | 74, 75 |
| MV-L, S. aureus Φ MR11 | amidase, endopeptidase | + (nasal cavity, intraperitoneal) | 33 |
| LysH5, S. aureus Φ H5 | amidase, endopeptidase | − | 73 |
| LysWMY, S. Warneri M Φ WMY | amidase, endopeptidase | − | 102 |
| Ply3626, C. perfringens Φ 3626 | amidase | − | 82 |
| CD27 lysin, C. difficile Φ CD27 | amidase | − | 80 |
| PlyV12, E. faecalis Φ 1 | amidase | − | 76 |
| Av-1 lysin, A. naeslundi Φ Av-1 | putative amidase/muramidase | − | 103 |
| LysgaY, L. gasseri Φ gaY | muramidase | − | 104 |
+, Tested in vivo in murine sepsis model, with site of challenge; −, Not tested in vivo in murine sepsis model.
Conclusion
Lysins have enormous potential as effective antibacterials in the fight against infectious disease where multi-drug-resistance is prevalent. As bacteriophages are considered the most abundant biological entities on earth, they are a rich natural source of these enzymes. Bio-informatic and proteomic studies are likely to lead to new opportunities for domain swapping, construction of chimeras and the production of specifically engineered designer lysins with diverse applications.
Acknowledgements
Mark Fenton was supported by the Science Foundation Ireland Research Frontiers Program, Project Ref 06/RFP/BIM004.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/9818
References
- 1.Bergh O, Borsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 2.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.d'Herelle FH. Sur un microbe invisible antagoniste des bacilles dysenteriques. C R Acad Sci Gen. 1917;165:373–375. [Google Scholar]
- 4.Hankin EH. L'action bactericide des eaux de la jumna et du gange sur le vibrion du cholera. Ann Inst Pasteur. 1896;10:511. [Google Scholar]
- 5.Twort FW. An investigation on the nature of ultramicroscopic viruses. Lancet. 1915:1241–1246. [Google Scholar]
- 6.Hermoso JA, Garcia JL, Garcia P. Taking aim on bacterial pathogens. Curr Opin Microbiol. 2007;10:461–472. doi: 10.1016/j.mib.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 8.Infectious Diseases Society of America (IDSA), author Bad bugs, no drugs. As antibiotic discovery stagnates…a public health crisis brews. 2004:1–35. [Google Scholar]
- 9.Russel M, Linderoth NA, Sali A. Filamentous phage assembly: variation on a protein export theme. Gene. 1997;192:23–32. doi: 10.1016/s0378-1119(96)00801-3. [DOI] [PubMed] [Google Scholar]
- 10.Borysowski J, Weber-Dabrowska B, Gorski A. Bacteriophage endolysins as a novel class of antibacterial agent. Exp Biol Med. 2006;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- 11.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang IN, Deaton J, Young R. Holins: the protein clocks of bacterial infection. Annu Rev Microbiol. 2000;54:799–825. doi: 10.1146/annurev.micro.54.1.799. [DOI] [PubMed] [Google Scholar]
- 13.Young R, Blasi U. Holins: form and function in bacteriophage lysis. FEMS Microbiol Rev. 1995;17:191–205. doi: 10.1111/j.1574-6976.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Loessner MJ. Bacteriophage endolysins-current state of research and applications. Curr Opin Microbiol. 2005;8:480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Loessner MJ, Schneider A, Scherer S. A new procedure for efficient recovery of DNA, RNA and proteins from Listeria cells by rapid lysis with a recombinant bacteriophage endolysin. Appl Environ Microbiol. 1995;61:1150–1152. doi: 10.1128/aem.61.3.1150-1152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson D, Loomis L, Fischetti VA. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci USA. 2001;98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeffler JM, Nelson D, Fischetti VA. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 18.Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer M, Vukov N, Scherer S, Loessner MJ. The murein hydrolase of the bacteriophage phi3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl Environ Microbiol. 2002;68:5311–5317. doi: 10.1128/AEM.68.11.5311-5317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol. 2005;187:7161–7164. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loessner MJ, Maier SK, Daubek-Puza H, Windlinger G, Scherer S. Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J Bacteriol. 1997;179:2845–2851. doi: 10.1128/jb.179.9.2845-2851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoong P, Schuch R, Nelson D, Fischetti VA. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J Bacteriol. 2004;186:4808–4812. doi: 10.1128/JB.186.14.4808-4812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunin CM. Resistance to antimicrobial drugs—a worldwide calamity. Ann Intern Med. 1993;18:557–561. doi: 10.7326/0003-4819-118-7-199304010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 25.European Antimicrobial Resistance Surveillance system, author. EARSS Annual Report 2005–2006. :1–47.
- 26.Diaz E, Lopez R, Garcia JL. Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc Natl Acad Sci USA. 1990;87:8125–8129. doi: 10.1073/pnas.87.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia P, Garcia JL, Garcia E, Sanchez-Puelles JM, Lopez R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990;86:81–88. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- 28.Fischetti VA. The use of phage lytic enzymes to control bacterial infections. In: Kutter E, Sulakvelidze A, editors. Bacteriophages: Biology and Applications. Boca Raton: CRC Press; 2004. pp. 321–334. [Google Scholar]
- 29.Loessner MJ, Kramer K, Ebel F, Scherer S. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol. 2002;44:335–349. doi: 10.1046/j.1365-2958.2002.02889.x. [DOI] [PubMed] [Google Scholar]
- 30.Lopez R, Garcia E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes and bacteriophage. FEMS Microbiol Rev. 2004;28:553–580. doi: 10.1016/j.femsre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Fischetti VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 2008;11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarre WW, Ton-That H, Faull KF, Schneewind O. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a D-alanyl-glycine endopeptidase activity. J Biol Chem. 1999;274:15847–15856. doi: 10.1074/jbc.274.22.15847. [DOI] [PubMed] [Google Scholar]
- 33.Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage ΦMR11. J Infect Dis. 2007;196:1237–1247. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 34.Nelson D, Schuch R, Cahales P, Zhu S, Fischetti VA. PlyC: A multimeric bacteriophage lysin. Proc Natl Acad Sci USA. 2006;103:10765–10770. doi: 10.1073/pnas.0604521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermoso JA, Monterroso B, Albert A, Galan B, Ahrazem O, Garcia P, et al. Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1. Structure. 2003;11:1239–1249. doi: 10.1016/j.str.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Sass P, Bierbaum G. Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol. 2007;73:347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loessner MJ, Gaeng S, Wendlinger G, Maier SK, Scherer S. The two-component lysis system of Staphylococcus aureus bacteriophage twort: a large TTG-start holin and an associated amidase endolysin. FEMS Microbiol Lett. 1998;162:265–274. doi: 10.1111/j.1574-6968.1998.tb13008.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Q, Fischetti VA. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl Microbiol Biotechnol. 2007;74:1284–1291. doi: 10.1007/s00253-006-0771-1. [DOI] [PubMed] [Google Scholar]
- 39.Horgan M, O'Flynn G, Garry J, Cooney J, Coffey A, Fitzgerald GF, et al. The phage lysin, LysK, can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl Environ Microbiol. 2009;75:872–874. doi: 10.1128/AEM.01831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low LY, Yang C, Perego M, Osterman A, Liddington RC. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J Biol Chem. 2005;280:35433–35439. doi: 10.1074/jbc.M502723200. [DOI] [PubMed] [Google Scholar]
- 41.Briers Y, Schmelcher M, Loessnen MJ, Hendrix J, Engelborghs Y, Volckaert G, et al. The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144. Biochem Biophys Res Commun. 2009;383:187–191. doi: 10.1016/j.bbrc.2009.03.161. [DOI] [PubMed] [Google Scholar]
- 42.Cheng X, Zhang X, Pflugrath JW, Studier FW. The structure of bacteriophage T7 lysozyme, a zinc amidase and an inhibitor of T7 RNA polymerase. Proc Natl Acad Sci USA. 1994;91:4034–4038. doi: 10.1073/pnas.91.9.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagermann M, Matthews BW. Crystal structures of a T4-lysozyme duplication-extension mutant demonstrate that the highly conserved beta-sheet region has low intrinsic folding propensity. J Mol Biol. 2002;316:931–940. doi: 10.1006/jmbi.2001.5376. [DOI] [PubMed] [Google Scholar]
- 44.Korndorfer IP, Danzer J, Schmelcher M, Zimmer M, Skerra A, Loessner MJ. The crystal structure of the bacteriophage PSA endolysin reveals a unique fold responsible for specific recognition of Listeria cell walls. J Mol Biol. 2006;364:678–689. doi: 10.1016/j.jmb.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 45.Porter CJ, Schuch R, Pelzek AJ, Buckle AM, McGowan S, Wilce MC. The 1.6 A crystal structure of the catalytic domain of PlyB, a bacteriophage lysin active against Bacillus anthracis. J Mol Biol. 2007;366:540–550. doi: 10.1016/j.jmb.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 46.Alcantara EH, Kim DH, Do SI, Lee SS. Bi-functional activities of chimeric lysozymes constructed by domain swapping between bacteriophage T7 and K11 lysozymes. J Biochem Mol Biol. 2007;40:539–546. doi: 10.5483/bmbrep.2007.40.4.539. [DOI] [PubMed] [Google Scholar]
- 47.Croux C, Ronda C, Lopez R, Garcia JL. Interchange of functional domains switches enzyme specificity: construction of a chimeric pneumococcal-clostridial cell wall lytic enzyme. Mol Microbiol. 1993;9:1019–1025. doi: 10.1111/j.1365-2958.1993.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 48.Sanz JM, Garcia P, Garcia JL. Construction of a multifunctional pneumococcal murein hydrolase by module assembly. Eur J Biochem. 1996;235:601–605. doi: 10.1111/j.1432-1033.1996.00601.x. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan MM, Garcia JL, Lopez R, García P. Analysis of the catalytic domain of the lysin of the lactococcal bacteriophage Tuc2009 by chimeric gene assembling. FEMS Microbiol Lett. 1996;140:23–28. doi: 10.1111/j.1574-6968.1996.tb08309.x. [DOI] [PubMed] [Google Scholar]
- 50.Hanlon J. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. J Anti Microbial agents. 2007;30:118–128. doi: 10.1016/j.ijantimicag.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Jado I, Lopez R, Garcia E, Fenoll A, Casal J, Garcia P. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother. 2003;52:967–973. doi: 10.1093/jac/dkg485. [DOI] [PubMed] [Google Scholar]
- 52.Loeffler JM, Djurkovic S, Fischetti VA. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun. 2003;71:6199–6204. doi: 10.1128/IAI.71.11.6199-6204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCullers JA, Karlstrom A, Iverson AR, Loeffler JM, Fischetti VA. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3:28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischetti VA. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbial. 2005;13:496. doi: 10.1016/j.tim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother. 2005;49:4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh S, Shah A, Mond J. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob Agents Chemother. 2003;47:554–558. doi: 10.1128/AAC.47.2.554-558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia P, Lopez R, Ronda C, Garcia E, Tomasz A. Mechanism of phage induced lysis in pneumococci. J Gen Microbiol. 1983;129:479–487. doi: 10.1099/00221287-129-2-479. [DOI] [PubMed] [Google Scholar]
- 58.Fischetti VA. Novel method to control pathogenic bacteria on human mucous membranes. Ann N Y Acad Sci. 2003;987:207–214. doi: 10.1111/j.1749-6632.2003.tb06050.x. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita Y, Shibata Y, Nakano Y, Tsuda H, Kido N, Ohta M, et al. A novel gene required for rhamnose-glucose polysaccharide synthesis in Streptococcus mutans. J Bacteriol. 1999;181:6556–6559. doi: 10.1128/jb.181.20.6556-6559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker SC, Foster-Frey J, Donovan DM. The phage K enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbial Lett. 2008;287:185–191. doi: 10.1111/j.1574-6968.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- 61.Loffler JM, Fischetti VA. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother. 2003;47:375–377. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Cerrato V, Garcia P, Del Prado G, Garcia E, Gracia M, Huelves L, et al. In vitro actions of LytA, the major pneumococcal autolysin. With two bacteriophage lytic enzymes (Cpl-1 and Pal), cefotaxime and moxifloxacin against antibiotic-susceptible and -resistant Streptococcus pneumoniae strains. J Antimicrob Chemother. 2007;60:1159–1162. doi: 10.1093/jac/dkm342. [DOI] [PubMed] [Google Scholar]
- 63.Djurkovic S, Loeffler JM, Fischetti VA. Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme Cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. J Antimicrob Chemother. 2005;49:1225–1228. doi: 10.1128/AAC.49.3.1225-1228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng Q, Nelson D, Zhu Z. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother. 2005;49:111–117. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuzaki S, Rashel M, Uchiyama J, Sakurai S, Ujihara T, Kuroda M, et al. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J Infect Chemother. 2005;11:211–219. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 66.Brown PD, Lerner SA. Community-acquired pneumonia. Lancet. 1998;352:1295–1302. doi: 10.1016/S0140-6736(98)02239-9. [DOI] [PubMed] [Google Scholar]
- 67.Witzenrath M, Schmeck B, Doehn JM, Tschernig T, Zahlten J, Loeffler JM, et al. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med. 2009;37:779–780. doi: 10.1097/CCM.0b013e31819586a6. [DOI] [PubMed] [Google Scholar]
- 68.Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis. 2008;197:1519–1522. doi: 10.1086/587942. [DOI] [PubMed] [Google Scholar]
- 69.Cheng Q, Fischetti VA. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl Microbial Biotechnol. 2006;74:1284–1291. doi: 10.1007/s00253-006-0771-1. [DOI] [PubMed] [Google Scholar]
- 70.Gruet P, Maicent P, Berthelot X, Kaltsatos V. Bovine mastitis and intramammary drug delivery: review and perspectives. Adv Drug Deliv Rev. 2001;50:245–259. doi: 10.1016/s0169-409x(01)00160-0. [DOI] [PubMed] [Google Scholar]
- 71.European Antimicrobial Resistance Surveillance System (EARSS) management team, members of the advisory board, and national representatives of EARSS 2007, author. EARSS annual report 2006. The Netherlands: EARSS, Bilthoven; [Google Scholar]
- 72.Eady AE, Cove JH. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr Opin Infectious Dis. 2003;16:103–124. doi: 10.1097/00001432-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Obeso JM, Martinez B, Rodriguez A, Garcia P. Lytic activity of the recombinant staphylococcal bacteriophage phiH5 endolysin active against Staphylococcus aureus in milk. Int J Food Microbiol. 2008;128:211–218. doi: 10.1016/j.ijfoodmicro.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 74.Donovan DM, Lardeo M, Foster-Frey J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbial Lett. 2006;265:133–139. doi: 10.1111/j.1574-6968.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 75.Sass P, Bierbaum G. Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol. 2007;73:347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoong P, Schuch R, Nelson D, Fischetti VA. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J Bacteriol. 2004;186:4808–4812. doi: 10.1128/JB.186.14.4808-4812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Asher MS, Eitzen E. Anthrax as a biological weapon 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 78.Yoong P, Schuch R, Nelson D, Fischetti VA. PlyPH, a bacteriolytic enzyme with a broad pH range of activity and lytic action against Bacillus anthracis. J Bacteriol. 2006;188:2711–2714. doi: 10.1128/JB.188.7.2711-2714.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dancer SJ. How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet Infect Dis. 2004;4:611–619. doi: 10.1016/S1473-3099(04)01145-4. [DOI] [PubMed] [Google Scholar]
- 80.Mayer MJ, Narbad A, Gasson MJ. Molecular characterisation of Clostridium difficile bacteriophage and its cloned biologically active endolysin. J Bacteriol. 2008;20:6734–6740. doi: 10.1128/JB.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Byrnestad S, Synstad B, Granum PR. The Clostridium perfringens enterotoxin gene is a transposable element in type A human food poisoning strains. Microbiology. 1997;143:2109–2115. doi: 10.1099/00221287-143-7-2109. [DOI] [PubMed] [Google Scholar]
- 82.Zimmer M, Vukov N, Scherer S, Loessner MJ. The murien hydrolase of the bacteriophage phi3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl Environ Microbiol. 2002;68:5311–5317. doi: 10.1128/AEM.68.11.5311-5317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Courchesne NM, Parisien A, Lan CQ. Production and application of bacteriophage and bacteriophage-encoded lysins. Recent Pat Biotechnol. 2009;3:37–45. doi: 10.2174/187220809787172678. [DOI] [PubMed] [Google Scholar]
- 84.Wellenberg GJ, van der Poel WH, Van Oirschot JT. Viral infections and bovine mastitis: a review. Vet Microbiol. 2002;88:27–45. doi: 10.1016/s0378-1135(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 85.Celia LK, Nelson D, Kerr DE. Characterisation of a bacteriophage lysin (Ply700) from Streptococcus uberis. Vet Microbiol. 2007;130:107–117. doi: 10.1016/j.vetmic.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Lun ZR, Wang QP, Chen QG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7:201–209. doi: 10.1016/S1473-3099(07)70001-4. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Sun JH, Lu CP. Purified phage lysin LySMP: an extensive spectrum of lytic activity for swine streptococci. J Curr Microbiol. 2009;58:609–615. doi: 10.1007/s00284-009-9379-x. [DOI] [PubMed] [Google Scholar]
- 88.Zhang C, Ning Y, Zhang Z, Song L, Qui H, Gao H. In vitro antimicrobial susceptibility of Streptococcus suis strains isolated from clinically healthy sows in China. Vet Microbiol. 2008;131:386–392. doi: 10.1016/j.vetmic.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 89.Hoopes JT, Stark CJ, Kim HA, Sussman DJ, Donovan MD, Nelson DC. Use of a bacteriophage lysin, PlyC as an enzyme disinfectant against Streptococcus equi. Appl Environ Microbiol. 2009:1388–1394. doi: 10.1128/AEM.02195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sweeney CR, Whitlock RH, Meirs DA, Whitehead SC, Barningham SO. Complications associated with Streptococcus equi infection on a horse farm. J Am Vet Med Assoc. 1987;191:1446–1448. [PubMed] [Google Scholar]
- 91.Gaeng S, Scherer S, Neve H, Loessner MJ. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl Environ Microbiol. 2000;66:2951–2958. doi: 10.1128/aem.66.7.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Ruyter PGGA, Kuipers OP, Meijer WC, de Vos VM. Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat Biotechnol. 1997;15:976–979. doi: 10.1038/nbt1097-976. [DOI] [PubMed] [Google Scholar]
- 93.During K, Porsch P, Fladung M, Lörz H. Transgenic potato plants resistant to the phytopathogenic bacterium Erwinia carotovora. Plant J. 1993;3:587–598. [Google Scholar]
- 94.Kim WS, Salm H, Geider K. Expression of bacteriophage ϕEa1h lysozyme in Escherichia coli and its activity in growth inhibition of Erwinia amylovora. Microbiology. 2004;150:2707–2714. doi: 10.1099/mic.0.27224-0. [DOI] [PubMed] [Google Scholar]
- 95.During K, Porsch P, Mahn A, Brinkmann O, Gieffers W. The non enzymatic microbicidal activity of lysozymes. FEBS Lett. 1999;449:93–100. doi: 10.1016/s0014-5793(99)00405-6. [DOI] [PubMed] [Google Scholar]
- 96.Rosovitz MJ, Leppla SH. Virus deals anthrax a killer blow. Nature. 2002;418:825–826. doi: 10.1038/418825a. [DOI] [PubMed] [Google Scholar]
- 97.Kretzer JW, Lehmann R, Schmelcher M, Banz M, Kim KP, Korn C, et al. Use of high-affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells. Appl Environ Microbiol. 2007;73:1992–2000. doi: 10.1128/AEM.02402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koller T, Nelson D, Nakata M, Kreutzer M, Fischetti VA, Glocker MO. PlyC, a novel enzyme for compartment dependent proteomics of group A Streptococci. Proteomics. 2007;8:140–148. doi: 10.1002/pmic.200700001. [DOI] [PubMed] [Google Scholar]
- 99.Pritchard DG, Dong S, Baker JR, Engler EA. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology. 2004;150:2079–2087. doi: 10.1099/mic.0.27063-0. [DOI] [PubMed] [Google Scholar]
- 100.Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl Environ Microbiol. 2007;73:7150–7154. doi: 10.1128/AEM.01783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takac M, Witte A, Blasi U. Functional analysis of the lysis genes of Staphylococcus aureus phage P68 in Escherichia coli. Microbiology. 2005;151:2331–2342. doi: 10.1099/mic.0.27937-0. [DOI] [PubMed] [Google Scholar]
- 102.Yokoi KJ, Kawahigashi N, Uchida M, Sugahara K, Shinohara M, Kawasaki KI. The two component cell lysis genes holWMY and lysWMY of the Staphylococcus warneri M phage WMY: cloning, sequencing, expression and mutational analysis in Escherichia coli. Gene. 2005;351:97–108. doi: 10.1016/j.gene.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 103.Delisle AL, Barcak GJ, Guo M. Isolation and expression of the lysis genes of Actinomyces naeslundii phage Av-1. Appl Environ Microbiol. 2006;72:1110–1117. doi: 10.1128/AEM.72.2.1110-1117.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sugahara K, Yokai KJ, Nakamura Y, Nishino T, Yamakawa A, Taketo A. Mutational and biochemical analysis of the endolysin LysgaY encoded by the Lactobacillus gasseri JCM phage Φ gaY. Gene. 2007;404:41–52. doi: 10.1016/j.gene.2007.08.023. [DOI] [PubMed] [Google Scholar]