Abstract
Autism is the most commonly studied of a spectrum of developmental disorders that are believed to be neurobiologically based but which, at this point, for lack of good biomarkers, are defined purely by behavior. In the last 20 years, the definition of autism has shifted in emphasis from extreme aloofness and positive signs of abnormality in repetitive and sensori-motor behaviors to a greater awareness of the importance of more subtle reciprocal social-communication deficits as core features. Standard diagnostic instruments were developed for research purposes to acquire information both through caregiver interviews and direct clinical observation. Use of these instruments in clinical practice resulted in major improvements which in turn affected research results. These results yielded further improvements that led to changes in clinical practice over time. The synergism between research and clinical practice in the understanding of autism is discussed.
Keywords: autism, assessment, ADI-R and ADOS
The constellation of behaviors that we call autism today is the result of astute observations by an eminent clinician, Leo Kanner (1943), of unusual patterns of social, communication, cognitive and motor development co-occurring in a small number of children. At about the same time, but without knowledge of Kanner, Hans Asperger (1944), a German pediatrician, wrote about similar, though not identical, patterns in boys he had seen. Through systematic studies, many aspects of these behaviors are now more carefully delineated and better understood. This includes knowing that not all individuals with autism have generally strong intelligence (Fombonne, 2005), that there are genetic components to autism which are sometimes, but not always, familial (Abrahams & Geschwind, 2008), and that autism is not just a disorder of childhood but a truly developmental disorder that affects development and is itself manifested differently across the lifespan (Lord & Spence, 2006).
Despite the urgent search for biomarkers and genetic loci for autism in the last 20 years (Abrahams & Geschwind, 2008), clinicians cannot wait for biology to change behavior for most people with autism. Basic research in brain function and genetics provides hope for improvements and prevention on a scale that current behaviorally-focused interventions and comprehensive treatments cannot yet offer. Nevertheless, with the continually increasing evidence of biological heterogeneity in autism (Abrahams & Geschwind, 2008; Lord & Spence, 2006), neurobiological answers seem far away except perhaps for a small subset of individuals with very specific genetic anomalies. Thus, we are back to behavior.
The focus of this paper is on how the measurement of social-communicative behavior in children and adults with autism was affected by research aims and goals in clinical practice, and how the two forces, research and practice, converged and diverged to make these measures better. Having had the opportunity to work with persons with autism in clinical and research settings for more than 40 years, one goal of the paper is to argue that there is a place for research not only for clinicians but by clinicians. Currently there are many calls for establishing evidence-bases for psychological practices (American Psychiatric Association (APA), 2005). As practice manuals are written, innovative research designs developed, and effect sizes analyzed, it is important to remember to use the knowledge from clinical practice and research to create better measures and better treatments, not simply justify what has already been done. This is not a call for all psychologists to become scientist-practitioners, but rather an account of ways in which innovations in assessment of children and adults with autism occurred within both research and clinical contexts.
Diagnostic characterization of autism
Autism is the most-studied disorder within a group of conditions now officially categorized as Pervasive Developmental Disorders or PDD (APA, 1994; World Health Organization [WHO], 1992). In response to parent and professional advocacy, the term PDD has now been commonly replaced in both research and professional communications by the term, Autism Spectrum Disorders (ASD), which will be used throughout this paper. ASDs include a number of different subtypes, including autism, PDD-NOS (Pervasive Developmental Disorder-Not Otherwise Specified), Asperger Disorder and several other more specific conditions. Because proposals are currently under consideration by the neurodevelopmental workgroup of DSM-V (APA, 2010) and just beginning to be raised by workgroups for the next International Classification of Diseases (ICD 11) that may reorganize these subtypes, for the purposes of this paper, the term autism will be used interchangeably with ASD. Unless specified, no distinctions among different subtypes within the autism spectrum will be made.
Autism is defined by deficits in very basic social and communication skills, differences in the way those skills are used in reciprocal social interaction and communication and by a heterogeneous list of behaviors that share repetitive or restricted features. In DSM-IV and ICD 10, social and communication features in autism are considered separately. Thus, three domains define autism (social, communication, restricted/repetitive behaviors and interests). One example of a communication deficit in autism, severe delays in expressive language level, can be separated from social skills relatively clearly. However, for the most part, specific examples of communication deficits in ASD, such as difficulties in reciprocal conversation, limited engagement in social chat, unusual intonation, limited gestures and deficits in imitation and play, are equally part of social factors as well as communication (Snow, Lecavalier, & Houts, 2009) except when nonverbal status is considered separately (Tadevosyan-Leyfer et al., 2003; Kamp-Becker, Ghahreman, Smidt, Remschmidt, 2008).
Basic social behaviors such as eye contact, facial expressions, and amount of social overtures to caregivers are highly related to more contextually-defined social skills such as offering to share, offering comfort, response to others’ approaches and participating in group activities, as well as relationships such as friendships (Gotham, Risi, Pickles, & Lord, 2007; Lord, Rutter, & Le Couteur, 1994). Moreover, nonverbal communication items on the Autism Diagnostic Interview – Revised (ADI-R) have been found to correlate very highly with social behaviors (Snow, et al., 2009).
Consequently, the most recent proposal for DSM V-has been to define ASD according to two domains: reciprocal social-communication and a broad domain of restricted, repetitive interests and behaviors (APA, 2010), with the caveat that, at a minimum, expressive language level and chronological age, as markers for developmental levels, must be taken into account before attempting to exemplify these domains for an individual child or adult. For example, while failure to learn to imitate a caregiver’s hand actions in a familiar song (e.g.,” The Wheels on the Bus”) may contribute to the diagnosis of autism in a two year-old as part of a social-communication deficit, a verbally fluent 13 year-old with ASD may be able to imitate hand movements, but does not spontaneously imitate friends or role models. The reverse may also be true. In an 18 month-old, complex mannerisms such as jumping up and down and flapping her hands in excitement are not a behavior specific to autism, but in a verbally articulate 13 year-old, such behaviors would be a sign of possible ASD, to be considered along with other behaviors. Consequently, in order to increase sensitivity (correctly identifying persons with the disorder) and specificity (correctly ruling out persons without the disorder), clinicians must always consider developmental factors in ASD. These factors are most easily proxied by expressive language level and age (Gotham et al., 2007).
There is currently widespread recognition that the repetitive, restricted behaviors (RRBs) that are used to define autism represent a very heterogeneous group that have distinct associations with intellectual disabilities (Bishop, Richler, & Lord, 2006), different trajectories over development (Richler, Huerta, Bishop, & Lord, 2010), and overlap in a variety of ways with other developmental disorders (e.g., Bishop, Gahagan, & Lord, 2007). Recent conceptualizations of RRBs have primarily broken them down into two factors: repetitive sensori-motor behaviors and insistence on sameness (Turner, 1999; Bishop et al., 2006) with a recent proposal to add a third factor of intense, circumscribed interests (Lam, Bodfish, & Piven, 2008). Almost all children with ASD have some kind of repetitive sensori-motor behavior (Bishop et al., 2006; Turner, 1999) with a significant minority of children having particular difficulties with insistence on sameness. Repetitive sensori-motor behaviors tend to develop relatively early (by age 4) and decrease with age except for children with autism and severe intellectual disabilities, whereas behaviors involving reactions to change tend to remain more consistent over time (Richler et al., 2010). Unusual preoccupations (e.g., Secretaries of the Navy, power tools, train schedules) and circumscribed interests that may be socially acceptable (Disney movies, animé, dinosaurs) but are so intense that they interfere with daily life, have tended to group with sensori-motor repetitive behaviors in young children and/or less verbal persons and then form their own factor in more verbally-skilled children and adults, in part depending on how the behaviors are described or coded (Richler et al., 2010).
Numerous studies have suggested that there is a broader autism phenotype in some parents and siblings that may occur as developmental language abnormalities, executive functioning deficits, milder social difficulties or specific personality characteristics (Landa et al., 1992; Piven, Palmer, Jacobi, Childress & Arndt, 1997; Virkud, Todd, Abbacchi, Zhang, & Constantino, 2008). Studies comparing children with autism to children with other developmental disorders that affect social skills have found that the most consistent differences have been in basic social skills, whereas more contextually-defined social behaviors are less specific to autism (Bishop et al., 2007). Recent studies using more direct measures of neurological function have supported the idea that there may be very basic deficits in social cognitive processing in autism such as the processing of faces or biological motion (Schultz et al., 2000). However, similar measures have also been linked to more complex phenomena, such as joint attention and Theory of Mind (Abell et al., 1999). Interpretation of many of these phenomena for clinical purposes of targeting and planning treatments has to consider the general cognitive demands of the situations children with ASD face – how much are face-processing deficits specific to faces or related to cognitive demands to process certain levels of complexity (Schultz et al., 2000)?
Important factors that affect quality of life for persons with autism and their families
Most of the publicity about autism in the past few years has stressed its unique impact on individuals and families (Schieve, Blumberg, Rice, Visser, & Boyle, 2007). Yet, when considering the outcome of persons with autism in terms of independence and quality of life, factors not unique to autism become equally important. The most well-studied of these is intellectual disabilities, which overlaps considerably with expressive language level. There are a small number of individuals with autism who have strong nonverbal problem-solving skills who cannot speak. However, not being able to use language fluently, especially when accompanied by very poor social skills, limits independence greatly even in a person with other strengths.
One of the major scientific shifts in autism research in the 1970’s was awareness that many, but not all, children diagnosed with autism at that time also had general intellectual disabilities (Rutter & Lockyer, 1967). This meant that findings from earlier studies in which children with autism were compared to typically developing children could not be interpreted as specific to autism because the differences could have been accounted for by intellectual disabilities alone. This changed the clinical conceptualization of autism from an emotional problem or basic deficit of sensory processing (Ornitz, 1971) to a cognitive or language disorder (Hermelin & O’Connor, 1967; Rutter, 1978). Autism moved out of consideration as an emotional disturbance and into the awareness of more cognitively and behaviorally oriented clinicians.
Shifts in conceptualizing autism
In the 1960’s and 1970’s, autism research proceeded on three fronts related to the description of core features. One was a behavioral focus on defining behaviors that would be changed through operant learning. There was little emphasis on clinical diagnosis or measurement of general functioning (Lovaas, 1987). The second research front was primarily led by child psychiatrists in centers where clinical diagnosis was taken quite seriously. This included work by Rutter at the Maudsley Hospital (Lockyer & Rutter, 1969) and Cohen at Yale (Cohen, Capurolo, & Shaywitz, 1976), as well as Rapin, a child neurologist (Rapin & Allen, 1983). A third movement was led by psychologists who began to develop standardized measures for screening and describing children with autism. The leader was Schopler, who developed the Childhood Autism Rating Scale (CARS; Schopler, Reichler, DeVellis, & Daly, 1980), the first commonly used clinician-completed measure of autism, and the Psychoeducational Profile (PEP; Schopler & Reichler, 1979), a direct assessment that emphasized cognitive and behavioral functioning. The focus of the CARS was to discriminate children with autism from other children. Hence, a number of behaviors not specific to autism, such as intelligence and language level, were included. The PEP was a comprehensive clinical instrument intended to lead directly to programming sessions in which parents were taught how to work with their children on individualized goals. At about the same time, Rimland, an experimental psychologist, introduced the first widely available questionnaire, the E-2 (Rimland, 1971) used to identify children with autism.
For much of this time, autism was not distinguished in formal psychiatric frameworks from childhood psychoses. Gradually, specific criteria for autism began to evolve, first as proposed by the National Society for Autistic Children (Ritvo & Freeman, 1978), now known as the Autism Society of America, by Rutter (1978), and then by the American Psychiatric Association in the Diagnostic and Statistical Manual III (APA, 1980). These criteria all alluded to social deficits in terms of general, rather extreme statements, such as aloofness and lack of social awareness, and then variously focused on severe communication delays, repetitive behaviors, insistence on sameness and unusual reactions to sensations. This resulted in diagnostic instruments that stressed unusual behaviors and implied the complete absence of social behavior and verbal communication (e.g., Rimland, 1971; Schopler et al., 1980). Because it is easier, particularly in checklists, to evaluate the presence of “positive” or odd behaviors rather than the “negative” diminution of different social or communicative reciprocal behaviors, the scales tended to emphasize easily observable, atypical behaviors that were particularly common in children with intellectual disabilities and autism. The CARS was adopted widely by clinicians, especially in school settings.
Yet conceptualizations of autism were changing rapidly. Wing proposed a triad of deficits in language comprehension, social deficits and lack of imaginary play (Wing & Gould, 1978). Other researchers began to focus on children with autism who did not have intellectual disabilities, with the logic that these children were the key to understanding the nature of “pure” autism (Bartak, Rutter & Cox, 1975).
The interest in the neurobiological underpinnings of autism was also expanding, in part because of the links identified between autism and seizures (see Lord & Spence, 2006), and because of the growing interest in the genetics of autism, stimulated by twin studies (Folstein & Rutter, 1977). There was also increasing research interest in combining samples across different clinical centers. However, studies suggested that clinical diagnoses at the major centers were not comparable to the CARS or E-2 (Venter, Lord & Schopler, 1992), making this possibility a challenge. In addition, because of the disparate ways in which the three different perspectives (behavioral, psychiatric, psychometric) defined autism, new researchers who were not behaviorists and not from the major child psychiatry centers had a very difficult time receiving research funding because they could not justify their clinical diagnoses as being equivalent to those from Yale, UCLA, or the Maudsley.
It was out of this research need that the specific clinician-based instruments, the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview (ADI) were developed. Rutter had used semi-structured clinical interviews within his teaching clinic for many years, working from interviews developed for the Isle of Wight studies of the relationship between neurological dysfunction and behavior problems (Rutter, Tizard, & Whitmore, 1970). Following on the twin study that found high levels of concordance for autism symptoms in identical twins, Rutter designed a family study that assessed the occurrence of similar symptoms in siblings and parents of children with autism (Bolton, Pickles, Murphy, & Rutter, 1998). Because there were so many new observations about the nature of the behavioral abnormalities in autism, including joint attention and specific language abnormalities, the goal undertaken in the ADI (Le Couteur et al., 1989) was to create a comprehensive account of both early history and current behavior through a semi-structured caregiver interview.
Development of the Autism Diagnostic Observation Scale (ADOS)
As a psychologist trained at UCLA by behaviorists, in North Carolina using the CARS and PEP, and at the Maudsley, Lord, with her colleagues (Lord et al., 1989) proposed the need for an additional standardized measure, particularly of social communication, which would be based on direct interaction with and observation by a clinician. The idea was to use the social and communication skills of the clinician to create standardized, but individualized, contexts in which to observe a child’s reciprocal interaction with an unfamiliar but engaging person. The first version of the ADOS was created (Lord, Rutter, DiLavore, & Risi, 2000) to be used with children 5 years and older in Rutter’s first family genetics study (Bolton et al., 1998) and in a follow-up study of verbal adolescents in North Carolina (Venter et al., 1992).
Another turning point was access to the then new option of making inexpensive, permanent records through videotape. Awareness that the understanding of the nature of autism was evolving meant that a video library offered the possibility of going back to re-score behaviors that might not have been identified initially as important. Although the option to videotape the ADOS was an important aspect of its development, from a clinical point of view, one of the key priorities in its design was to create a way to structure a social interaction with a child that could be scored by a clinician in real-time during a typical office visit. Clinical experience had convinced the authors that requiring a practitioner to carry out a standardized interaction and then go back and code a video was not feasible in usual practice. The PEP (Schopler & Reichler, 1979) and age-graded intelligence tests provided models. Thus, a key part of the design of the ADOS was that the activities of the clinician were organized so that he or she could interact with the child while taking brief notes. In the original ADOS, activities were presented, the child’s response to the activity coded immediately and, at the very end of the assessment, the clinician completed summary codes. Empirical research provided a number of examples of contexts in which children with autism consistently behaved differently than children with other disabilities, for example, in describing other people (Wolff & Barlow, 1979), playing with dolls and representational objects (see Lewis & Boucher, 1988), and narrating stories (see Loveland, McEvoy, Kelley, & Tunali, 1990). Table 1 shows examples of the activities from the original ADOS and later versions.
Table 1.
Activities from the original ADOS, PL-ADOS and current ADOS
| Tasks | Original ADOS |
PL-ADOS, ADOS (Toddler) |
ADOS Modules |
||
|---|---|---|---|---|---|
| M2 | M3 | M4 | |||
| Free play/breaks | X | X | X | X | X |
| Response to joint attention | X | X | |||
| Bubble play/teasing/balloon | X | X | |||
| Social smile/imitation | X | X | |||
| Birthday party/bath | X | ||||
| Snack | X | X | |||
| Construction task | X | X | X | X | |
| Make believe/interactive play | X | X | |||
| Conversation/cartoons | X | X | X | X | |
| Teaching toothbrushing | X | X | X | X | |
| Picture/book | X | X | X | X | |
| Reporting event/emotions | X | X | X | ||
| Social difficulties/create a story | X | X | |||
| Plans and dreams | X | ||||
The question was then how to create standardized “presses” (Murray, 1938) for social and communicative behaviors that clinicians could use during a 30 – 45 minute interaction. The model was influenced by Schopler ‘s concept of “emerging” behaviors (Schopler & Reichler, 1979). In the PEP, items such as imitating actions with objects or matching letters are initially administered in a standardized fashion. If a child cannot pass an item, the clinician makes the task easier in ways such as backward chaining or reducing the number of alternatives. If the child can then complete the task, an “emerging” score is applied. The clinician records what he/she did to help the child accomplish the task, yielding information to be used later in programming recommendations.
In the ADOS, this approach was modified in order to allow for standardized observations of reciprocal social-behaviors. A hierarchy of behaviors that the clinician follows was specified, then coded to indicate how far down the hierarchy the clinician had to move before the child responded. For example, in setting up a context for imaginative play, a clinician first introduces a set of action figures and potential props; then, if the child does not create imaginative sequences, the clinician demonstrates a simple sequence of play. If the child does not respond, then the clinician enters play with him or her, and if necessary, asks the child to choose a figure. If the child still does not play imaginatively, the clinician hands the child a figure, takes one himself or herself, and initiates a play sequence (such as having the figure jump in a puddle represented by a shiny disk) and invites the child to join. By specifying the sequence of actions of the clinician within each activity, similar contexts in which to observe relevant behaviors can be created by different clinicians, with room still left for the child’s individual interests.
Standardization had to not only include what the clinician and the child did, but directions as to what the clinician should observe. The child’s responses to specific activities comprised some of the codes (Lord et al., 2000). Additional ADOS codes were based on clinical descriptions of symptoms and previous research (Lockyer & Rutter, 1969). Beyond describing what the clinician should be looking for, how these observations were quantified also needed to be standardized and diagnostic algorithms created.
One of the most clinically interesting findings of the data analysis for the diagnostic algorithms was that, though items describing social and communication behaviors were highly correlated, specificity was improved (i.e., false positive diagnoses decreased) by requiring a child to meet cut-offs on both communication and social deficits separately. Because repetitive behaviors observed during the ADOS were not highly correlated with parent reports of these behaviors, the recommendation was made that clinicians note the presence of such behaviors but be careful not to interpret their absence, given the relatively short period of time and limited contexts of the observation.
Feedback quickly emerged that sometimes even well-established clinicians had difficulty either giving and/or coding the ADOS in a standardized fashion. Some clinicians rapidly became skilled in administering the items, but had difficulty using the specific and sometimes rather arbitrary ADOS codes. Other clinicians were good observers and conceptually sophisticated, but had little experience successfully engaging children with ASD in activities. In almost all cases, with practice and feedback, the clinicians learned to administer the scale. Funding from NIMH for research-training provided the foundation for the development of clinical training workshops as well.
From Research to Practice (the ADI)
In clinics, first at Glenrose Hospital in Edmonton, Alberta, and then in the newly created Greensboro TEACCH Center in North Carolina, staff conducted family-based individualized assessments of children and adults suspected of having autism. Goals were to identify strengths and weaknesses, work with families to understand and make decisions about programming, and make formal diagnoses primarily to access appropriate services. A database of core measures which almost all families agreed to join, was already in place using the PEP and CARS, which were employed by all TEACCH centers across the state. It was relatively easy to add the standard measures of the ADI and ADOS to the clinical protocol at the Greensboro Center.
Although the original function of the measures was to have standard data across UK and US sites for research purposes, clinical benefits of the new measures quickly emerged. The ADI was so long (about 4 hours at that point) that it required an extra visit for the parents and/or caregivers, without the child underfoot, resulting in a more relaxed and pleasant interchange than most assessments where clinicians are trying to talk to caregivers and observe the child at the same time. The ADI gave the clinicians an excellent picture of the child, through the parents’ eyes, as well as domain scores in the three areas (social, communication, repetitive) that define autism.
The ADI does not replace a medical history or a physical exam but does include questions about early behaviors and how the child has changed over time. Caregivers almost always reported their responses to these behaviors as they described them. Although caregivers’ descriptions did not always match what was later observed in the clinic, they gave clinicians a broader sense of the child, beyond what was typically acquired through teacher forms and phone calls. This was particularly important for young children, more complex cases, or cases when diagnosis was questioned by family members or service providers.
From Practice Back to Research (the ADI-R)
The greatest difficulty with the original ADI, which remains true to a lesser extent with the ADI-R, is that it was very long, and it was laid out so that items about early development were asked first, followed by a block of questions about current development. This sometimes felt cumbersome. In addition, much was happening in research about young children with autism (Mundy et al., 1994). Because the original ADI had been intended for children 5 years old and up, there were not sufficient questions about preschool children.
In the end, in part because of the impetus of an NIMH funded longitudinal study of children referred for possible autism at age 2, clinical researchers in North Carolina revised the ADI to be used with younger children (Lord, Shulman, & DiLavore, 2004). Questions were re-written and reorganized so that, for older children, the same question was asked about a child for a specific early time period and then immediately repeated about current behavior. This reduced the time of the administration to about 2 hours. A slightly longer version is now published as the ADI-R (Rutter, Le Couteur, & Lord, 2003).
Over the years, many clinicians and researchers have had much to say about individual questions on the ADI, and revisions have been made. In addition, where research has met practice has been in the necessity of not just including specific items that directly yield scores for algorithm diagnoses, but in continuing to ask open-ended questions that allow families to describe their children in their own words, without the onus of having to attribute a deficit to their child. These open-ended items provide additional information in a more useful way than relying completely on more pointed questions, giving the clinician a better picture of the referred child or adult as an individual within a family (see Rutter et al., 2003).
From Research to Practice (the ADOS)
The ADOS originally had roots in both the PEP and in informal modifications of experimental tasks created by developmental psychologists and child psychiatrists (see Mundy et al., 1994; Lewis & Boucher, 1988; Wolff & Barlow, 1979). Once the original ADOS was created, standard test kits and protocols provided a framework within which to interact with a child. That is, by not having to invent activities as they went along, clinicians could spend their energy thinking about the child, and then, whenever necessary, modify tasks within the hierarchical options offered in the ADOS. Caregivers, teachers and other service providers who came to watch the ADOS, usually through one-way mirrors, were enthusiastic about the new information that could be obtained about a child’s social-communication and play in an unfamiliar, but positive, fairly relaxed, setting.
From Practice to Research (the ADOS)
However, the ADOS was only intended for children 5 years and up with relatively fluent speech. Moreover, in research, high correlations were found between children’s verbal levels and ADOS scores, especially on the “activity-based” items. Autism clinics were beginning to get many referrals for children under age 5, and most of these children were not yet fluent speakers. Consequently, a modification of the ADOS appropriate for 3 and 4 year-olds was begun in preparation for the early diagnosis study that would recruit 2 year-olds. In this case, DiLavore, a special educator and clinical researcher, led the development of the Pre-Linguistic Autism Diagnostic Observation Schedule (PL-ADOS) (DiLavore, Lord, & Rutter, 1995). Although initially the plan was to begin with the original ADOS tasks, find bigger, non-swallowable toys more appropriate for preschool children, and reduce the amount of language required, it was quickly clear that the physical structure of the original ADOS was not appropriate. Two and 3 year-olds do not usually sit at a table for an hour while an adult hands them different toys. Nor is this an appropriate situation in which to evaluate reciprocal social behavior and spontaneous communication. Another clinically-based change was that very young children were often much more comfortable with their caregivers in the room.
Unexpectedly, it was also found that having caregivers witness and participate in the PL-ADOS was valuable for them. After having discussions with families during the ADI of whether their children ever showed them items or initiated joint attention or smiled at them spontaneously to share enjoyment, the PL-ADOS provided a way for clinicians to demonstrate for families exactly what they were talking about, and for caregivers to see for themselves what their child did and did not do in response to the social- communicative “presses” for interaction. When a child did not respond, a family member was asked if the child typically behaved differently in a more familiar environment. For several of the PL-ADOS items, the protocol included a caregiver trying to elicit a particular response to whatever he or she commonly did at home (e.g. how did a parent typically get a child to smile, without touching him or her). The ADOS is organized around materials that are fun for most children with each activity ending in a positive way (by using errorless learning techniques if the standard administration was not successful). Thus, clinicians can move, ideally, seamlessly from task to task with neither caregivers nor children distressed by repeated failures.
From Practice Back to Research (the ADOS)
The field of autism was changing in many ways in the mid-1990’s. There was greater acknowledgement that many children met some criteria, without necessarily fitting the standard conception of classic autism. These children, particularly those with less severe language delays and no intellectual disabilities, were first referred to as having atypical autism or Pervasive Developmental Disorder- Not Otherwise Specified (PDD-NOS), and later sometimes given Asperger Syndrome diagnoses (Buitelaar & van der Gaag, 1998; Volkmar & Klin, 2000). They needed services, their families wanted information, and they sometimes fell between the cracks when instruments developed from research focusing on identifying more severe cases of autism were used. Clinically, there was a growing number of referrals of older children and adolescents who often arrived for an evaluation with other diagnoses such as attention deficits, anxiety disorders or disruptive behavior disorders, but were now suspected of having undiagnosed PDD-NOS or Asperger Syndrome. A concern arising from clinicians was that, by having only a single “autism” threshold on the ADOS, children who had significant social-communication difficulties that were on the autism spectrum were being excluded from services.
Research quickly showed that children under 4 who had beginning phrases or even more language were not correctly characterized on the PL-ADOS, as compared to broader clinical diagnoses (Lord, Risi, DiLavore, Shulman, Thurm, & Pickles, 2006). In addition, empirical studies demonstrated that various aspects of development, most obviously expressive language, but also other aspects of communication and nonverbal intelligence, contributed to performance by children with autism on standard social-cognitive tasks increasingly being used to help understand the thinking of children with autism (Happé, 1995).
Developmental changes in autism, and the spectrum of autistic disorders were increasingly seen as critical to the understanding of individual differences and trajectories that might be influenced by different treatments. In addition, factor analytic and longitudinal studies (Lord et al., 2006) were showing that repetitive restricted behaviors, even from brief observations such as the ADOS, contributed to prognosis, suggesting that inclusion of RRBs in an ADOS algorithm that used a total across all three domains, would make it more accurate, and provide a way of replacing the relatively artificial distinction between social and communication scores in the original ADOS. In the end, the confluence of these factors resulted in one major and one minor change in how the ADOS was organized and then, later, additional changes in how scores could be interpreted.
First, the PL-ADOS and original ADOS were consolidated into what was initially called the ADOS-G (for Generic) and now is referred to as the ADOS (Lord, et al., 2000). A modular format was used in which a clinician selects one module out of four, based on a combination of the referred patient’s age and language level. Four modules were proposed: Module 1 (children with no words or single words), Module 2 (children with simple sentences), Module 3 (children with fluent speech) and Module 4 (older adolescents and adults with fluent speech). About half the tasks in each consecutive module overlap with tasks in the previous module and about two-thirds of the behavior codings overlap, allowing direct comparison of observations made at earlier points in development. This was a first attempt to fill in the gaps by providing ways to assess older, less verbally fluent children and younger more verbally fluent children, as well as adolescents and adults for whom toys were not appropriate.
Second, coding was shifted from a combination of codes that directly described a child’s behavior in a particular task to almost all summary codes (e.g. eye contact, quality of social overtures), completed after the ADOS is finished. This significantly reduced the effect of IQ on ADOS scores and also better discriminated ASD from other developmental disorders (Lord et al., 2000).
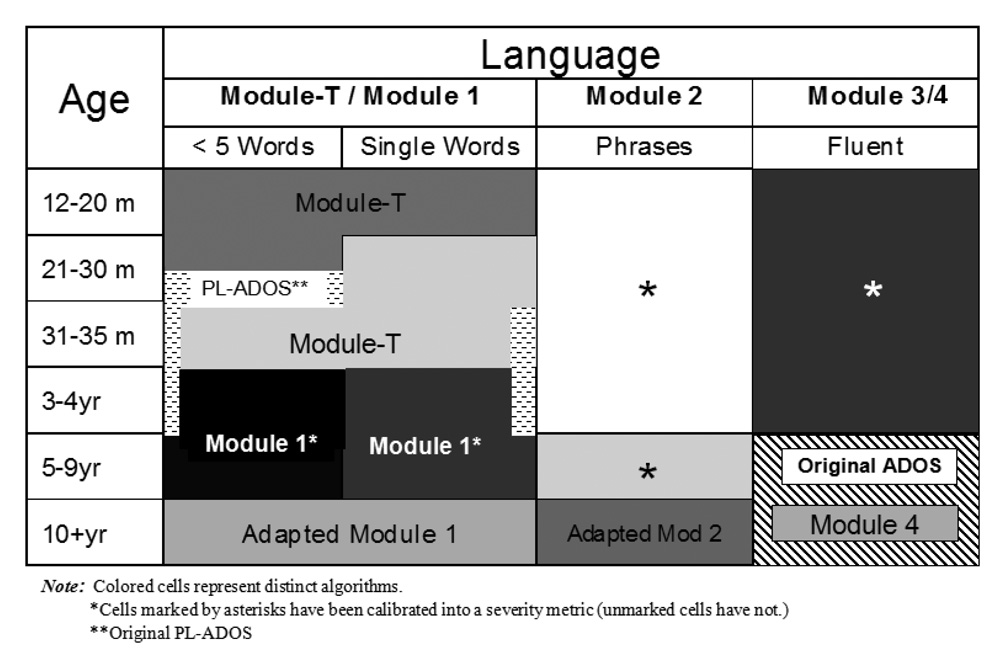
With the modular approach, it was then possible to revise diagnostic algorithms to be even more specific to different combinations of language level and age (Gotham et al., 2008). As shown in Figure 1, there are now 5 different modules, including a new Toddler Module (Luyster et al, 2008), each of which has 1 – 3 algorithms. A 7 year-old who speaks in simple phrases is given Module 2 and scored using a different algorithm than a 3 year-old with the same language level because research showed that for children 5 and older, different behaviors discriminate children who have limited language with and without autism than younger children. These additional algorithms increased the specificity with which the ADOS characterizes children with PDD-NOS or ASD (Gotham et al., 2008).
Figure 1.
ADOS Modules and Forms by Language and Age Groups
Third, from these new algorithms, calibrated severity scores were developed that allow a child’s performance on the ADOS to be ranked as compared to a large sample of children with ASD of similar age and expressive language level (Gotham, Pickles, & Lord, 2009). Thus, a child receives a score from 1 (no evidence of ASD) to 10 (severe ASD symptoms observed during the ADOS), which recognizes that ASD is truly a quantifiable dimension (Constantino & Todd, 2000) or, in all likelihood, dimensions (APA, 2010). A child’s performance on the ADOS can thus be compared across time, even if the child changes modules. In addition, trajectories of change in social-communication behaviors described in the ADOS, which may have important clinical implications for prognosis and response to treatment, can begin to be mapped across development.
Immediate Plans
A number of current projects are underway, in response to both clinical and research needs. A research version of a Toddler ADOS Module, intended for toddlers who are between about 12 – 30 months of age and are walking is now complete (Luyster et al, 2009) and will be available clinically soon. Adapted versions of Module 1 and 2 specifically intended for adolescents and adults with limited language are being pilot-tested. Algorithms for toddlers and children under age 4 on the ADI-R are now in preparation. Autism Speaks has been active in promoting translations and training for the instruments in countries around the world. While validity studies have been conducted in several western European countries and South America (Papanikolaou et al., 2009; Vrancic et al., 2002), there is still much to be learned about cultural differences in using different diagnostic methods. Most exciting is that Wakschlag and colleagues (Wakschlag et al, 2008) have developed an instrument, the Disruptive Behavior Disorder Observation Scale (DB-DOS), whose origins began with the ADOS, to use observational methods to diagnose disruptive behaviors in preschool children. The ADOS now has a cousin!
Developing a briefer, easier to administer face-to-face and/or telephone interview, based on more modular versions of the ADI-R (following the concept of age and language modules from the ADOS) is underway with the research goal of providing more rapid screening for neurobiological studies. Such measures could also be used in clinic visits to increase efficiency, while still yielding the advantages of a dedicated time for a caregiver interview as part of a standard clinical assessment. The development of an instrument, based on the tenets of the ADOS, but focused on yielding a more extensive sample of spontaneous expressive language to use in programming was funded by the National Institute of Deafness and Other Communication Disorders (NIDCD) and is now being normed. More information is needed from epidemiological samples to test and, if necessary, correct the calibrations proposed for the ADOS. The many large, publicly accessible datasets (e.g., the Simons Simplex Collection, www.sfari.org) offer new opportunities.
The interchange between clinical experience and knowledge continues to challenge the conceptualizations of ASD and autism, and to demand that clinicians and researchers concerned about families and children and adults with autism keep learning. The opportunity to work within a clinical setting and to see children and adults referred for ASD, both new families and especially those seen over time, never fails to be inspiring, and serves as a reminder of how much more there is to a person than any assessment can measure. The movement back and forth between clinical practice and research continues, with input from clinical researchers, both past and present, and clinicians at the University of Michigan Autism and Communication Disorders Center (UMACC) as well as collaborators in North Carolina, Chicago and the UK. The joy of discovery and the hope of being useful as a clinical psychologist and a clinical researcher are dependent on the opportunity to work with dedicated and skilled colleagues, students and families and children and adults with ASD.
ACKNOWLEDGEMENTS
Thanks are extended to Kathy Hatfield, Marisela Huerta, Kathryn Larson, Chad Tiernan, and Mary Yonkovit for help in preparation of this manuscript, to NIMH (R01 MH081757, R01 MH081873) for support during its preparation, and to all the UMACC staff for their contributions to all of these efforts. Dr. Lord receives royalties for the ADOS and ADI-R from Western Psychological Services. She donates all royalties related to clinical efforts and research projects at UMACC to Have Dreams, a not for profit agency that serves children and adults with ASD.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/AMP
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Frith U. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Abrahams B, Geschwind D. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9:341. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd. Washington, DC: Author; 1980. [Google Scholar]
- American Psychiatric Association Diagnostic and statistical manual of mental disorders 19944thWashington, DC: Author [Google Scholar]
- American Psychiatric Association. American Psychological Association statement: Policy statement on evidence-based practice in psychology. 2005 Retrieved from http://www.apa.org.proxy.lib.umich.edu/practice/guidelines/evidence-based.pdf.
- American Psychiatric Association. Proposed draft revisions to DSM disorders and criteria. 2010 Retrieved from http://www.dsm5.org.
- Asperger H. Die “autistichen Psychopathen” im Kindersalter. Archive für psychiatrie und Nervenkrankheiten. 1944;117:76–136. [Google Scholar]
- Bartak L, Rutter M, Cox A. A comparative study of infantile autism and specific developmental receptive language disorder: I. The children. British Journal of Psychiatry. 1975;126:127–145. doi: 10.1192/bjp.126.2.127. [DOI] [PubMed] [Google Scholar]
- Bishop S, Gahagan S, Lord C. Re-examining the core features of autism: A comparison of autism spectrum disorder and fetal alcohol spectrum disorder. Journal of Child Psychology & Psychiatry. 2007;48:1111–1121. doi: 10.1111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Lord C. Restricted and repetitive behaviors and non-verbal IQ in children with autism spectrum disorders. Journal of Child Neuropsychology. 2006;12:247–267. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective, and other psychiatric disorders: Patterns of familial aggregation. Psychological Medicine. 1998;28:385–395. doi: 10.1017/s0033291797006004. [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ. Diagnostic rules for children with PDD-NOS and multiple complex developmental disorder. Journal of Child Psychology & Psychiatry. 1998;39:911–919. [PubMed] [Google Scholar]
- Cohen DJ, Caparulo B, Shaywitz B. Primary childhood aphasia and childhood autism: Clinical, biological, and conceptual observations. Journal of the American Academy of Child Psychiatry. 1976;15(4):604–645. doi: 10.1097/00004583-197601540-00002. [DOI] [PubMed] [Google Scholar]
- Constantino J, Todd R. Genetic structure of reciprocal social behavior. The American Journal of Psychiatry. 2000;157:2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M. The Pre-Linguistic Autism Diagnostic Observation Schedule. Journal of Autism & Developmental Disorders. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Rutter ML. Infantile autism: A genetic study of 21 twin pairs. Journal of Child Psychology and Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The changing epidemiology of autism. Journal of Applied Research in Intellectual Disabilities. 2005;18:281–294. [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism & Developmental Disorders. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, Lord C. A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(6):642–651. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Happé FGE. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Development. 1995;66:843–855. [PubMed] [Google Scholar]
- Hermelin B, O’Connor N. Remembering of words by psychotic and subnormal children. British Journal of Psychology. 1967;58:213–218. doi: 10.1111/j.2044-8295.1967.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Kamp-Becker I, Ghahreman M, Smidt J, Remschmidt H. Dimensional structure of the autism phenotype: Relations between early development and current presentation. Journal of Autism and Developmental Disorders. 2008;39:557–571. doi: 10.1007/s10803-008-0656-5. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. The Nervous Child. 1943;2:217–250. [Google Scholar]
- Lam KSL, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behaviors in autism that differ in familiarity and association with other symptoms. Journal of Child Psychology & Psychiatry. 2008;49:1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Piven J, Wzorek MM, Gayle JO, Cloud D, Chase G, Folstein S. Social language use in parents of autistic individuals. Psychological Medicine. 1992;22:245–254. doi: 10.1017/s0033291700032918. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan JD. Autism Diagnostic Interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lewis V, Boucher J. Spontaneous, instructed and elicited play in relatively able autistic children. British Journal of Developmental Psychology. 1988;6(4):325–339. [Google Scholar]
- Lockyer L, Rutter M. A five- to fifteen-year follow-up study of infantile psychosis: Psychological aspects. British Journal of Psychiatry. 1969;115:865–882. doi: 10.1192/bjp.115.525.865. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore P, Shulman C, Thurm A, Pickles A. Autism from two to nine years of age. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism & Developmental Disorders. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, California: Western Psychological Services; 2000. [Google Scholar]
- Lord C, Shulman C, DiLavore P. Regression and word loss in autistic spectrum disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2004;45:936–955. doi: 10.1111/j.1469-7610.2004.t01-1-00287.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Spence S. Autism spectrum disorder: Phenotype and diagnosis. In: Moldin SO, Rubenstein JL, editors. Understanding autism: From basic neuroscience to treatment. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2006. pp. 1–23. [Google Scholar]
- Lovaas O. Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology. 1987;55(1):3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- Loveland KA, McEvoy RE, Kelley ML, Tunali B. Narrative story telling in autism and Down's syndrome. British Journal of Developmental Psychology. 1990;8:9–23. [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, DiLavore P, Pierce K, Lord C. The Autism Diagnostic Observation Schedule - Toddler Module: A new module of a standardized diagnostic measure for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(9):1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in young children with autism. Development and Psychopathology. 1994;6:389–401. [Google Scholar]
- Murray HA. Explorations in personality. New York: Oxford; 1938. [Google Scholar]
- Ornitz EM. Childhood autism: A disorder of sensorimotor integration. In: Rutter M, editor. Infantile autism: Concepts, characteristics, and treatment. Edinburgh & London: Churchill Livingstone; 1971. pp. 50–68. [Google Scholar]
- Papanikolaou K, Paliokosta E, Houliaras G, Vgenopoulou S, Giouroukou E, Pehlivanidis A, Tsiantis I. Using the Autism Diagnostic Interview-Revised and the Diagnostic Observation Schedule-Generic for the diagnosis of autism spectrum disorders in a Greek sample with a wide range of intellectual abilities. Journal of Autism and Developmental Disorders. 2009;39:414–420. doi: 10.1007/s10803-008-0639-6. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: Evidence from a family history of multiple-incidence autism families. The American Journal of Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Rapin I, Allen DA. Developmental language disorders: Nosologic considerations. In: Kirk U, editor. Neuropsychology of language, reading, and spelling. New York: Academic Press; 1983. pp. 155–184. [Google Scholar]
- Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Developmental Psychopathology. 2010;22:55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimland B. The differentiation of childhood psychoses: An analysis of checklists for 2,218 psychotic children. Journal of Autism and Childhood Schizophrenia. 1971;1:161–174. doi: 10.1007/BF01537955. [DOI] [PubMed] [Google Scholar]
- Ritvo E, Freeman BJ. Current research on the syndrome of autism. Journal of Autism and Childhood Schizophrenia. 1978;8:162–167. doi: 10.1007/BF01538044. [DOI] [PubMed] [Google Scholar]
- Rutter M. Diagnosis and definition of childhood autism. Journal of Autism and Childhood Schizophrenia. 1978;8:139–161. doi: 10.1007/BF01537863. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview–Revised (ADI–R) manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Rutter M, Lockyer L. A five- to fifteen-year follow-up study of infantile psychosis: I. Description of sample. British Journal of Psychiatry. 1967;113:1169–1182. doi: 10.1192/bjp.113.504.1169. [DOI] [PubMed] [Google Scholar]
- Rutter M, Tizard J, Whitmore K. Education, Health and Behaviour. London: Longmans; 1970. Eds. [Google Scholar]
- Schieve LA, Blumberg SJ, Rice C, Visser SN, Boyle C. The relationship between autism and parenting stress. Pediatrics. 2007;119:S114–S121. doi: 10.1542/peds.2006-2089Q. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler R. Psychoeducational Profile: Individualized assessment and treatment for autistic and developmentally delayed children. Baltimore: University Park Press; 1979. [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood autism rating scale (CARS) Journal of Autism and Developmental Disorders. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Snow AV, Lecavalier L, Houts C. The structure of the Autism Diagnostic Interview-Revised: Diagnostic and phenotypic implications. Journal of Child Psychology and Psychiatry. 2009;50:734–742. doi: 10.1111/j.1469-7610.2008.02018.x. [DOI] [PubMed] [Google Scholar]
- Tadevosyan-Leyfer O, Dowd M, Mankoski R, Winklosky B, Putnam S, McGrath L, Folstein SE. A principal components analysis of the Autism Diagnostic Interview-Revised. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(7):864–872. doi: 10.1097/01.CHI.0000046870.56865.90. [DOI] [PubMed] [Google Scholar]
- Turner M. Annotation: Repetitive behavior in autism: A review of psychological research. Journal of Child Psychology & Psychiatry. 1999;40:839–849. [PubMed] [Google Scholar]
- Venter A, Lord C, Schopler E. A follow-up study of high-functioning autistic children. Journal of Child Psychology & Psychiatry. 1992;33:489–507. doi: 10.1111/j.1469-7610.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American Journal of Medical Genetics: B Neuropsychiatric Genetics. 2008;150B:328–334. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Klin A. Diagnostic issues in Asperger syndrome. In: Klin A, Volkmar FK, Sparrow SS, editors. Asperger syndrome. New York: The Guildford Press; 2000. pp. 25–71. [Google Scholar]
- Vrancic D, Nanclares V, Soares D, Kulesz A, Mordzinski C, Plebst C, Starkstein S. Sensitivity and specificity of the Autism Diagnostic Inventory- Telephone Screening in Spanish. Journal of Autism and Developmental Disorders. 2002;32(4):313–320. doi: 10.1023/a:1016335003256. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Hill C, Danis B, Leventhal BL, Keenan K, Carter AS. Observational assessment of preschool disruptive behavior, Part II: Validity of the Disruptive Behavior Diagnostic Observation Schedule (DB-DOS) Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(6):632–641. doi: 10.1097/CHI.0b013e31816c5c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of Autism and Developmental Disorders. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Wolff S, Barlow A. Schizoid personality in childhood: A comparative study of schizoid, autistic and normal children. Journal of Child Psychology and Psychiatry. 1979;20:29–46. doi: 10.1111/j.1469-7610.1979.tb01704.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization International classification of diseases and related health problems 199210thGeneva, Switzerland: Author [Google Scholar]