Abstract
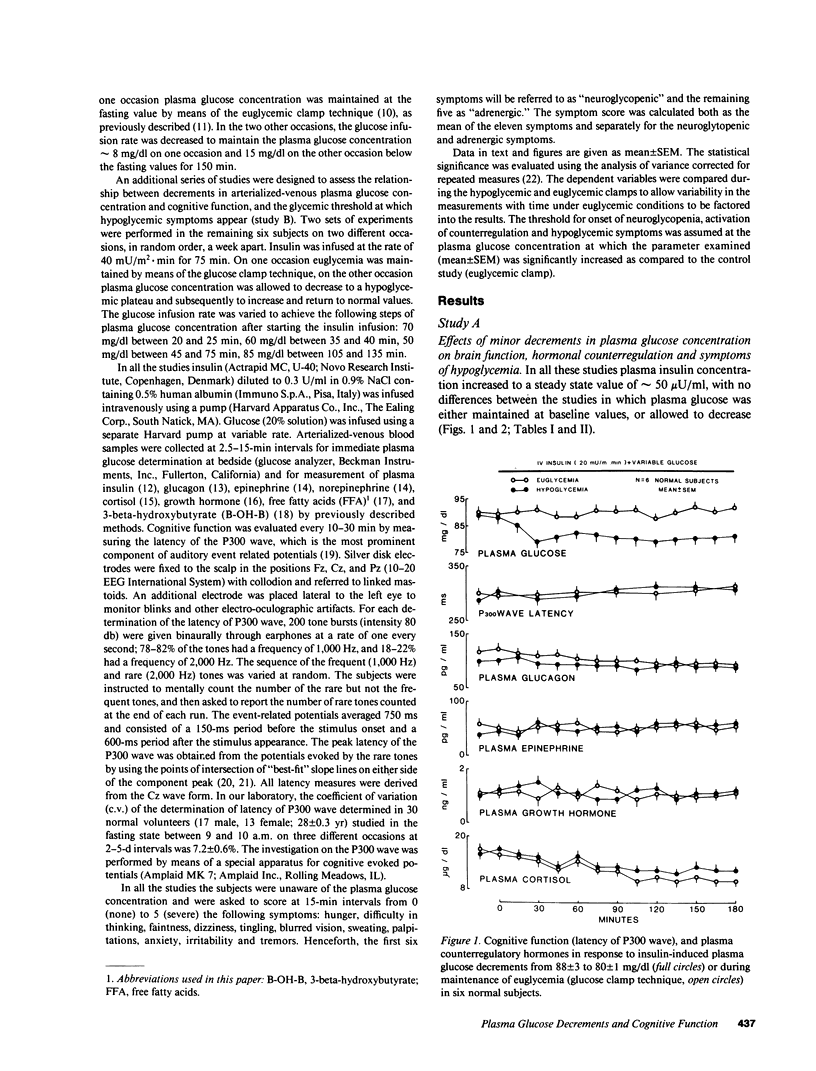
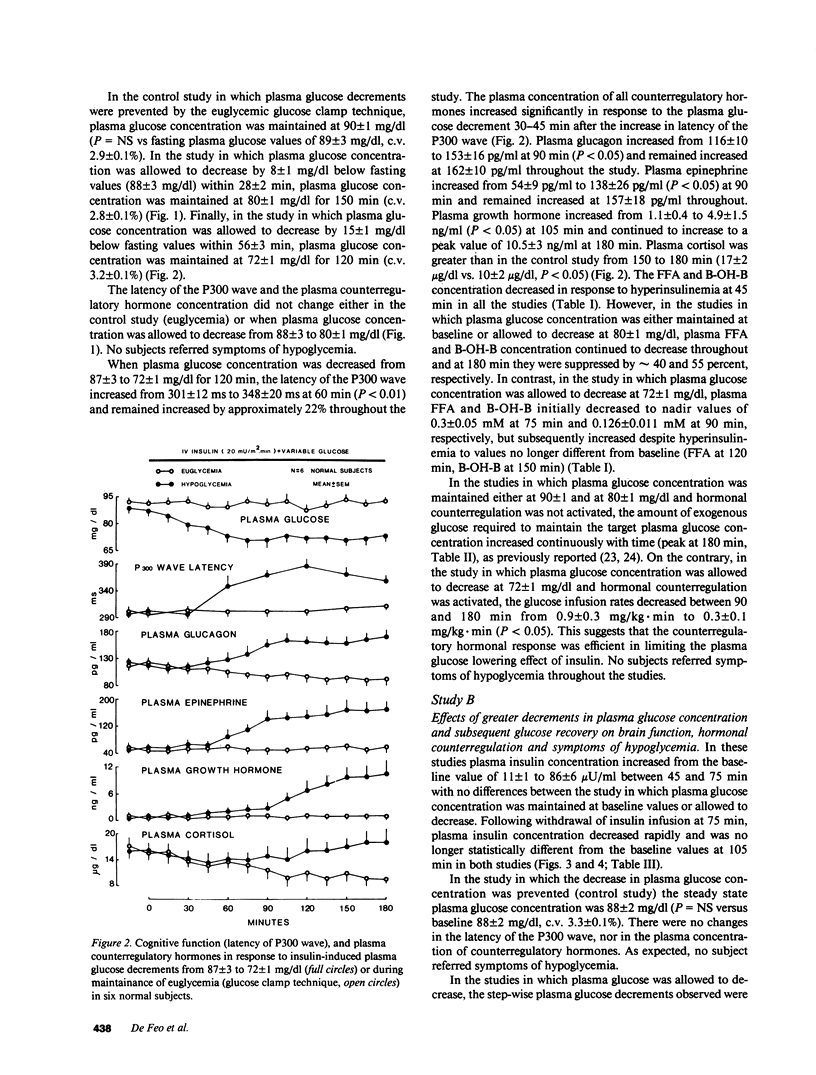
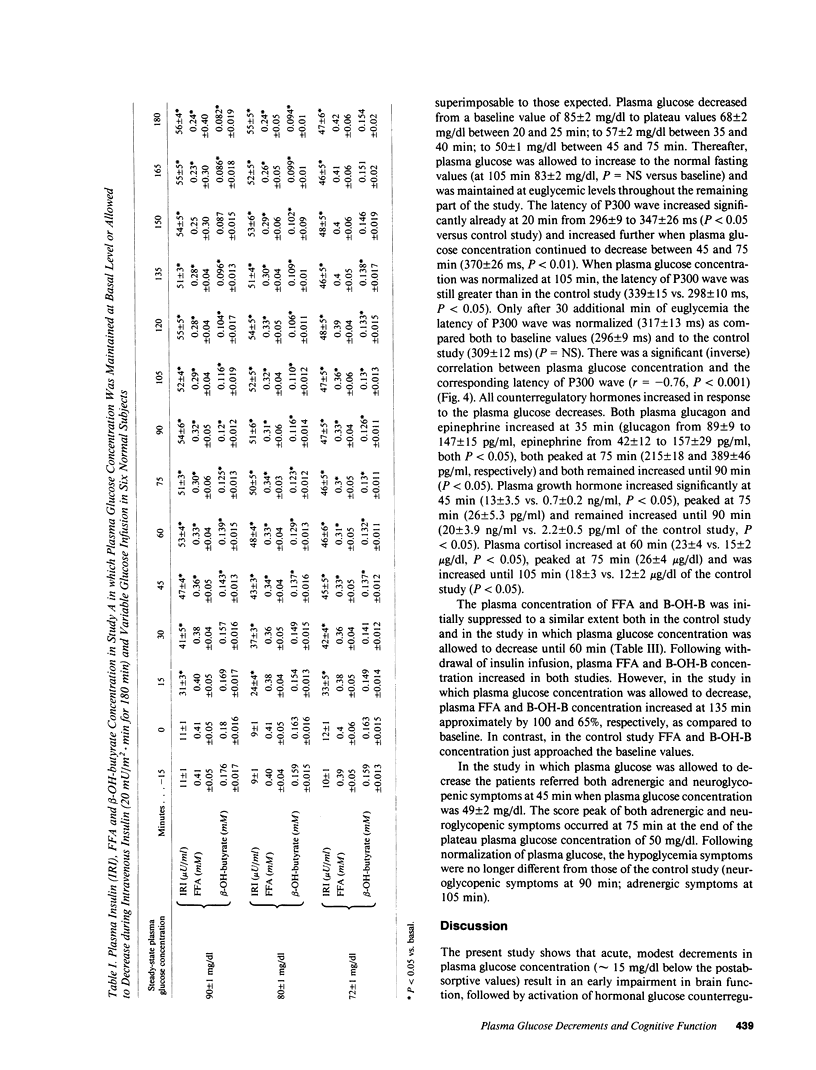
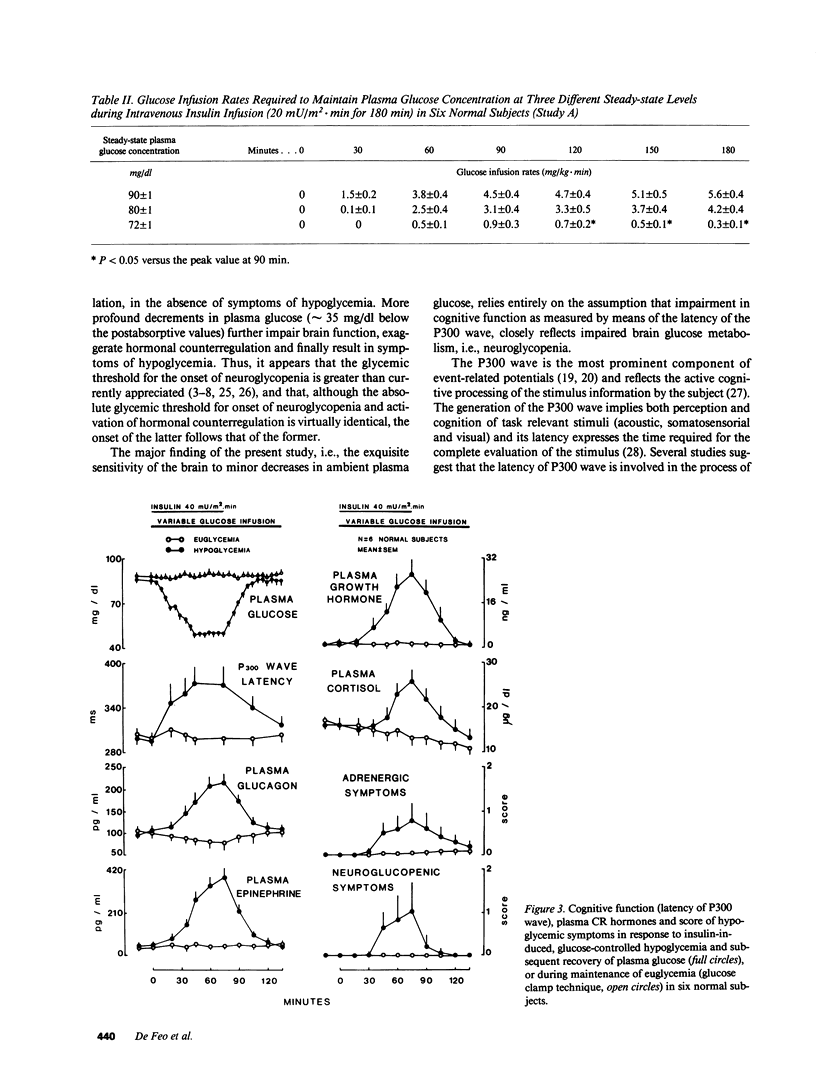
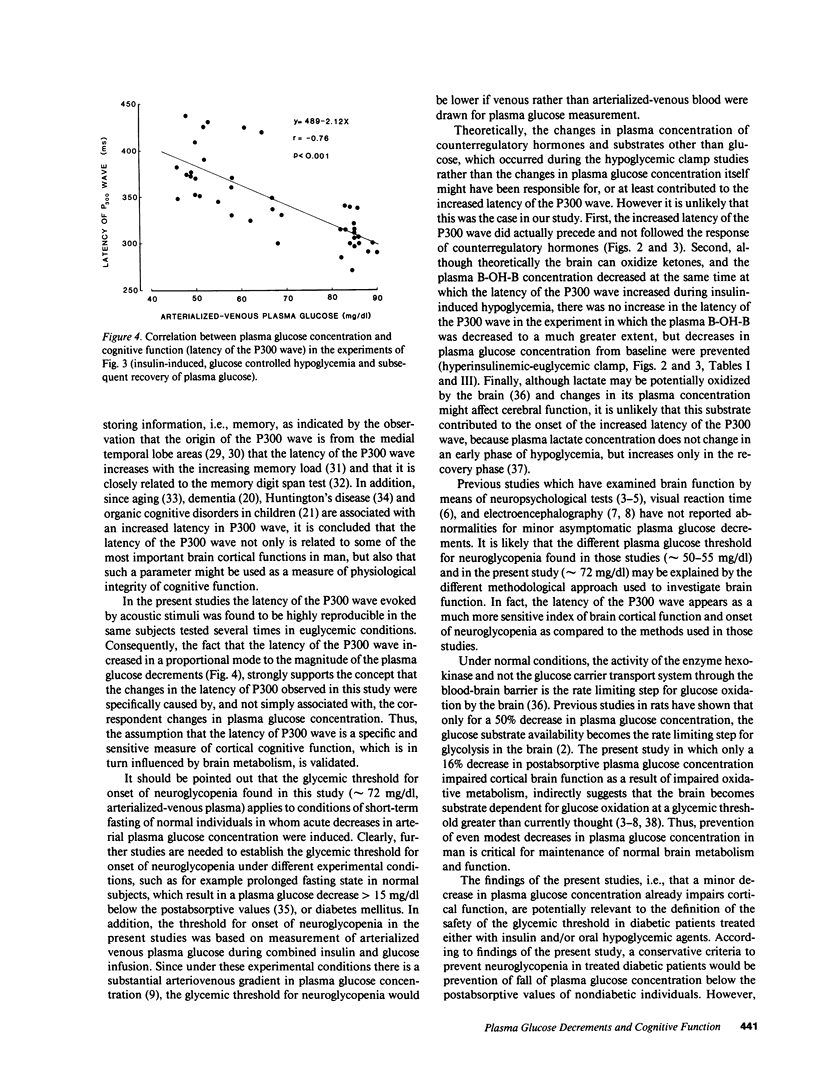
To establish the glycemic threshold for onset of neuroglycopenia (impaired cognitive function, measured by the latency of the P300 wave), activation of hormonal counterregulation and hypoglycemic symptoms, 12 normal subjects were studied either under conditions of insulin-induced, glucose-controlled plasma glucose decrements, or during maintenance of euglycemia. A decrement in plasma glucose concentration from 88 +/- 3 to 80 +/- 1 mg/dl for 150 min did not result in changes in the latency of the P300 wave nor in an activation of counterregulatory hormonal response. In contrast, a greater decrement in plasma glucose concentration from 87 +/- 3 to 72 +/- 1 mg/dl for 120 min caused an increase in the latency of the P300 wave (from 301 +/- 12 to 348 +/- 20 ms, P less than 0.01), a subsequent increase in all counterregulatory hormones but no hypoglycemic symptoms. Finally, when plasma glucose concentration was decreased in a stepwise manner from 88 +/- 2 to 50 +/- 1 mg/dl within 75 min, the increase in the latency of the P300 wave was correlated with the corresponding plasma glucose concentration (r = -0.76, P less than 0.001). The glycemic threshold for hypoglycemic symptoms was 49 +/- 2 mg/dl. Thus, in normal man the glycemic threshold for neuroglycopenia (72 +/- 1 mg/dl) is greater than currently thought; the hormonal counterregulation follows the onset of neuroglycopenia; the hypoglycemic symptoms are a late indicator of advanced neuroglycopenia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam N., Collins G. I. Late components of the visual evoked potential to search in short-term memory. Electroencephalogr Clin Neurophysiol. 1978 Feb;44(2):147–156. doi: 10.1016/0013-4694(78)90261-4. [DOI] [PubMed] [Google Scholar]
- Amiel S. A., Tamborlane W. V., Simonson D. C., Sherwin R. S. Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med. 1987 May 28;316(22):1376–1383. doi: 10.1056/NEJM198705283162205. [DOI] [PubMed] [Google Scholar]
- Arias P., Kerner W., Zier H., Navascués I., Pfeiffer E. F. Incidence of hypoglycemic episodes in diabetic patients under continuous subcutaneous insulin infusion and intensified conventional insulin treatment: assessment by means of semiambulatory 24-hour continuous blood glucose monitoring. Diabetes Care. 1985 Mar-Apr;8(2):134–140. doi: 10.2337/diacare.8.2.134. [DOI] [PubMed] [Google Scholar]
- Bergenstal R. M., Polonsky K. S., Pons G., Jaspan J. B., Rubenstein A. H. Lack of glucagon response to hypoglycemia in type I diabetics after long-term optimal therapy with a continuous subcutaneous insulin infusion pump. Diabetes. 1983 May;32(5):398–402. doi: 10.2337/diab.32.5.398. [DOI] [PubMed] [Google Scholar]
- Bolli G. B., Gottesman I. S., Cryer P. E., Gerich J. E. Glucose counterregulation during prolonged hypoglycemia in normal humans. Am J Physiol. 1984 Aug;247(2 Pt 1):E206–E214. doi: 10.1152/ajpendo.1984.247.2.E206. [DOI] [PubMed] [Google Scholar]
- Bolli G., De Feo P., Perriello G., De Cosmo S., Compagnucci P., Santeusanio F., Brunetti P., Unger R. H. Mechanisms of glucagon secretion during insulin-induced hypoglycemia in man. Role of the beta cell and arterial hyperinsulinemia. J Clin Invest. 1984 Apr;73(4):917–922. doi: 10.1172/JCI111315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrall R. J., Frier B. M., Davidson N. M., French E. B. Hormonal and substrate responses during recovery from hypoglycaemia in man during beta 1-selective and non-selective beta-adrenergic blockade. Eur J Clin Invest. 1981 Aug;11(4):279–283. doi: 10.1111/j.1365-2362.1981.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Gerich J. E. Glucose counterregulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl J Med. 1985 Jul 25;313(4):232–241. doi: 10.1056/NEJM198507253130405. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Da Prada M., Zürcher Simultaneous radioenzymatic determination of plasma and tissue adrenaline, noradrenaline and dopamine within the femtomole range. Life Sci. 1976 Oct 15;19(8):1161–1174. doi: 10.1016/0024-3205(76)90251-4. [DOI] [PubMed] [Google Scholar]
- De Feo P., Perriello G., De Cosmo S., Ventura M. M., Campbell P. J., Brunetti P., Gerich J. E., Bolli G. B. Comparison of glucose counterregulation during short-term and prolonged hypoglycemia in normal humans. Diabetes. 1986 May;35(5):563–569. [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Doberne L., Greenfield M. S., Schulz B., Reaven G. M. Enhanced glucose utilization during prolonged glucose clamp studies. Diabetes. 1981 Oct;30(10):829–835. doi: 10.2337/diab.30.10.829. [DOI] [PubMed] [Google Scholar]
- Finley W. W., Faux S. F., Hutcheson J., Amstutz L. Long-latency event-related potentials in the evaluation of cognitive function in children. Neurology. 1985 Mar;35(3):323–327. doi: 10.1212/wnl.35.3.323. [DOI] [PubMed] [Google Scholar]
- Gjedde A., Crone C. Blood-brain glucose transfer: repression in chronic hyperglycemia. Science. 1981 Oct 23;214(4519):456–457. doi: 10.1126/science.7027439. [DOI] [PubMed] [Google Scholar]
- Goodin D. S., Squires K. C., Henderson B. H., Starr A. Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalogr Clin Neurophysiol. 1978 Apr;44(4):447–458. doi: 10.1016/0013-4694(78)90029-9. [DOI] [PubMed] [Google Scholar]
- Goodin D. S., Squires K. C., Starr A. Long latency event-related components of the auditory evoked potential in dementia. Brain. 1978 Dec;101(4):635–648. doi: 10.1093/brain/101.4.635. [DOI] [PubMed] [Google Scholar]
- Halgren E., Squires N. K., Wilson C. L., Rohrbaugh J. W., Babb T. L., Crandall P. H. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events. Science. 1980 Nov 14;210(4471):803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Herold K. C., Polonsky K. S., Cohen R. M., Levy J., Douglas F. Variable deterioration in cortical function during insulin-induced hypoglycemia. Diabetes. 1985 Jul;34(7):677–685. doi: 10.2337/diab.34.7.677. [DOI] [PubMed] [Google Scholar]
- Hillyard S. A., Kutas M. Electrophysiology of cognitive processing. Annu Rev Psychol. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Holmes C. S., Hayford J. T., Gonzalez J. L., Weydert J. A. A survey of cognitive functioning at difference glucose levels in diabetic persons. Diabetes Care. 1983 Mar-Apr;6(2):180–185. doi: 10.2337/diacare.6.2.180. [DOI] [PubMed] [Google Scholar]
- Holmes C. S., Koepke K. M., Thompson R. G., Gyves P. W., Weydert J. A. Verbal fluency and naming performance in type I diabetes at different blood glucose concentrations. Diabetes Care. 1984 Sep-Oct;7(5):454–459. doi: 10.2337/diacare.7.5.454. [DOI] [PubMed] [Google Scholar]
- Lager I., Attvall S., Blohmé G., Smith U. Altered recognition of hypoglycaemic symptoms in type I diabetes during intensified control with continuous subcutaneous insulin infusion. Diabet Med. 1986 Jul-Aug;3(4):322–325. doi: 10.1111/j.1464-5491.1986.tb00772.x. [DOI] [PubMed] [Google Scholar]
- MATTINGLY D. A simple fluorimetric method for the estimation of free 11-hydroxycorticoids in human plasma. J Clin Pathol. 1962 Jul;15:374–379. doi: 10.1136/jcp.15.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall A. L., Fixman L. B., Fleming N., Tornheim K., Chick W., Ruderman N. B. Chronic hypoglycemia increases brain glucose transport. Am J Physiol. 1986 Oct;251(4 Pt 1):E442–E447. doi: 10.1152/ajpendo.1986.251.4.E442. [DOI] [PubMed] [Google Scholar]
- McCall A. L., Millington W. R., Wurtman R. J. Metabolic fuel and amino acid transport into the brain in experimental diabetes mellitus. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5406–5410. doi: 10.1073/pnas.79.17.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Okada Y. C., Kaufman L., Williamson S. J. The hippocampal formation as a source of the slow endogenous potentials. Electroencephalogr Clin Neurophysiol. 1983 Apr;55(4):417–426. doi: 10.1016/0013-4694(83)90130-x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Polich J., Howard L., Starr A. P300 latency correlates with digit span. Psychophysiology. 1983 Nov;20(6):665–669. doi: 10.1111/j.1469-8986.1983.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Pramming S., Thorsteinsson B., Stigsby B., Binder C. Glycaemic threshold for changes in electroencephalograms during hypoglycaemia in patients with insulin dependent diabetes. Br Med J (Clin Res Ed) 1988 Mar 5;296(6623):665–667. doi: 10.1136/bmj.296.6623.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramming S., Thorsteinsson B., Theilgaard A., Pinner E. M., Binder C. Cognitive function during hypoglycaemia in type I diabetes mellitus. Br Med J (Clin Res Ed) 1986 Mar 8;292(6521):647–650. doi: 10.1136/bmj.292.6521.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard W. S. Psychophysiology of P300. Psychol Bull. 1981 May;89(3):506–540. [PubMed] [Google Scholar]
- Robinson P. J., Rapoport S. I. Glucose transport and metabolism in the brain. Am J Physiol. 1986 Jan;250(1 Pt 2):R127–R136. doi: 10.1152/ajpregu.1986.250.1.R127. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Nudleman K., Starr A. Cognitive evoked potentials (P300) in early Huntington's disease. Arch Neurol. 1985 Oct;42(10):984–987. doi: 10.1001/archneur.1985.04060090066016. [DOI] [PubMed] [Google Scholar]
- Schwartz N. S., Clutter W. E., Shah S. D., Cryer P. E. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. 1987 Mar;79(3):777–781. doi: 10.1172/JCI112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson D. C., Tamborlane W. V., DeFronzo R. A., Sherwin R. S. Intensive insulin therapy reduces counterregulatory hormone responses to hypoglycemia in patients with type I diabetes. Ann Intern Med. 1985 Aug;103(2):184–190. doi: 10.7326/0003-4819-103-2-184. [DOI] [PubMed] [Google Scholar]
- Sutton S., Braren M., Zubin J., John E. R. Evoked-potential correlates of stimulus uncertainty. Science. 1965 Nov 26;150(3700):1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]


