Abstract
Golgins are coiled-coil proteins involved in Golgi architecture and function. A complex of golgins (p115, GM130 and giantin), together with the rab1 GTPase and cis-Golgi SNAREs, helps to mediate fusion processes at the entry face of the Golgi apparatus. The C-terminal acidic domain of p115 binds specifically to GM130 and giantin. However, deletion of this domain in vivo appears to have no effect on exocytic transport when using an RNAi depletion/rescue approach (Puthenveedu MA, Linstedt AD. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proceedings of the National Academy of Sciences of the United States of America 2004;101(5):1253–1256). Here we have used a different approach, introducing a tobacco etch virus (tev) protease cleavage site into p115 so that the C-terminal domain can be rapidly and specifically released in vivo by micro-injection of the tev protease. The results show that cleavage inhibits exocytic transport to the cell surface.
Keywords: Golgi, golgin, tether, COPI vesicles, tobacco etch virus (tev) protease
Introduction
The Golgi receives the entire output of newly-synthesized cargo proteins from the ER, modifies any bound oligosaccharides, and then distributes them to their final destinations (1–4). Transport through the Golgi is mediated by COPI vesicles that bud from one cisternal membrane carrying Golgi enzymes and/or cargo, and fuse with the next cisterna on the pathway in either the anterograde or retrograde direction (5, 6).
Fusion is a multi-layered process involving tethers, small GTPases (rabs, ARFs and ARLs) and SNAREs (7–10). Tethers are thought to mediate the initial contact between COPI vesicles and the recipient cisternal membrane. They include multi-protein complexes and long coiled-coil proteins (11–15). Vesicle tethering is followed by docking and fusion, a process mediated by SNAREs and accessory proteins (1, 16–18).
Tethers for intra-Golgi transport include those coiled-coil proteins that belong to the golgin family (13, 19, 20). The best characterized set are those that are thought to operate at the entry face of the Golgi and include p115, GM130 and giantin (12, 21, 22). These form a large complex together with the rab1 GTPase (which binds to each of these golgins) and cis-Golgi SNAREs (22–26).
The precise sequence of events is still unclear but one plausible model is the following: GM130 is present exclusively on cis-Golgi membranes, bound to the rab1 GTPase and the GRASP matrix protein (through a C-terminal PDZ-like domain) (24, 25, 27, 28). GM130 is also bound to the Golgi t-SNARE, syntaxin5 (26). The golgin tether p115 also binds to cis-Golgi membranes through binding to the rab1 GTPase and this reveals a cryptic binding site at the C-terminus for the N-terminus of GM130 (Figure 1) (22, 29, 30). The C-terminal domain of p115 can also bind to giantin, a membrane-anchored golgin that (unlike GM130) is also found on COPI vesicles (31, 32). This suggests that p115 (bound to rab1 and GM130) could act to capture COPI vesicles, bringing them to cis-Golgi membranes. It could also capture other membranes that contain giantin, such as the ER to Golgi carrier vesicles that arrive at the cis-Golgi. The binding of p115 to GM130 also releases syntaxin5 which could then interact with the v-SNAREs on the COPI vesicles, a process catalyzed by p115 (26, 33). This assembly of cis-Golgi SNAREs would eventually lead to membrane fusion (1, 34).
Figure 1. Schematic of p115 modified with the tev protease recognition site.
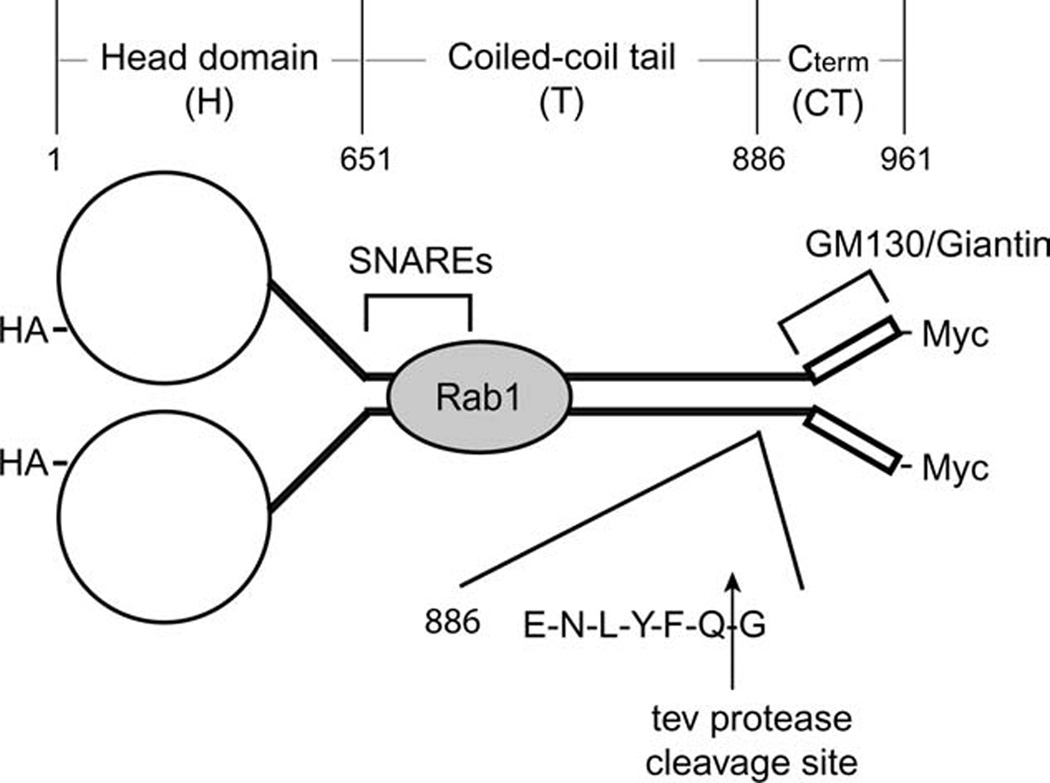
Wild-type p115 comprises an N-terminal globular head domain (H), followed by a putative coiled-coil tail (T), ending in a C-terminal (CT) acidic domain. Binding partners and the mapped binding sites on p115 are indicated. The tev protease recognition site (ENLYFQG) was inserted between the tail and CT after position 886. For detection purposes, the constructs had either an N-terminal HA- and/or a C-terminal Myc-tag.
This scenario is one of several possible models that fit the existing data. However, they all point to the importance of p115 in catalyzing the assembly of cis-Golgi SNARE complexes and the interaction of the C-terminal acidic domain with GM130 and giantin. The importance of the interaction between GM130 and p115 is further emphasized by the fact that mitotic phosphorylation of GM130 at a single serine in the N-terminal domain is sufficient to prevent binding to the C-terminal domain of p115 (29, 35). This should prevent the capture and therefore fusion (but not budding) of COPI vesicles, helping to explain the dramatic vesiculation of the Golgi that occurs when animal cells enter mitosis (12, 36, 37).
The essential role of p115 in exocytic transport has been demonstrated using a number of techniques (38–42). The most recent used RNAi to assess the importance of p115 binding to SNAREs and to the golgins, GM130 and giantin (43). Depletion of p115 led to an almost complete inhibition of transport of the VSV-G protein. Transport was restored by expression of wild-type p115 (encoded in an RNAi-resistant manner) but not with a mutant p115 lacking the SNARE-binding site. This confirmed the importance of p115 in SNARE-mediated fusion in vivo. Interestingly, transport was also rescued using a mutant p115 lacking the C-terminal domain. This suggested that the interaction of p115 with GM130 and giantin was not important for transport, despite the wealth of data characterizing this interaction.
One explanation was to suggest that the interaction amongst the golgins was important for fidelity and efficiency, rather than obligatory (22). Another, however, is that the methodology used might somehow suppress the importance of this interaction. We therefore decided to take a completely different in vivo approach, exploiting the properties of a highly specific protease to cleave the C-terminal domain from p115 in vivo. The results show that the C-terminal domain of p115 is important for exocytic transport.
Results and Discussion
Introduction of a tev protease cleavage site into p115
p115 is a parallel homo-dimer comprising a globular head domain (H) followed by a coiled-coil tail (T), ending with an acidic C-terminus that binds to GM130 and giantin (Figure 1).
In order to study the role played by the C-terminal domain (CT), a protease cleavage site was introduced between this domain and the coiled-coil tail. The cleavage site for the tobacco etch virus (tev) protease was chosen since it is highly specific for a hepta-peptide sequence (Figure 1) that is not found in several databases including, most importantly for the present paper, the human protein database. Tev protease has been used in other fields of research to selectively cleave individual proteins (with the introduced heptapeptide) within the normal cellular context (44–46).
In vitro cleavage of modified p115
By introducing the tev cleavage site it should be possible to express tev-modified p115 (p115(tev)) in HeLa cells and cleave it using micro-injected tev protease. As a control, it was shown that micro-injection of the tev protease into HeLa cells alone had no discernible effect on viability or cell proliferation (data not shown).
To show that the introduction of the tev protease cleavage site allowed removal of the CT, wild-type bovine p115 (p115) and p115(tev) were stably expressed in HeLa cells. To permit detection, these constructs were tagged with HA at the N-terminus and Myc at the C-terminus. Cells were lysed in detergent, immuno-precipitated using anti-HA-agarose, treated (or not) with tev protease, then fractionated by SDS-PAGE followed by Western blotting using anti-HA. As shown in Figure 2A, the p115 was not affected by treatment with the tev protease. The p115(tev), however, was cleaved almost completely, decreasing in molecular weight from 115kDa to ~100kDa. This cleaved protein co-migrated with HT (a p115 construct comprising the head domain and coiled-coil tail but not the CT, and tagged with HA at the N-terminus), suggesting that the CT of p115(tev) had been removed by the tev protease.
Figure 2. Cleavage of modified p115 in vitro.
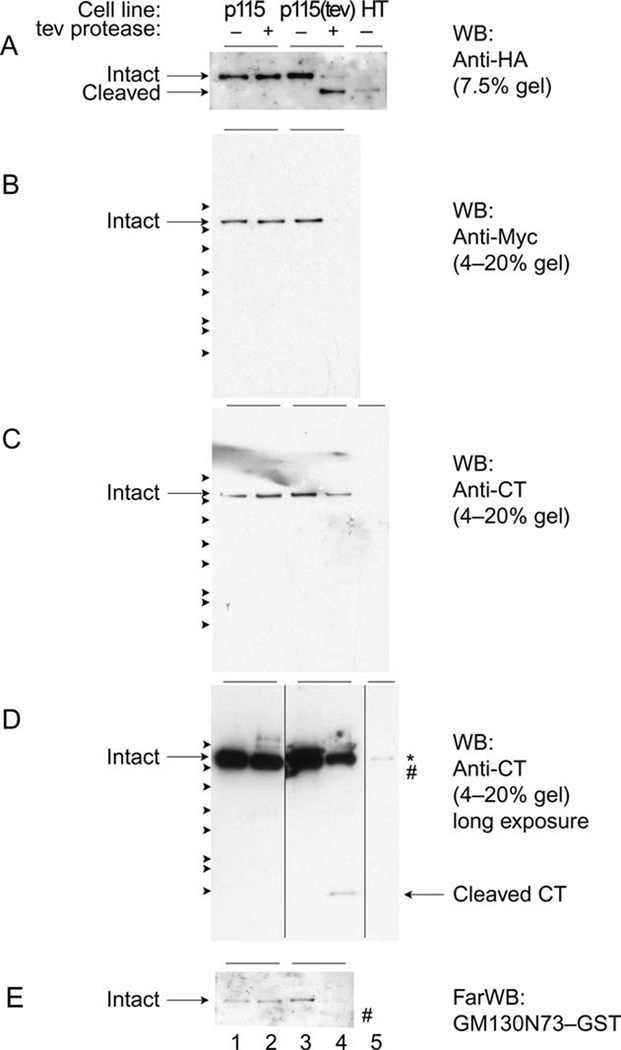
Wild-type p115 (p115) and tev-modified p115 (p115(tev)), tagged at both ends (HA at the N-terminus, Myc at the C-terminus), were stably expressed in HeLa cells. An N-terminal fragment (HT, 1–886; HA-tagged at the N-terminus) was also stably expressed. Tagged proteins were immuno-precipitated from cell lysates using anti-HA-agarose, treated with (+) or without (−) tev protease, and fractionated by SDS-PAGE followed by Western blotting (WB) with anti-HA (A; detection of N-termini), with anti-Myc (B; detection of C-termini) or with anti-CT antibodies (C and D (longer exposure); detection of cleaved CT fragment (arrow in D) and endogenous p115 (asterisk in D)).
E. Fractionated samples were also probed by Far Western blotting using the N-terminal 73 amino acids of GM130 fused to GST (GM130N73-GST). Arrowheads in panels B, C and D indicate protein standards (kDa, from top): 150, 100, 75, 50, 37.5, 25, 20 and 10kDa. # indicates the position of HT.
Cleavage of the C-terminus was confirmed by Western blotting using anti-Myc antibodies. As shown in Figure 2B, the C-terminal Myc-tag was detected on p115 with or without treatment with tev protease. However, the Myc-tag on p115(tev) was not detected after protease treatment suggesting very efficient cleavage of p115(tev).
The cleaved CT fragment (~10kDa) could not be detected using the anti-Myc antibodies so blots of the same samples were probed with an antibody raised to CT. As shown in Figure 2D (arrow), a ~10kDa protein was detected (after long exposure) but only after treatment of p115(tev) with the tev protease, strongly suggesting that this is the C-terminal cleaved fragment.
The specificity of the antibody for CT was confirmed using the HT immuno-precipitates probed with the anti-CT antibodies (Figure 2C,D). HT was not detected, even after a long exposure (Figure 2D, lane 5, #), only endogenous p115 (Figure 2D, lane 5, asterisk). The latter was likely present because it had formed a heterodimeric complex with HA-tagged HT during biosynthesis. Comparison with the stably expressed p115 (wild-type and tev-modified) suggests that these are present at levels 10–20 times higher than the endogenous protein. This does, however, assume that the formation of the HT-p115 hetero-dimer is as efficient as the p115 homo-dimer. It also assumes that the antibody raised to the bovine p115 recognizes the human CT as efficiently. This seems likely, since they are 84.2% identical in sequence. However, further experiments will be needed to show this.
The functionality of p115(tev) and the cleaved product was tested using the N-terminal 73 amino acid fragment of GM130 (fused to GST; GM130N73-GST), which has been shown to bind to the CT of p115 using a variety of methods including Far Western blotting (29). Blots probed with GM130N73-GST showed binding to wild-type p115 and p115(tev). The latter bound GM130N73-GST as well as wild-type p115, suggesting that the introduction of the tev cleavage site did not affect binding efficiency (Figure 2E, cf. lanes 1 and 3). However, after treatment of p115(tev) with tev protease, GM130N73-GST was no longer able to bind, showing that cleavage leads to a non-functional p115, at least with regards to GM130 binding.
In vivo cleavage of modified p115
To show that cleavage of p115(tev) could also occur in vivo, HeLa cells stably expressing wild-type p115 or p115(tev) (both tagged with HA at the N-terminus, Myc at the C-terminus) were micro-injected with tev protease. Two hours after protease injection, cells were fixed, permeabilized and processed for immunofluorescence microscopy. Cells were double-labeled for Myc (at the C-terminus of p115) and either the Golgi marker giantin or the coiled-coil tail of p115 (anti-p115T which recognizes HT, not CT and gave more consistent results than anti-HA).
As shown in Figure 3 (top panels), in cells expressing wild-type p115, HT and CT co-localized in the Golgi region before or after protease injection. However, in cells expressing p115(tev), the labeling for HT disappeared in injected cells whereas that for CT remained in the Golgi region, as shown by double-labeling with the Golgi marker giantin (Figure 3, bottom panels). These results suggest that cleavage of p115(tev) into two parts (HT and CT) leads to retention of the CT in the Golgi region and dispersal of the N-terminal HT part into the cytoplasm. This was unexpected both because of the small size of CT (~10kDa) and the fact that earlier data suggested that HT localizes to the Golgi region (30). However, it seems that this depends on whether HT is stably or transiently expressed in cells. When transiently expressed, HT bound to the Golgi region (and ER exit sites) in agreement with earlier studies. When stably expressed, HT was present as diffuse cytoplasmic staining (Suppl. Figure 1). What remains unclear is why stable versus transient expression should yield such a difference though it does help explain why, after tev injection, no HT staining of the Golgi region was observed.
Figure 3. Cleavage of modified p115 in vivo.
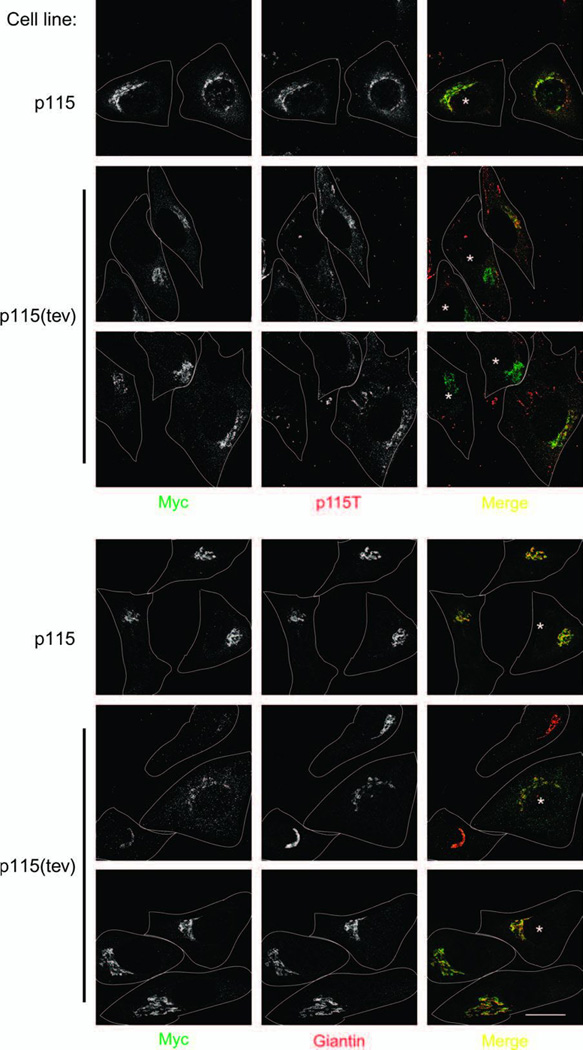
HeLa cells stably expressing p115 or p115(tev), tagged at both ends (HA at N-terminus, Myc at the C-terminus), were micro-injected with tev protease (asterisks in right panels). After 2 h at 37°C, cells were fixed, permeabilized and labeled with (left panels) anti-Myc antibodies and either (top, middle panels) anti-p115T (which recognizes the coiled-coil tail, not the CT domain of p115), or (bottom, middle panels) the Golgi marker, giantin. The merged images are on the right (Myc: green, other markers: red). Cell boundaries outlined in white. Bar, 20 µm.
In vivo cleavage of modified p115 inhibits cargo transport
The ability to cleave p115(tev) in vivo allowed a test of its role in exocytic transport. CD8 was chosen as a well-characterized exocytic marker and the cDNA encoding this plasma membrane protein was micro-injected into HeLa cells with or without tev protease and in the presence of cycloheximide. After one hour at 37°C, to allow accumulation of CD8 mRNA, the cycloheximide was removed to allow protein synthesis and transport of the newly-synthesized CD8 to the cell surface. Cells were then fixed, permeabilized and labeled for total CD8.
As shown in Figure 4A, in HeLa cells transiently expressing wild-type p115, there was no effect on the pattern of staining in cells injected with the tev protease. CD8 was clearly seen in the Golgi region and on the cell surface. However, in cells transiently expressing p115(tev), injection of tev protease had a dramatic effect on the staining pattern. CD8 accumulated in the nuclear envelope and associated peripheral ER, suggestive of an inhibition of transport in the early part of the exocytic pathway (43, Alvarez, 1999 #32).
Figure 4. Cleavage of modified p115 in vivo inhibits cargo transport.
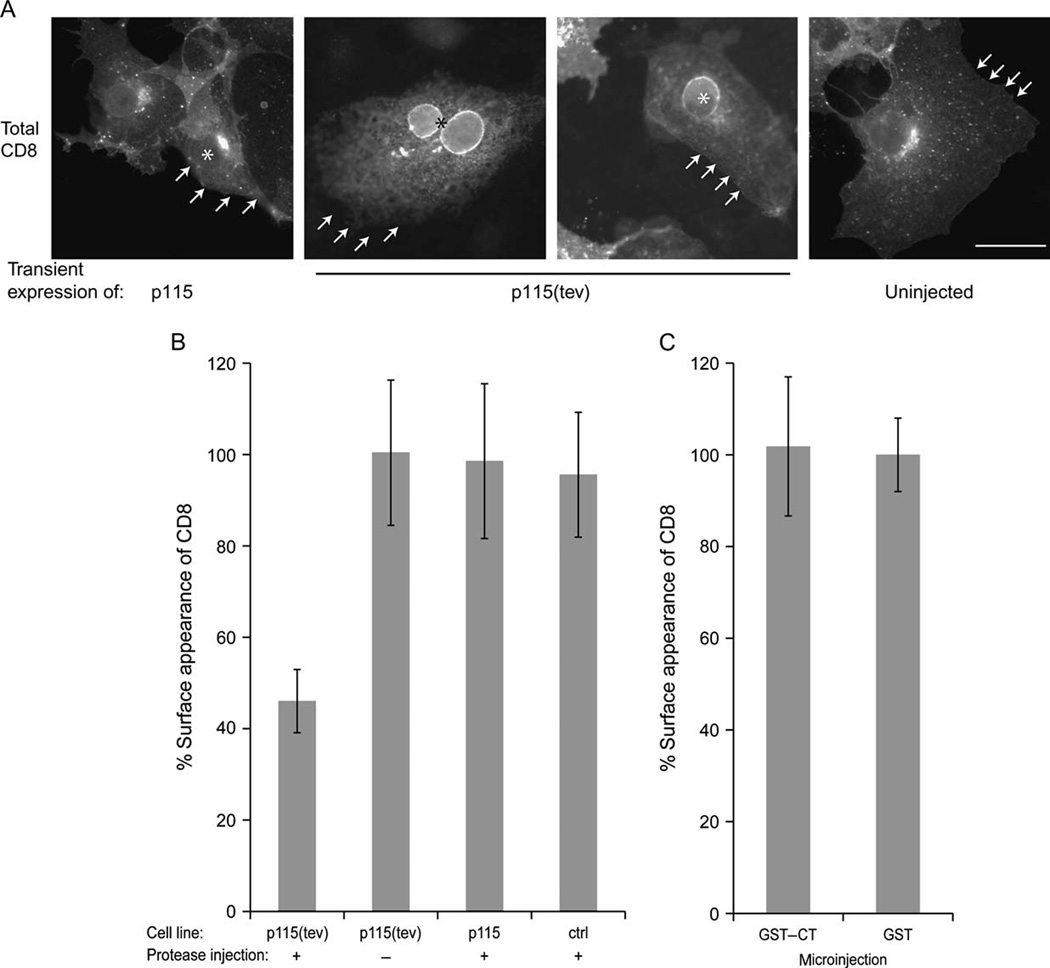
(A) HeLa cells transiently expressing p115 or p115(tev) were micro-injected (asterisks), in the presence of cycloheximide, with the cDNA encoding a cell-surface marker, CD8, together with the tev protease. After 1 h at 37 °C to accumulate CD8 mRNA, the cycloheximide was removed, and cells incubated for 2.5 h at 37 °C with a 45-min cycloheximide chase at the end of the incubation. Cells were fixed, permeabilized and labeled with anti-CD8 polyclonal antibodies to visualize total CD8. Arrows mark the cell periphery. Bar, 20 µm.
(B) HeLa cells stably expressing p115 or p115(tev), or transfected with the vector alone (ctrl), were micro-injected as in (A). Cells were fixed and labeled for surface and total CD8, the ratio being determined for each cell. The results were normalized to the mean value of p115(tev) without protease, set to 100%, and expressed as the mean ± SD (n=15~20 cells).
C. HeLa cells were micro-injected with cDNA encoding CD8 and either GST or GST-CT (~3 mg/ml) and processed as in B. The results were normalized to the mean value of GST, set to 100%, and expressed as the mean± SD (n=15~20 cells).
In order to quantitate this inhibition, stable HeLa cell lines were generated, expressing wild-type p115 or p115(tev), or transfected with vector alone. After micro-injection of the cDNA encoding CD8, with or without the tev protease, the surface and total CD8 were measured. As shown in Figure 4B, the percentage surface expression of CD8 was not affected by injection of p115-expressing cells with tev protease. However, micro-injection of cells stably expressing p115(tev) had less than 50% of the cell surface staining for CD8, compared with cells that had not been injected with the tev protease. In vivo cleavage of p115(tev) should generate both HT and CT. Since HT does not inhibit transport (43) but CT is retained at the Golgi, this suggests that CT might be a dominant negative inhibitor of exocytic transport. This was tested by microinjection of GST or GST-CT, together with cDNA encoding CD8, to permit measurement of exocytic transport. As shown in Figure 4C, CT had no significant effect on the cell surface appearance of CD8. This despite the fact that the calculated concentration of CT in the cytoplasm (~0.3 mg/ml) would be 5–10 times that of the endogenous p115 protein (47). This in turn raises the interesting possibility that CT has to be generated in situ (bound to GM130/giantin) in order to exert its inhibitory effects.
It is clear that these results are in contrast to those by Linstedt and colleagues who could find no effect on transport when p115 was depleted then rescued with p115 lacking the C-terminal domain (43). One reason might be, as suggested by us earlier, that the C-terminal domain is regulatory rather than obligatory (22). This could explain why there is still 50% transport after cleavage though we cannot yet eliminate the possibility that this might instead reflect residual p115 (endogenous and/or the p115(tev)). Another reason might be the method used to study the role of the acidic domain. RNAi is a powerful technique but has limitations as does any other. Even the same RNAi method applied to GM130 depletion has given contradictory results (26, 48, 49). This makes it even more important to use different methods to look at the same problem. The use of tev protease described here emphasizes the value of using a protease to rapidly and selectively cleave one protein species in vivo and determine the consequences. The data show clearly that the C-terminal domain of p115 is needed for efficient exocytic transport strongly suggesting that the interaction with GM130 and giantin is important. The protease cleavage approach should be a useful complement to other perturbation methods used in the membrane traffic field.
Materials and Methods
Antibodies
Polyclonal antibodies against the C-terminal domain of bovine p115 (anti- CT) were raised using the C-terminal 76 residues fused to GST (GST-CT) as an antigen. Polyclonal antibodies to the coiled-coil tail and C-terminal domain (residues 636–962 of human p115) were a gift from Dennis Shields (Albert Einstein College of Medicine of Yeshiva University). They were passed through a column of immobilized GST-CT to generate anti-p115T antibodies that recognize the coiled-coil tail (T) but not the CT of expressed p115 constructs.
Other antibodies were: anti-Sec31 (Fred Gorelick (Yale University)), monoclonal anti-HA (16B12, BAbCO, Richmond, CA), monoclonal anti-Myc (9E10, Abcam, Cambridge, MA), polyclonal anti-Myc (A-14, Santa Cruz Biotechnology, Santa Cruz, CA), anti-p115 (clone 46, BD Biosciences, San Jose, CA), polyclonal anti-giantin (50), monoclonal anti-CD8 (eBioscience, San Diego, CA), polyclonal anti-CD8 (C-19, Santa Cruz Biotechnology), monoclonal anti-gamma tubulin (GTU-88, Sigma, St. Louis, MO), Alexa-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) and HRP-conjugated secondary antibodies (Pierce, Rockford, IL or Sigma).
Plasmids and recombinant proteins
Bovine p115 cDNA (22) was tagged with HA (YPYDVPDYA) and Myc (EQKLISEEDL) by PCR and subcloned into pQCXIN (for retrovirus production, Clontech, Mountain View, CA) or pcDNA3.1 (for transient expression, Invitrogen). The tev protease cleavage site (ENLYFQG) was introduced by nested PCR. C-terminal truncation of p115 was generated by site-directed mutagenesis. The N-terminal 73 amino acid residues of GM130 fused to GST (GM130 N73-GST) and GST-CT were purified as described (22, 51). For the transport assay, the human CD8 mammalian expression plasmid was used (50, 52).
Expression of tagged p115 in HeLa cells
To generate tagged p115 stable cell lines, GP2–293 cells were co-transfected with the pQCXIN vector, along with a vector expressing VSV-G protein, using Lipofectamin 2000 (Invitrogen) (53). After 36 h, medium containing recombinant retrovirus was collected and used to infect HeLa cells. Twenty-four hrs post infection, stable lines were selected with DMEM supplemented with 10% FBS and 0.8 g/l G418 (Invitrogen). For transient expression, the tagged p115 cDNA in pcDNA3.1 was transfected using FuGENE 6 (Roche, Basel, CH, Switzerland) according to the manufacturer's protocol.
Immunoprecipitation and Western blotting
Cell lysates were prepared with IP buffer (10 mM HEPES-KOH, pH 7.4, 100 mM KCl, 0.1 mM dithiothreitol (DTT), 2.5 mM MgCl2, 1% Triton X-100 and the protease inhibitor cocktail from Roche). After incubation for 10 min on ice, the extract was clarified by centrifugation at 135,000 g for 20 min. For immunoprecipitation, the supernatants (~100 µg protein) were incubated with anti-HA agarose beads (Sigma) for 30 min at 4°C. After washing three times with IP buffer, the beads were washed three times with tev buffer (50 mM Tris-HCl (pH 8.0), 0.5 mM EDTA, 1 mM DTT). Proteins on the beads were then incubated with/without tev protease (5 U, Invitrogen) for 1 h at 30°C. The samples were fractionated by SDS-PAGE followed by Western blotting with antibodies indicated in the figures or Far Western blotting using GM130N73-GST (29).
Micro-injection and immunofluorescence microscopy
For micro-injection and immunofluorescence, cells were cultured on 12 mm glass coverslips. Desalted tev protease (~4 U/µl) together with Alexa-conjugated dextran (1 µg/µl) as an injection marker was injected into cytoplasm. After the micro-injection, the cells were incubated for 2 h at 37°C. Cells on coverslips were fixed with 3.7% PFA in PBS for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. The cells were blocked with 4% BSA in PBS for 15 min, and then incubated for 15 min with primary antibodies diluted in 4% BSA in PBS. The cells were then washed three times with PBS, and incubated for 15 min with secondary antibodies conjugated to Alexa fluorophors (Invitrogen). After washing the cells, the coverslips were mounted on microscope slides and viewed using a Zeiss LSM510-Meta confocal or an Axioplan upright microscope (Carl Zeiss, Thornwood, NY).
Transport assay and quantification
The surface appearance of CD8 was monitored and quantified as previously described (50, 52) with minor modifications. After micro-injection of CD8 cDNA with/without tev protease or GST/GST-CT (~3 mg/ml), cells on coverslips were transferred to DMEM containing 100 µg/ml cycloheximide and incubated for 1 h at 37 °C to accumulate CD8 mRNA. Cells were then washed free of cycloheximide, and synthesis and transport of CD8 allowed to proceed for ~2.5 h with a 45-min cycloheximide chase at the end of the incubation, before being fixed and processed for immunofluorescence microscopy.
Supplementary Material
HeLa cells expressing HT (the N-terminal HA-tagged 1–886 fragment of p115) were fixed, permeabilized, and labeled with anti-HA (middle panels) and either anti-giantin or antibodies to the ER exit site marker, Sec31 (left panels). The merged images are shown in the right panels (HA is in green, other markers are in red). Bar, 20 µm. Note that transiently expressed HT co-localized partially with the Golgi region (arrowhead) and Sec31, whereas stably expressed HT did not.
Acknowledgments
We thank Ira Mellman, Susan Ferro-Novick, Fred Gorelick and the Warren/Mellman/Ferro-Novick group for discussion and support, and Fred Gorelick and Dennis Shields for generous provision of reagents, Sandra Maday and Thomas Gniadek for critical reading of the manuscript. This work was funded by the National Institutes of Health (AG030101 and GM060919) and the American Heart Association.
References
- 1.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116(2):153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 2.Barlowe C. Traffic COPs of the early secretory pathway. Traffic. 2000;1(5):371–377. doi: 10.1034/j.1600-0854.2000.010501.x. [DOI] [PubMed] [Google Scholar]
- 3.Watson P, Stephens DJ. ER-to-Golgi transport: form and formation of vesicular and tubular carriers. Biochim Biophys Acta. 2005;1744(3):304–315. doi: 10.1016/j.bbamcr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 5.Pelham HR, Rothman JE. The debate about transport in the Golgi--two sides of the same coin? Cell. 2000;102(6):713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 6.Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307(5712):1095–1098. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer SR. Unsolved mysteries in membrane traffic. Annu Rev Biochem. 2007;76:629–645. doi: 10.1146/annurev.biochem.76.061705.130002. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12(5):671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sztul E, Lupashin V. Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol. 2006;290(1):C11–C26. doi: 10.1152/ajpcell.00293.2005. [DOI] [PubMed] [Google Scholar]
- 11.Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115(Pt 13):2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 12.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 13.Gillingham AK, Munro S. Long coiled-coil proteins and membrane traffic. Biochim Biophys Acta. 2003;1641(2–3):71–85. doi: 10.1016/s0167-4889(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 14.Oka T, Krieger M. Multi-component protein complexes and Golgi membrane trafficking. J Biochem. 2005;137(2):109–114. doi: 10.1093/jb/mvi024. [DOI] [PubMed] [Google Scholar]
- 15.Ungar D, Oka T, Krieger M, Hughson FM. Retrograde transport on the COG railway. Trends Cell Biol. 2006;16(2):113–120. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Söllner TH. Regulated exocytosis and SNARE function (Review) Mol Membr Biol. 2003;20(3):209–220. doi: 10.1080/0968768031000104953. [DOI] [PubMed] [Google Scholar]
- 17.Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744(3):493–517. [PubMed] [Google Scholar]
- 18.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 19.Gleeson PA, Lock JG, Luke MR, Stow JL. Domains of the TGN: coats, tethers and G proteins. Traffic. 2004;5(5):315–326. doi: 10.1111/j.1398-9219.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 20.Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744(3):383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Linstedt AD, Jesch SA, Mehta A, Lee TH, Garcia-Mata R, Nelson DS, Sztul E. Binding relationships of membrane tethering components. The giantin N terminus and the GM130 N terminus compete for binding to the p115 C terminus. J Biol Chem. 2000;275(14):10196–10201. doi: 10.1074/jbc.275.14.10196. [DOI] [PubMed] [Google Scholar]
- 22.Beard M, Satoh A, Shorter J, Warren G. A cryptic Rab1-binding site in the p115 tethering protein. J Biol Chem. 2005;280(27):25840–25848. doi: 10.1074/jbc.M503925200. [DOI] [PubMed] [Google Scholar]
- 23.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289(5478):444–448. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 24.Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis--Golgi tethering. Traffic. 2001;2(4):268–276. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- 25.Weide T, Bayer M, Koster M, Siebrasse JP, Peters R, Barnekow A. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2001;2(4):336–341. doi: 10.1093/embo-reports/kve065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diao A, Frost L, Morohashi Y, Lowe M. Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. J Biol Chem. 2007 doi: 10.1074/jbc.M708401200. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131(6 Pt 2):1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr FA, Nakamura N, Warren G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. Embo J. 1998;17(12):3258–3268. doi: 10.1093/emboj/17.12.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89(3):445–455. doi: 10.1016/s0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 30.Nelson DS, Alvarez C, Gao YS, Garcia-Mata R, Fialkowski E, Sztul E. The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J Cell Biol. 1998;143(2):319–331. doi: 10.1083/jcb.143.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sönnichsen B, Lowe M, Levine T, Jamsa E, Dirac-Svejstrup B, Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140(5):1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesa GM, Seemann J, Shorter J, Vandekerckhove J, Warren G. The amino-terminal domain of the golgi protein giantin interacts directly with the vesicle-tethering protein p115. J Biol Chem. 2000;275(4):2831–2836. doi: 10.1074/jbc.275.4.2831. [DOI] [PubMed] [Google Scholar]
- 33.Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol. 2002;157(1):45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols BJ, Pelham HR. SNAREs and membrane fusion in the Golgi apparatus. Biochim Biophys Acta. 1998;1404(1–2):9–31. doi: 10.1016/s0167-4889(98)00044-5. [DOI] [PubMed] [Google Scholar]
- 35.Lowe M, Rabouille C, Nakamura N, Watson R, Jackman M, Jamsa E, Rahman D, Pappin DJ, Warren G. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94(6):783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 36.Lucocq JM, Warren G. Fragmentation and partitioning of the Golgi apparatus during mitosis in HeLa cells. Embo J. 1987;6(11):3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137(6):1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barroso M, Nelson DS, Sztul E. Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc Natl Acad Sci U S A. 1995;92(2):527–531. doi: 10.1073/pnas.92.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapperstein SK, Walter DM, Grosvenor AR, Heuser JE, Waters MG. p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc Natl Acad Sci U S A. 1995;92(2):522–526. doi: 10.1073/pnas.92.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez C, Fujita H, Hubbard A, Sztul E. ER to Golgi transport: Requirement for p115 at a pre-Golgi VTC stage. J Cell Biol. 1999;147(6):1205–1222. doi: 10.1083/jcb.147.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seemann J, Jokitalo EJ, Warren G. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol Biol Cell. 2000;11(2):635–645. doi: 10.1091/mbc.11.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthenveedu MA, Linstedt AD. Evidence that Golgi structure depends on a p115 activity that is independent of the vesicle tether components giantin and GM130. J Cell Biol. 2001;155(2):227–238. doi: 10.1083/jcb.200105005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puthenveedu MA, Linstedt AD. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc Natl Acad Sci U S A. 2004;101(5):1253–1256. doi: 10.1073/pnas.0306373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehrmann M, Bolek P, Mondigler M, Boyd D, Lange R. TnTIN and TnTAP: mini-transposons for site-specific proteolysis in vivo. Proc Natl Acad Sci U S A. 1997;94(24):13111–13115. doi: 10.1073/pnas.94.24.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 2000;103(3):375–386. doi: 10.1016/s0092-8674(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 46.Pauli A, Althoff F, Oliveira RA, Heidmann S, Schuldiner O, Lehner CF, Dickson BJ, Nasmyth K. Cell-Type-Specific TEV Protease Cleavage Reveals Cohesin Functions in Drosophila Neurons. Dev Cell. 2008;14(2):239–251. doi: 10.1016/j.devcel.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waters MG, Clary DO, Rothman JE. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J Cell Biol. 1992;118(5):1015–1026. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8(3):238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- 49.Marra P, Salvatore L, Mironov A, Jr., Di Campli A, Di Tullio G, Trucco A, Beznoussenko G, Mironov A, De Matteis MA. The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol Biol Cell. 2007;18(5):1595–1608. doi: 10.1091/mbc.E06-10-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelletier L, Jokitalo E, Warren G. The effect of Golgi depletion on exocytic transport. Nat Cell Biol. 2000;2(11):840–846. doi: 10.1038/35041089. [DOI] [PubMed] [Google Scholar]
- 51.Satoh A, Beard M, Warren G. Preparation and characterization of recombinant golgin tethers. Methods Enzymol. 2005;404:279–296. doi: 10.1016/S0076-6879(05)04026-7. [DOI] [PubMed] [Google Scholar]
- 52.Yu S, Satoh A, Pypaert M, Mullen K, Hay JC, Ferro-Novick S. mBet3p is required for homotypic COPII vesicle tethering in mammalian cells. J Cell Biol. 2006;174(3):359–368. doi: 10.1083/jcb.200603044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maday S, Anderson E, Chang HC, Shorter J, Satoh A, Sfakianos J, Fölsch H, Anderson JM, Walther Z, Mellman I. A PDZ-binding motif controls basolateral targeting of syndecan-1 along the biosynthetic pathway in polarized epithelial cells. Traffic. 2008 doi: 10.1111/j.1600-0854.2008.00805.x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HeLa cells expressing HT (the N-terminal HA-tagged 1–886 fragment of p115) were fixed, permeabilized, and labeled with anti-HA (middle panels) and either anti-giantin or antibodies to the ER exit site marker, Sec31 (left panels). The merged images are shown in the right panels (HA is in green, other markers are in red). Bar, 20 µm. Note that transiently expressed HT co-localized partially with the Golgi region (arrowhead) and Sec31, whereas stably expressed HT did not.


