Abstract
GRASP65 (Golgi reassembly and stacking protein of 65 KDa) is a cis-Golgi protein with roles in Golgi structure, membrane trafficking and cell signalling. It is cleaved by caspase-3 early in apoptosis, promoting Golgi fragmentation. We now show that cleavage is needed for Fas-mediated apoptosis: expression of caspase-resistant GRASP65 protects cells, whereas expression of membrane proximal caspase-cleaved GRASP65 fragments dramatically sensitises cells. GRASP65 coordinates passage through the Golgi apparatus of proteins containing C-terminal hydrophobic motifs, via its tandem PDZ type ‘GRASP' domains. Fas/CD95 contains a C-terminal leucine–valine pairing so its trafficking might be coordinated by GRASP65. Mutagenesis of the Fas/CD95 LV motif reduces the number of cells with Golgi-associated Fas/CD95, and generates a receptor that is more effective at inducing apoptosis; however, siRNA-mediated silencing or expression of mutant GRASP65 constructs do not alter the steady state distribution of Fas/CD95. We also find no evidence for a GRASP65–Fas/CD95 interaction at the molecular level. Instead, we find that the C-terminal fragments of GRASP65 produced following caspase cleavage are targeted to mitochondria, and ectopic expression of these sensitises HeLa cells to Fas ligand. Our data suggest that GRASP65 cleavage promotes Fas/CD95-mediated apoptosis via release of C-terminal fragments that act at the mitochondria, and we identify Bcl-XL as a candidate apoptotic binding partner for GRASP65.
Keywords: apoptosis, GRASP65, Golgi apparatus, Fas/CD95
The secretory pathway comprises sequential membrane compartments that regulate the synthesis, post-translational processing and targeting of soluble and membrane proteins.1 Maintaining the correct distribution of lipids and proteins between the organelles of the secretory pathway is essential for cellular function. This is achieved through the actions of vesicular–tubular transport intermediates that traffic between compartments, and factors that control the fusion of these with acceptor membranes.1 Data predict that pathways exist to recognise errors in the secretory pathway function, and to transduce these signals to apoptotic cell death pathways,2, 3, 4, 5 although the underlying mechanisms are poorly understood. Strong evidence suggests that the biosynthetic and endocytic pathways are disrupted by caspase activity during apoptosis2, 6, 7, 8, 9, 10, 11; for example, the Golgi apparatus fragments and scatters throughout the peripheral cytoplasm during the execution phase following caspase cleavage of many resident proteins including golgin-160,9 p115,10 giantin8 and GRASP65.11 Importantly, caspase-2-mediated cleavage of golgin-160 has been shown to be necessary for an efficient apoptotic response in cells challenged with TNFα, TRAIL and Fas ligand,12 suggesting that caspase cleavage of Golgi proteins is a prerequisite for apoptosis in certain contexts.
The Golgi stacking factor, GRASP65, is an important regulator of Golgi structure and function. It is required for both the establishment13 and maintenance14 of Golgi structural integrity, it coordinates passage of cargo through the Golgi,15, 16, 17 and it is implicated in the accurate post-translational processing of plasma membrane proteins.14 Properties associated with the C-terminus of GRASP65 contribute to growth factor signalling during interphase,18 and regulate Golgi reorientation in migrating cells.19 In addition, CDK1/cyclin-B-mediated phosphorylation at the C-terminus of GRASP65 establishes a platform for binding of polo-like kinase,20 thereby integrating mitotic kinase signalling with the timely passage through pro-metaphase.20, 21, 22, 23, 24 These findings demonstrate that GRASP65 is an important component of signalling pathways at the Golgi.25 GRASP65 structure/function is altered rapidly and efficiently by caspase cleavage at the early stages of apoptosis.11 GRASP65 has three caspase-3 sites within its membrane distal C-terminal signalling domain.11 We previously demonstrated that expression of caspase-resistant GRASP65 delays apoptotic Golgi fragmentation, but did not examine its wider implications.11 To determine whether caspase cleavage of GRASP65 is important for effective apoptotic signalling, we have examined the apoptotic responses of cells expressing mutant forms of GRASP65. We find that caspase cleavage of GRASP65 is needed for apoptosis in cells treated with Fas ligand, and that cells expressing membrane proximal GRASP65 cleavage fragments are sensitised. Our investigations suggest that GRASP65 cleavage contributes to apoptotic signalling via release of membrane distal caspase cleavage fragments that translocate to the mitochondria and engage downstream execution phase processes, and we identify Bcl-XL as a candidate apoptotic GRASP65 binding partner.
Results
Caspase cleavage of GRASP65 is required for some forms of apoptosis
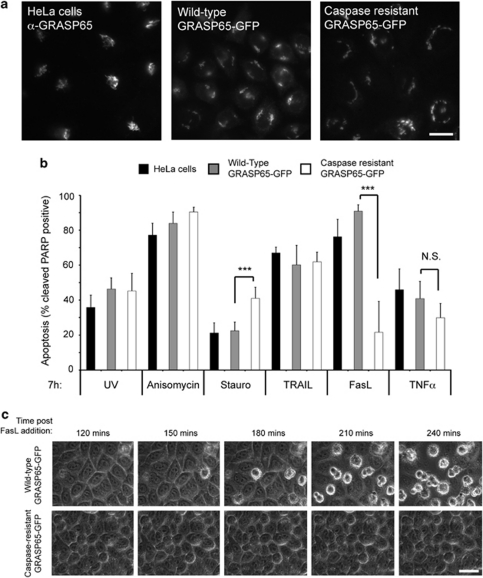
Many Golgi proteins are cleaved by caspases during apoptosis, leading to Golgi disassembly and arresting membrane traffic.5, 8, 9, 10, 11, 12, 26, 27, 28 We tested whether caspase cleavage of GRASP65 is required for apoptosis by measuring cell death (cleaved PARP) in HeLa cell lines stably expressing wild-type or caspase-resistant GRASP65–GFP11 (Figure 1a and b). The rates of apoptosis were similar between these cell lines under treatment with UV, anisomycin, TRAIL or TNFα; however, caspase-resistant GRASP65–GFP cells were more sensitive to staurosporine (Figure 1b), and were protected from Fas-ligand-induced apoptosis (Figure 1b and c; Supplementary Video 1). The differential responses of these cell lines to staurosporine are still under investigation. In this study, we have focussed on GRASP65 and the Fas/CD95 pathway, as a further example of how caspase action at the Golgi apparatus propagates cell death signals from surface receptors.12
Figure 1.
Expression of caspase-resistant GRASP65 protects cells from Fas-mediated apoptosis. (a) Fluorescence images of GRASP65 in standard Hela cells (labelled with GRASP65 antibodies) and HeLa cells stably expressing wild-type or caspase-resistant GRASP65–GFP. Bar=20 μm. (b) Apoptosis responses of standard HeLa cells, and HeLa cells stably expressing wild-type or caspase-resistant GRASP65–GFP to various apoptotic stimuli. Apoptotic cells were scored by immunofluorescence detection of caspase-cleaved PARP. Data show means±S.D. for a minimum of three experiments. Students' t-test: ***P<0.001, NS, not significant. (c) Time-matched frames from time lapse movies of HeLa cells stably expressing wild-type and caspase-resistant GRASP65–GFP treated with Fas ligand, comparing the rates of apoptosis (rounded, blebbing profiles) between these cell lines (frames correspond to Supplementary Video 1). Bar=20 μm
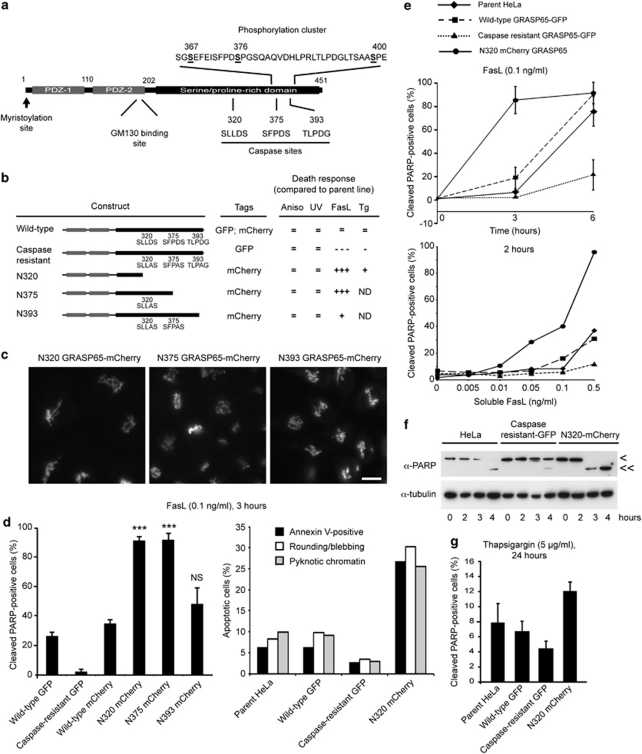
GRASP65 contains three caspase sites within its C-terminal serine/proline-rich domain (D320; D375; D393 in rat;11 Figure 2a). This domain is responsible for Golgi organisation, but is not required for GRASP65 dimerisation.29 To examine the roles of the C-terminus of GRASP65 in Fas/CD95 cell death signalling, we generated HeLa cell lines stably expressing membrane proximal caspase-cleavage fragments of GRASP65 fused to mCherry (N320, N375, N393: Figure 2b and c). These constructs were targeted normally to the Golgi apparatus (Figure 2c), and the cell lines showed no morphological or proliferative defects (data not shown). However, when treated with Fas ligand, cells expressing the two shortest caspase fragments (N320; N375) died at dramatically accelerated rates compared with the wild-type GRASP65–GFP cell line and wild-type GRASP65–mCherry cells (Figure 2d). Cells expressing the longest form of caspase-cleaved GRASP65 (N393) were only marginally sensitised (Figure 2d), suggesting that factors between amino acids 320 and 393 are responsible. Assessment of the kinetics of Fas-mediated apoptosis showed that expression of N320 GRASP65–mCherry accelerated the apoptotic rate considerably (Figure 2e and f). Similarly, the N320 GRASP65–mCherry cell line was highly sensitised to low doses of Fas ligand (2 h treatment; Figure 2e).
Figure 2.
Caspase cleavage of GRASP65 sensitises cells to Fas ligand and thapsigargin. (a) Schematic of the basic structure of GRASP65 showing the two PDZ ‘GRASP' domains and serine/proline-rich domain containing phosphorylation and caspase sites. (b) Summary of the relative sensitivities of the different GRASP65-expressing HeLa cell lines to different apoptotic stimuli. ND, not determined. (c) Fluorescence images of fields of HeLa cells stably expressing truncated GRASP65 constructs with mCherry tags. Bar=10 μm. (d) Responses of the cell lines shown to Fas ligand (FasL). N320–mCherry and N375–mCherry cell lines are dramatically sensitised. Data to the left show means±S.D. for a minimum of three experiments. Students' t-test compared with wild-type GRASP65–mCherry cells: ***P<0.001, NS, not significant. (e) Above, time-course of apoptosis of the different GRASP65-expressing cell lines treated with Fas ligand (0.1 ng/ml). Below, dose responses of the GRASP65 cell lines to Fas ligand (2 h treatment). (f) Immunoblot showing PARP cleavage profiles of standard HeLa cells, caspase-resistant GRASP65–GFP cells and N320 GRASP65–mCherry cells. <, Full-length PARP; <<, caspase-cleaved PARP. (g) Responses of various GRASP65 cell lines to thapsigargin treatment
Expression of caspase-resistant golgin-160 protects cells from the secretory pathway stress.12 We measured apoptosis in cells treated with the sarco/endoplasmic reticulum Ca2+ ATPase inhibitor, thapsigargin (Figure 2g). Caspase-resistant GRASP65 reduced apoptosis rates compared with parental HeLa cells and the wild-type GRASP65–GFP expressing cell line (although not statistically significant; Figure 2g). In addition, HeLa cells stably expressing N320 GRASP65–mCherry were sensitised to apoptosis (Figure 2g), pointing to a general role for GRASP65 cleavage in the secretory pathway stress and Fas/CD95 signalling.
A C-terminal PDZ-binding motif coordinates Fas/CD95 Golgi retention
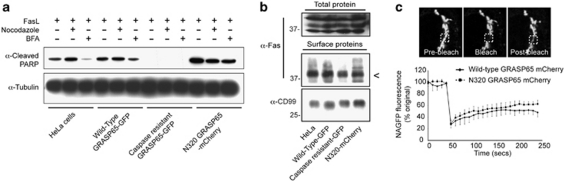
Apoptotic Fas/CD95 signalling requires biosynthetic membrane trafficking. In cells treated with Fas ligand in the presence of brefeldin A (BFA) apoptotic efficiency was clearly reduced (as judged by immunoblotting with anti-cleaved PARP antibodies; Figure 3a). Notably, the steady state levels of Fas/CD95 at the plasma membrane were similar between mutant GRASP65 cell lines, suggesting that Fas/CD95 is not retained internally in any of these (Figure 3b). GRASP65 generates lateral linkages between adjacent Golgi stacks,14 a property that is essential for accurate lateral distribution of enzymes and for efficient sialylation of surface proteins14 – an important feature of the functional Fas receptor.30 In cells expressing wild-type GRASP65–mCherry or N320 GRASP65–mCherry, lateral diffusion of the Golgi enzyme β1,2N-acetylglucosaminyltransferase I (NA–GFP) was similar (Figure 3c). This suggests that the Golgi apparatus is intact in N320 GRASP65–mCherry expressing cells (Figure 3c) – a scenario that differs from cells silenced for GRASP55 or GM130, which show deficiencies in lateral Golgi enzyme mobility.14, 31
Figure 3.
Fas/CD95 trafficking and Golgi architecture in GRASP65 cell lines. (a) Immunoblot of cleaved PARP showing the influence of BFA and nocodazole treatment on apoptosis in GRASP65 cell lines treated with Fas ligand (FasL; 6 h). (b) Immunoblot of total and surface Fas/CD95 following biotinylation enrichment from the GRASP65 expressing cell lines shown. <, Surface Fas/CD95. (c) Fluorescence recovery after photobleaching (FRAP) analysis of Golgi structural integrity in wild-type and N320 GRASP65–mCherry cells. The trace shows the recovery of NAGFP fluorescence (%) into the bleached region (hatched box in the example images). Data are means ± S.D. for wild-type GRASP65–GFP and N320 GRASP65–mCherry of three cells and six cells, respectively
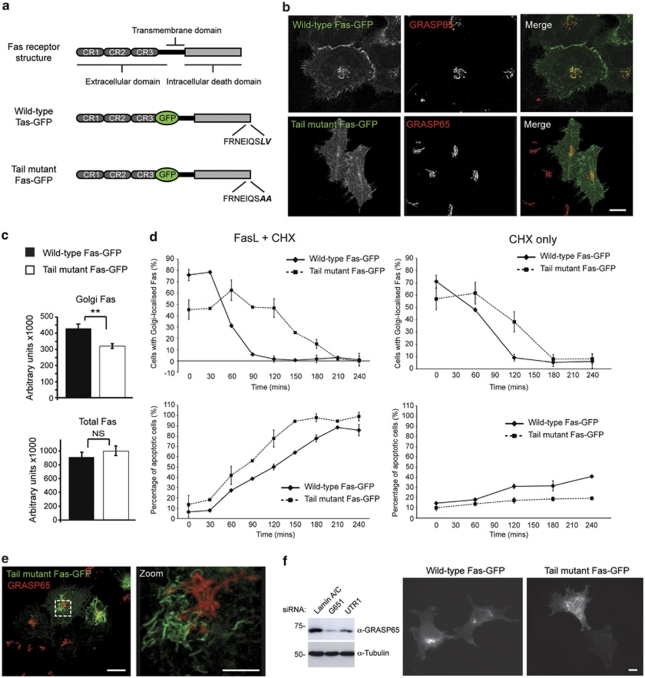
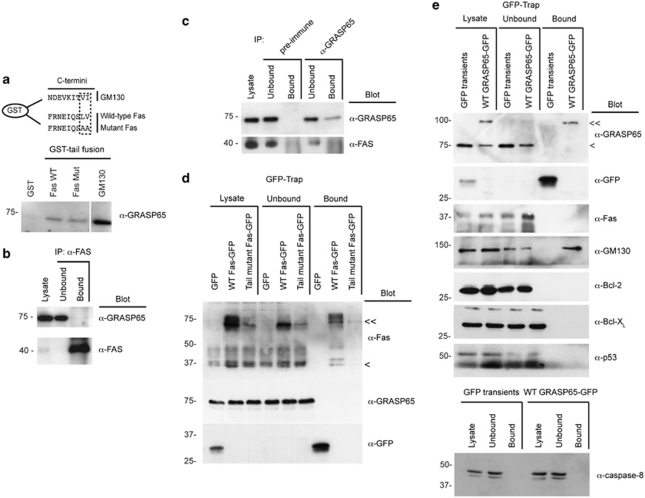
Truncation of 15 amino acids from the C-terminus of Fas/CD95 sensitises cells to Fas ligand.32 Fas/CD95 terminates with a leucine–valine (LV) motif through which it binds to the tandem PDZ domains of Fas-associated phosphatase, Fap-1 – an interaction that is important for its intracellular targeting.33 Indeed, overexpression of Fap-1 is a feature of cancers that show resistance to Fas ligand.33 At steady state, Fas/CD95 is found at significant levels at the Golgi apparatus, and mobilisation of this Golgi pool is a prerequisite for effective Fas-mediated apoptosis.34 Importantly, GRASP65 has been found in complex with Fas/CD95 and caspase-8,35 suggesting that it might mediate the trafficking of the Fas/CD95 DISC complex. GRASP65 possesses tandem PDZ domains (Figure 2a), through which it coordinates trafficking of p24 cargo adaptors, TGFα,15 CD8α and Frizzled416 via tandem C-terminal acidic residues. To test whether GRASP65 interacts with Fas/CD95 and influences Fas/CD95 biosynthetic trafficking, we prepared wild-type and LV-to-AA tail mutants of Fas/CD95 with luminal/extracellular membrane-proximal GFP tags (Figure 4a), and compared their subcellular locations and apoptosis-inducing capabilities (Figure 4b–d). Each construct showed the same distribution pattern with evidence for cell surface localisation and for a Golgi pool, and the expression levels of each construct were similar (Figure 4b and c); however, Golgi-localised Fas–GFP fluorescence levels were significantly greater for wild type than for tail mutant constructs (Figure 4c). Interestingly, we found that tail mutant Fas–GFP was released more slowly from the Golgi than wild-type Fas–GFP (Figure 4d). Despite this, mutant Fas–GFP was more cytotoxic than its wild-type counterpart (Figure 4d). Interestingly, in cells expressing high levels of mutant Fas–GFP, we often observed filamentous juxtanuclear structures (Figure 4e). These were of similar dimensions to GRASP65-labelled Golgi membranes (Figure 4e), but did not co-localise with markers of the Golgi apparatus, TGN or endocytic compartment (Supplementary Figure 1), suggesting that they represent a novel compartment exaggerated by the presence of mutant Fas–GFP.
Figure 4.
The C-terminus of Fas/CD95 mediates trafficking through the Golgi apparatus. (a) Schematic of the Fas/CD95 constructs used (CR, coiled coil region). (b) Fluorescence images of Fas–GFP transiently expressed in HeLa cells. The Golgi was labelled with GRASP65 antibodies. Bar=20 μm. (c) Quantitation of total cellular and Golgi-associated wild-type and tail mutant Fas–GFP measured by fluorescence microscopy (arbitrary units). **P<0.01; NS, not significant. (d) Analysis of the proportion of cells with Golgi-associated Fas–GFP (above) and the rate of cell death (below) in response to cycloheximide (CHX) in the absence (right) and presence (left) of Fas ligand, in cells expressing wild-type of tail mutant Fas–GFP. Data show means ± S.D. for three experiments. (e) Analysis of the distribution of tail mutant Fas–GFP in HeLa cells. Note the tubular structures that lie adjacent to, but do not overlap with GRASP65 staining. Further examples of co-localisation with cellular compartments are shown in Supplementary Figure 1. Bar=20 μm (5 μm in zoom). (f) Effect of GRASP65 silencing on Golgi-associated Fas/CD95. Immunoblot showing efficiency of GRASP65 silencing with two oligonucleotides (UTR1, untranslated region 1), and examples images of wild-type and tail mutant Fas–GFP in a GRASP65 silencing background. Bar=10 μm
If GRASP65 coordinates Fas/CD95 trafficking, then we would anticipate a change in the steady state localisation of Fas/CD95 in GRASP65-suppressed cells; however, the distribution patterns of wild-type and tail mutant GFP–Fas were not substantially different in GRASP65-silenced cells (compare Figure 4b and f). To assess binding between GRASP65 and Fas/CD95 in vitro, we produced GST-tagged Fas/CD95 tail constructs (Figure 5a) and used these to probe HeLa cell lysates.15 For these experiments, a tail construct of GM130 was used as a positive control (Figure 5a). We observed strong binding of GRASP65 to GM130 tail, but only weak binding to Fas/CD95 tail constructs (Figure 5a). Significantly, we observed no apparent differences between wild-type and tail mutant Fas/CD95 constructs (Figure 5a). We next immunoprecipitated GRASP65 and Fas/CD95 from HeLa cell lysates; however, the corresponding candidate partner protein was not detected in the bound fractions (Figure 5b and c). We then transfected HeLa cells with wild-type or tail mutant Fas–GFP, and subjected cell lysates to GFP–Trap immunoprecipitation36 (Figure 5d). In parallel, we tested lysates from HeLa cells stably expressing wild-type GRASP65–GFP11 (Figure 5e). We probed Fas–GFP immune complexes with GRASP65 antibodies and the GRASP65–GFP immune precipitates with antibodies recognising Fas/CD95, GM130, and Bcl-2, Bcl-XL and p53 (three candidate GRASP65 binding partners identified by antibody array; Supplementary Figure 2; Figure 5d and e). Although wild-type Fas–GFP was efficiently isolated, we did not detect GRASP65 in these immune complexes (Figure 5d). Similarly, GRASP65–GFP isolates were negative for Fas/CD95 and for caspase-8, although these were enriched for GM130 (Figure 5e). Also, although we were routinely able to clear the cell lysates of GRASP65–GFP, we never detected Bcl-2, Bcl-XL or p53 in the GRASP65–GFP complexes (Figure 5e).
Figure 5.
GRASP65 and Fas/CD95 do not directly interact at the Golgi apparatus. (a) GST-tail pull-down assay. Comparison of the C-terminal sequence of GM130 – a protein known to interact with GRASP65 – and the fusion proteins used in the assay (above). Example tail-binding assay showing strong binding of GRASP65 to the C-terminus of GM130, but only weak binding to both wild-type and mutant tail fusions of Fas/CD95 (below). (b and c) Immunoprecipitation of endogenous Fas/CD95 (b) and GRASP65 (c) and immunoblotting for the respective candidate binding partner. (d and e) GFP–Trap immunoprecipitations of HeLa cells transiently expressing wild-type or tail mutant Fas–GFP (d), and GFP–Trap immunoprecipitations of HeLa cells stably expressing GRASP65–GFP (e) immunoblotted using the antibodies shown. <, Endogenous Fas/CD95 or GRASP65; <<, GFP-tagged Fas/CD95 or GRASP65
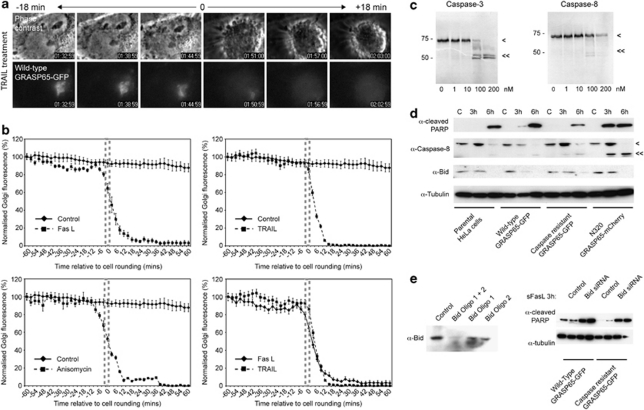
Cleavage of GRASP65 is an early event during apoptosis
To study the timing of GRASP65 cleavage, and to test whether different inducers of apoptosis promote cleavage with differing kinetics, we treated wild-type GRASP65–GFP HeLa cells with Fas ligand, anisomycin, or TRAIL, and measured Golgi-associated GRASP65–GFP fluorescence during apoptosis (Figure 6a and b). On caspase cleavage, GFP is released from membrane-associated GRASP65–GFP11 (Figure 6a), providing a quantitative read-out of GRASP65 cleavage. In Fas ligand and anisomycin-treated cells the mean time for complete loss of Golgi-associated GRASP65–GFP was similar (∼24 min; Figure 6b), whereas for TRAIL-treated cells, the mean time was much less (∼14 min; Figure 6b), possibly reflecting variations in the timing of caspase-3 activation (required for GRASP65 cleavage11). Notably, we routinely observed a decline in Golgi-associated GRASP65–GFP fluorescence 30–40 min before apoptotic execution exclusively in the Fas-ligand-treated cells, suggesting that advanced cleavage of a pool of GRASP65 might be a feature of Fas-mediated apoptosis. This implicates caspase-8, the initiator caspase that acts upstream of caspase-3 in the Fas/CD95 pathway. We tested cleavage of GRASP65 in vitro using recombinant caspase-8 (Figure 6c), and compared caspase-8 and Bid processing in wild-type and mutant GRASP65 cell lines (Figure 6d). GRASP65 was weakly cleaved by recombinant caspase-8 (Figure 6c), suggesting that caspase-8 might contribute to GRASP65 cleavage in living cells. Processing of caspase-8 was noticeably advanced in the N320 GRASP65–mCherry cell line (Figure 6d), consistent with the rate of Fas-mediated apoptosis in these cells. Importantly, caspase-8 processing was very similar between parental HeLa cells, and wild-type and caspase-resistant GRASP65–GFP cell–lines (Figure 6d), suggesting that the protection from Fas-mediated apoptosis afforded by caspase-resistant GRASP65 arises downstream of caspase-8 activation. In support of this, the rate of Bid cleavage in these cell lines was very similar (Figure 6d) implying a functional Fas/CD95 death receptor/caspase-8 activation pathway. Importantly, siRNA silencing of Bid expression did not delay Fas-mediated apoptosis in either wild-type or caspase-resistant GRASP65–GFP cell lines (Figure 6e), suggesting that amplification of the apoptotic response via Bid cleavage is not a prerequisite for apoptotic induction in this context. Together, these data support a role for early caspase cleavage and release of the C-terminus of GRASP65 in apoptotic signalling in the Fas/CD95 pathway.
Figure 6.
GRASP65 cleavage is an early event during apoptosis. (a) Time-lapse imaging of GRASP65–GFP caspase cleavage. To the top, phase contrast images of a TRAIL-treated HeLa cell before and after the onset of apoptosis; to the bottom, fluorescence images of Golgi-associated GRASP65–GFP and the loss of GFP signal from the Golgi following the onset of apoptosis. (b) Normalised fluorescence intensity traces of Golgi-associated GRASP65–GFP in HeLa cells treated with the apoptosis-inducing reagents shown. Traces are plotted relative to the onset of cell rounding (hatched boxes). Data show means ± S.D. for a minimum of five cells per treatment. (c) Autoradiographs of 35S-labelled GRASP65 translated in vitro and incubated for 90 min with increasing concentrations of recombinant caspase-3 or caspase-8. Full-length (<) and caspase-cleaved (<<) GRASP65 products are shown. (d) Immunoblot of different cell lines treated with Fas ligand. Full-length caspase-8 (<); processed caspase-8 (<<). (e) Influence of Bid silencing on Fas-mediated apoptosis in wild-type and caspase-resistant GRASP65–GFP cell lines. To the left, immunoblot of HeLa cells treated with silencing oligonucleotides against Bid. To the right, immunoblot comparing levels of cleaved PARP in Fas ligand-treated cells (as shown) silenced or not with a combination of Bid oligonucleotides 1 and 2
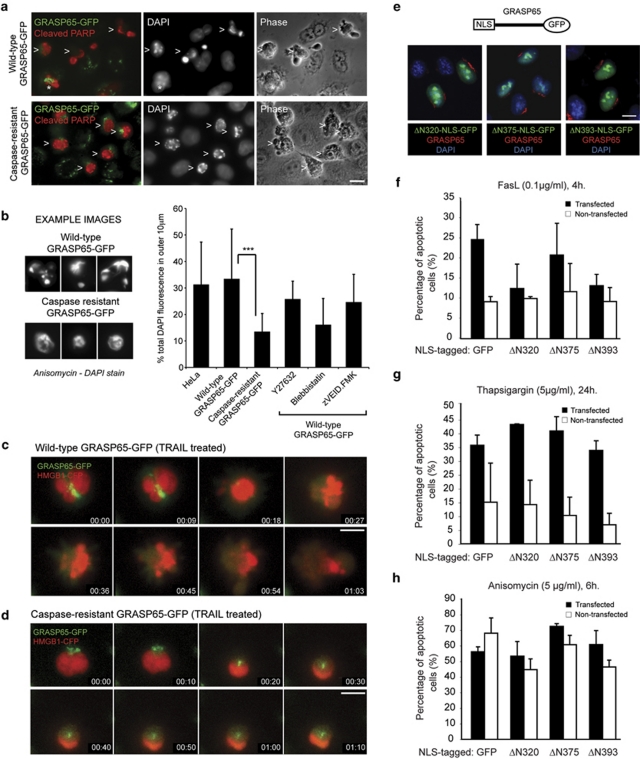
GRASP65 cleavage couples Golgi disruption to apoptotic nuclear disassembly
The C-terminus of GRASP65 has a low isoelectric point, which may direct GRASP65 cleavage products into the nucleus.24 Surprisingly, wild-type and caspase-resistant GRASP65–GFP stable cell lines show different apoptotic phenotypes, supporting a possible nuclear role for GRASP65 caspase products (Figure 7a–d): apoptotic wild-type GRASP65–GFP expressing cells had condensed, fragmented and scattered chromatin, consistent with late stages of nuclear disruption (Figure 7a and see Lane et al.11); meanwhile, apoptotic chromatin was retained within intact nuclei in apoptotic caspase-resistant GRASP65–GFP cells (Figure 7a). Consistent with this, ∼30% of 4′,6-diamidino-2-phenylindole (DAPI) fluorescence was found in the peripheral cytoplasm of apoptotic control and wild-type GRASP65–GFP expressing HeLa cells, compared with ∼12% in the caspase-resistant GRASP65 cell line (Figure 7b). Live-cell imaging of cells co-expressing GRASP65–GFP and the chromatin marker high-mobility group box 1 (HMGB1)–CFP supported these observations (Figure 7c and d; Supplementary Videos 2 and 3). These results are reminiscent of the effects of inhibition of actin/myosin II upon apoptotic cell remodelling,37 and indeed inhibition of myosin II activation (using the ROCK inhibitor, Y27632) or myosin II contractility (using blebbistatin), or prevention of nuclear envelope disruption (using the caspase-6 inhibitor, zVEID.FMK) reduced the peripheral redistribution of chromatin in apoptotic wild-type GRASP65–GFP cells to a similar extent (Figure 7b).
Figure 7.
Caspase cleavage of GRASP65 is required for effective apoptotic nuclear disruption. (a) Fluorescence images of wild-type and caspase-resistant GRASP65–GFP stable HeLa cells treated with anisomycin (6 h) and stained with anti-caspase-cleaved PARP antibodies and DAPI. (>) cells undergoing apoptosis; (*) a wild-type GRASP65–GFP expressing cell at a very early stage of apoptosis with Golgi-associated GRASP65–GFP fluorescence still apparent. Bar=10 μm. (b) Analysis of apoptotic chromatin redistribution. To the left, DAPI images of example apoptotic nuclei from HeLa cells stably expressing wild-type or caspase-resistant GRASP65–GFP. To the right, quantitation of DAPI fluorescence in the periphery of apoptotic HeLa cells in the absence or presence of Y27632 (ROCKI inhibitor: 100 μM), blebbistatin (myosin II inhibitor: 12.5 μM) or zVEID.FMK (caspase-6 inhibitor: 12.5 μM). Data show means±S.D. for 30 cells per treatment. Student's t-test: ***P<0.001. (c and d) Fluorescence time-lapse imaging of apoptotic cell re-organisation in HeLa cells stably expressing wild-type (c) or caspase-resistant (d) GRASP65–GFP. Cells were transiently transfected with HMGB1–CFP to label the nuclei. Bar=10 μm. (e–h) Nuclear targeting of GRASP65 caspase products does not sensitise cells to apoptosis. (e) Schematic of the constructs used in these experiments and example images of NLS- and GFP-tagged GRASP65 caspase products. Cells were co-stained with anti-GRASP65 antibodies and DAPI. Bar=10 μm. (f–h) Quantitation of apoptosis in HeLa cells transiently expressing NLS–GFP or NLS-tagged GRASP65 caspase products, treated with the apoptosis-inducing reagents as shown
To examine whether C-terminal GRASP65 cleavage products accumulate in the nucleus, we transfected cells with myc- or GFP-tagged C-terminal constructs (designated ΔN320, ΔN375, ΔN393). These were targeted to the cytoplasm and were not cytotoxic (data not shown; see below). To examine whether targeting these constructs to the nucleus would induce cytotoxicity, we inserted a tripartite nuclear localisation signal (NLS) upstream of the C-terminus of GRASP65 (Figure 7e).38 Each of the C-terminal fragments accumulated in the nucleus as expected without triggering obvious changes in nuclear organisation (Figure 7e), and none were cytotoxic (data not shown). We then treated HeLa cells transiently expressing these constructs with Fas ligand, anisomycin or thapsigargin, and monitored apoptosis rates (Figure 7f–h). None of the NLS-tagged constructs sensitised cells to apoptosis above the levels observed in NLS–GFP expressing control cells (Figure 7f–h). These data argue against a direct role for nuclear targeting of C-terminal GRASP65 caspase cleavage products in downstream apoptotic signalling.
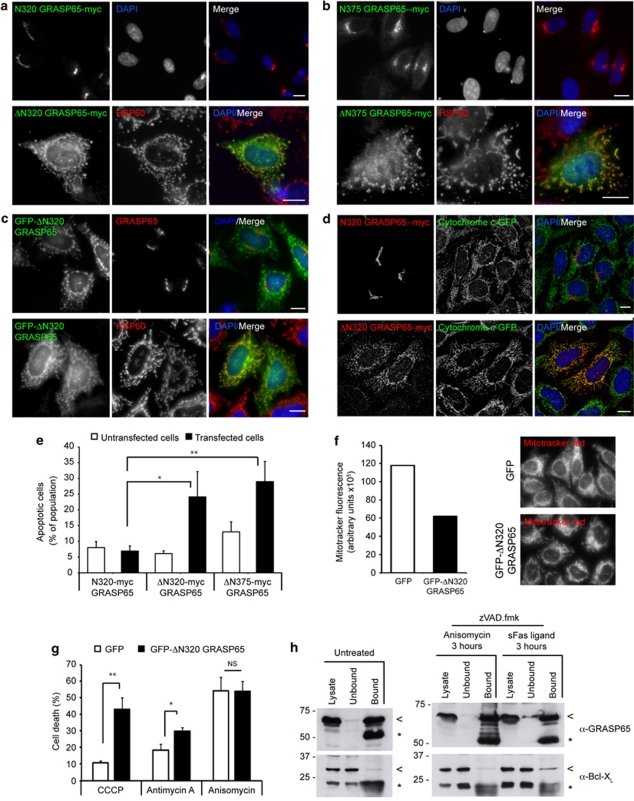
GRASP65 caspase cleavage products co-localise with mitochondria and sensitise cells to Fas ligand and mitochondrial poisons
On close inspection of the localisation of epitope-tagged GRASP65 C-terminal caspase products overexpressed in viable HeLa cells, a punctate/reticular cytoplasmic pattern was evident. Co-localisation with markers for mitochondria (e.g., HSP60) demonstrated that these fragments were targeted to the mitochondrial network (Figure 8a–c; Supplementary Figure 3). Mitochondrial co-localisation was seen for myc-tagged ΔN320 and ΔN375 (Figure 8a and b), but was not observed with myc-tagged ΔN393 (data not shown), suggesting that the presumed mitochondrial-targeting domain resides between the GRASP65 caspase sites (i.e., between amino acids 320 and 393). This is consistent with the Fas/CD95 cytotoxicity assays in the various GRASP65 truncation mutant cell lines (Figure 2d). Importantly, we also observed mitochondrial co-localisation in cells expressing GFP–ΔN320 (Figure 8c).
Figure 8.
GRASP65 caspase cleavage products are targeted to mitochondria and potentiate Fas-mediated apoptosis. (a, b) Immunofluorescence imaging of HeLa cells transiently expressing N320 GRASP65–myc or ΔN320 GRASP65–myc (a), or N375 GRASP65–myc or ΔN375 GRASP65–myc (b), stained with anti-myc antibodies, anti-HSP60 and DAPI. Bars=10 μm. (c) HeLa cells transiently expressing GFP–ΔN320, co-stained with anti-GRASP65 or anti-HSP60 antibodies and DAPI. Bar=10 μm. (d) Immunofluorescence images of cells HeLa cells stably expressing cytochrome c-GFP and transiently transfected with N320 GRASP65–myc or ΔN320 GRASP65–myc. Mitochondrial ΔN320 GRASP65–myc does not cause release of cytochrome c. (e) Transient expression of ΔN320–myc or ΔN375–myc sensitises cells to Fas-mediated apoptosis (2 h treatment). Apoptosis was assessed using cleaved PARP antibody. *P<0.05; **P<0.01. (f) The C-terminus of GRASP65 influences mitochondrial membrane potential. HeLa cells stably expressing GFP or GFP–ΔN320 GRASP65 were loaded with mitotracker red (15 min) then imaged using identical parameters (5 ms exposure). Aggregate fluorescence intensities for ∼120 cells were obtained. (g) The C-terminus of GRASP65 sensitises cells to apoptosis mediated by mitochondrial poisons. HeLa cells stably expressing GFP or GFP–ΔN320 GRASP65 were treated with the mitochondrial poisons CCCP (29 h) and antimycin A (29 h), or with anisomycin (6 h), and assessed for cell death by DAPI staining (condensed and/or pyknotic nuclei). Data show means of three independent experiments. *P<0.05; **P<0.01; NS, not significant. (h) GRASP65 binds to Bcl-XL during apoptotic commitment signalling after Fas ligand treatment. GRASP65 immunoprecipitation of untreated (to the left) and anisomycin or Fas ligand treated HeLa cells (to the right). Weak binding to Bcl-XL is observed in untreated and in anisomycin treated cells; however, robust Bcl-XL binding is observed in Fas-ligand-treated cells. Anisomycin and Fas ligand treated cells were treated with zVAD.FMK to prevent caspase activation and GRASP65 cleavage. (arrow, GRASP65 and Bcl-XL; asterisk, antibody heavy and light chains.)
One possible outcome of the association of the C-terminus of GRASP65 with mitochondria is to damage the outer membrane, leading to release of apoptotic factors and ensuing cell death. To test this, we transiently transfected cytochrome c–GFP expressing HeLa cells39 with N320 GRASP65–myc or ΔN320 GRASP65–myc, and labelled fixed cells with anti-myc antibodies (Figure 8d). Cells expressing ΔN320 GRASP65–myc showed no evidence of dislocation of cytochrome c–GFP (Figure 8d), making it unlikely that the presence of caspase cleavage products of GRASP65 at the mitochondria causes release of pro-apoptotic factors. Consistent with this observation, mitochondrial-associated GRASP65 ΔN320 and ΔN375 caspase cleavage fragments were not toxic when overexpressed in HeLa cells (data not shown). Interestingly, though, expression of GRASP65 ΔN320 and ΔN375 caspase cleavage fragments significantly sensitised HeLa cells to Fas ligand (Figure 8e), suggesting that the release of the C-terminus of GRASP65 from the Golgi apparatus promotes Fas-mediated apoptosis (note that for these experiments, we used Golgi-targeted GRASP65 N320–myc as a control, having confirmed that this construct did not potentiate the apoptosis response when transiently expressed; Figure 8e and data not shown).
Although mitochondrial association of the C-terminus of GRASP65 was not sufficient to cause release of cytochrome c (Figure 8d), its capacity to sensitise cells to Fas ligand (Figure 8e) indicated that mitochondrial physiology might nevertheless be compromised. To explore this, we generated HeLa cell lines stably expressing GFP or GFP–ΔN320 GRASP65 by lentiviral transduction and loaded these cell lines with mitotracker red for imaging using identical acquisition parameters. Mitochondrial fluorescence was consistently lower in the GFP–ΔN320 GRASP65 cells (Figure 8f). We next subjected these cell lines to the mitochondrial poisons carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and antimycin A (Figure 8g). Strikingly, the GFP–ΔN320 GRASP65 cells were highly sensitised to both CCCP and antimycin A, although GFP and GFP–ΔN320 GRASP65 cells died at similar rates upon treatment with anisomycin (Figure 8g). Together these observations suggest that C-terminal GRASP65 caspase cleavage products lower mitochondrial membrane potential rendering cells more sensitive to mitochondrial poisons such as CCCP and antimycin A, and to Fas ligand.
GRASP65 interacts with Bcl-XL during Fas/CD95-mediated apoptotic commitment signalling
It has previously been reported that Bcl-XL influences the relationships between GRASP65 and the Fas/CD95 disc complex.35 We therefore explored the possibility that GRASP65 interacts with Bcl-XL in viable cells and in cells primed to undergo apoptosis (i.e. during apoptotic commitment signalling; Figure 8h). We immunoprecipitated endogenous GRASP65 from untreated HeLa cell lysates and from lysates of HeLa cells treated whether with anisomycin or with Fas ligand for 3 h in the presence of zVAD.FMK (Figure 8h). This regime of treatments was established to enrich for cells arrested during the apoptotic commitment signalling phase, in which caspase cleavage of targets, such as GRASP65, had been prevented. GRASP65 bound very weakly to Bcl-XL in untreated cells and in cells treated with anisomycin (Figure 8h); however, in cells treated with Fas ligand, robust binding to Bcl-XL was observed (Figure 8h). This suggests that GRASP65 and Bcl-XL interact specifically during the commitment signalling phase of Fas/CD95-mediated apoptosis, providing a plausible mechanistic explanation for the influence of GRASP65 caspase cleavage on the susceptibility of cells to Fas ligand.
Discussion
GRASP65 is an important structural component of the Golgi apparatus with additional roles in membrane trafficking and cell signalling. In this study, we have shown that caspase cleavage of GRASP65 is needed for Fas/CD95 and thapsigargin-mediated apoptosis. A relationship between secretory pathway dysfunction, caspase cleavage of Golgi residents and effective apoptotic execution has previously been suggested for both golgin-1609, 12, 27, 28 and for p115,10 indicative of a common theme. How caspase cleavage of these proteins is coupled to efficient apoptotic progression remains uncertain. Being an important regulator of Golgi architecture11, 13, 14, 29, 31 and membrane trafficking,15, 16, 40 we initially predicted that GRASP65 cleavage would alter Fas/CD95 trafficking through the secretory and/or endocytic pathways, thereby affecting the cell-death-inducing capability of the Fas/CD95 population. Published evidence for a biochemical interaction between GRASP65 and Fas/CD95,35 and the presence of a C-terminal LV motif in Fas/CD95 (potentially able to bind to the tandem PDZ domains of GRASP6515, 16), supported this; however, although the C-terminus of Fas/CD95 coordinates its retention at the Golgi, we have been unable to obtain data to confirm this hypothesis. Instead, our results imply that GRASP65 influences apoptosis via release of C-terminal caspase cleavage fragments that translocate to mitochondria and sensitise cells to Fas/CD95-mediated apoptosis. Importantly, our data suggest that GRASP65 binds to Bcl-XL specifically during apoptotic commitment, suggesting a molecular pathway linking caspase action at the Golgi apparatus and the engagement of mitochondrial apoptosis signalling pathways.
The C-terminus of GRASP65 has a low isoelectric point suggested to be sufficient to drive caspase cleavage products into the nucleus.29 Independent studies have demonstrated this for p115 and golgin-160.10, 28 In the case of p115, this is thought to amplify the apoptotic response, because ectopic expression of this fragment was cytotoxic.10 We examined the potential for GRASP65 caspase fragments to translocate to the nucleus and influence apoptotic initiation; however, none of the C-terminal GFP- or myc-tagged cDNA constructs representing the three membrane distal caspase fragments (ΔN320; ΔN375; ΔN393) accumulated in the nuclei of healthy cells. Meanwhile, targeting of these fragments to the nucleus by insertion of a NLS did not decrease the viability of cells either in the absence or presence of apoptotic stimuli. Although these data suggest that GRASP65 caspase products do not influence events in the nucleus, caspase-resistant GRASP65–GFP cells show a marked reduction in chromatin redistribution into the peripheral cytoplasm. This is reminiscent of the influence of actin/myosin II inhibitors on the execution phase37 (see Figure 7b), implying a direct role for GRASP65 cleavage either in activating actin/myosin II contractility, in destabilising the nuclear envelope to facilitate chromatin release, or via a kinetic delay in apoptotic execution – the most likely scenario given the resistance to Fas ligand of cells stably expressing caspase-resistant GRASP65.
The two longest C-terminal GRASP65 caspase fragments (ΔN320; ΔN375) associate with mitochondria and sensitise HeLa cells to Fas ligand. This suggests that GRASP65 caspase products might influence cell viability via amplification of apoptotic signalling pathways at the mitochondria. We have used proteomics, yeast two-hybrid analyses and antibody array screens to identify GRASP65 interactors. Several candidates were identified in the antibody arrays, but none of these could be verified in our immunoprecipitation experiments of healthy cells. Our observations that cells over-expressing the longer C-terminal GRASP65 cleavage products were viable but became more susceptible to Fas ligand suggested that any potential interactions between GRASP65 and its candidate apoptotic binding partners might develop during the apoptotic commitment phase. We therefore re-examined the potential for GRASP65 to bind apoptotic factors during apoptotic commitment signalling. Importantly, we found that GRASP65 co-precipitates with Bcl-XL in cells primed to undergo apoptosis induced by Fas ligand, but not in viable cells or cells treated with anisomycin. This suggests that during Fas/CD95 signalling, Bcl-XL may be recruited to the Golgi via its association with GRASP65. A model in which Golgi recruitment of Bcl-XL during Fas/CD95 signalling has a cytoprotective affect that is alleviated by early caspase cleavage of GRASP65 is a possibility. In cells expressing caspase-resistant GRASP65, Bcl-XL association with the Golgi apparatus is prolonged, whereas the occupation of the Golgi apparatus by over-expressed truncated GRASP65 restricts the recruitment of Bcl-XL to the Golgi. Further investigations of the possible roles of Bcl-XL at the Golgi, and the molecular function of the C-terminus of GRASP65 at the mitochondria will be required to fully understand the linkages between secretory pathway function and apoptotic signalling.
Materials and Methods
Reagents and antibodies
Unless otherwise stated, reagents were obtained from Sigma (Poole, UK). Stock solutions of anisomycin (5 mg/ml), staurosporine (1 mM), DAPI (1 mg/ml), Fas ligand (soluble, FLAG tagged, Alexis Biochemicals, Exeter, UK: 0.1 mg/ml), TNFα (R&D Systems, Abingdon, UK: 100 μg/ml), nocodazole (5 mg/ml), thapsigargin (Calbiochem, Nottingham, UK: 5 mg/ml), BFA (Calbiochem: 5 mg/ml), Y27632 (Calbiochem: 100 mM), blebbistatin (Calbiochem: 100 mM), zVEID.FMK (Calbiochem: 12.5 mM) were stored at −20°C. Stock solutions of TRAIL (Calbiochem: 20 μg/ml) were stored at −80°C. Cell surface proteins were isolated and purified using a surface biotinylation kit (Pierce, Loughborough, UK) according to the manufacturer's instructions. The sheep anti-human GRASP65 antibody was raised against a recombinant GST fusion of human GRASP65 (amino acids 201-end), and affinity purified against His-tagged, recombinant full-length human GRASP65. The following antibodies were also used: monoclonal anti-PARP (Calbiochem), polyclonal anti-cleaved PARP (Promega, Southampton, UK), anti-caspase-8 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-Bid (BD Biosciences, Oxford, UK), anti-p53 (Upstate, Watford, UK), anti-Bcl-2 (Upstate), anti-Bcl-XL (Santa Cruz), anti-CD99 was a gift from Professor George Banting (Bristol, UK), anti-GFP (Covance, Princetown, NJ, USA), anti-α-tubulin (clone B-5-1-2), anti-acetylated α-tubulin (clone 6-11B-1), anti-TGN46 (AbD Serotec), anti-giantin (Covance), anti-GM130 (BD Biosciences), anti-β-COP (MAD; a gift from Professor Viki Allan, Manchester, UK), anti-ERGIC53 (Alexis Biochemicals), anti-calnexin (Stressgen, Exeter, UK), anti-EEA-1 (BD Biosciences), anti-transferrin receptor (Sigma), monoclonal anti-Fas (clone CH11; Upstate), polyclonal anti-Fas (Santa Cruz), anti-HSP60 (Sigma), anti-FLAG (M2; Sigma), anti-myc (clone 9E10; Sigma). Secondary antibodies for immunofluorescence (Alexa-488 and Alexa-594) were from Molecular Probes (Paisley, UK). HRP-tagged secondary antibodies were from Jackson Immunoresearch (Newmarket, UK).
Cell lines and apoptosis induction
Cells were maintained in DMEM containing 10% foetal bovine serum, at 37°C and 5% CO2. HeLa cell lines stably expressing wild-type and caspase-resistant GRASP65–GFP have been described previously.11 HeLa cells stably expressing wild-type and caspase-resistant GRASP65–mCherry, and mCherry fusions of GRASP65 C-terminal truncations (N320, N375, N393) were obtained following transient transfection by selecting positive clones after G418 treatment.11, 37 HeLa cells stably expressing GFP or GFP–ΔN320 GRASP65 were prepared by lentiviral trasduction (Lenti-X expression system; Clontech, Mountain View, CA, USA) and antibiotic selection. Cytochrome c-GFP stable HeLa cells39 were kindly provided by Professor Doug Green (St Jude Children's Research Hospital, Memphis, TN, USA). Cells were induced into apoptosis by treatment with 5 μg/ml anisomycin, UV irradiation (100 J/m2, Lane et al.11), 1 μM staurosporine, 20 ng/ml TRAIL (with 10 μg/ml cycloheximide), 100 ng/ml TNFα (with 10 μg/ml cycloheximide), 10 μM CCCP; 1 μg/ml antimycin A. For Fas-mediated apoptosis assays, cells were treated with FLAG-tagged, soluble Fas ligand (0.1 μg/ml) in the presence of 0.5 μg/ml anti-FLAG monoclonal antibody and 10 μg/ml cycloheximide. Cells were then incubated for the indicated times at 37°C, 5% CO2.
cDNA constructs and transfections
The human HMGB1–CFP construct has been described previously.6 Plasmid pCNG2 encoding a GFP fusion of the first 103 amino acids of NA–GFP was a gift from Professor Viki Allan (Manchester, UK). C-terminal CFP and YFP fusions of GRASP65 were made by colour switching the GRASP65–GFP construct. GRASP65–mCherry was made by PCR amplifying the mCherry sequence (a gift from Roger Tsien), and sub-cloning into GRASP65–GFP to replace GFP (the BamHI site in the rat GRASP65 ORF was first altered by site-directed mutagenesis). C-terminal GFP fusions of caspase-cleavage-mimicking truncations of GRASP65 (N320, N375, N393) were prepared by PCR amplification of the appropriate domains using caspase-resistant GRASP65–GFP as a template (hence all caspase sites within these constructs are mutated (D to A);11). These were then colour switched with mCherry (as described above). Nuclear-targeted GRASP65 constructs were prepared by inserting a tripartite nuclear localisation signal (see Moss et al.38) upstream of the C-terminal GRASP65 caspase-cleaved products in pEGFP (ΔN320, ΔN375, ΔN393–NLS–GFP). GFP- and myc-tagged GRASP65 C-terminal caspase products were prepared by PCR amplification and insertion into pEGFP and pcDNA 3.1 myc/his (Invitrogen, Paisley, UK), respectively. The GFP-Fas construct was prepared by insertion of GFP at an internal BamHI site upstream of the transmembrane domain of human CD95/Fas. GST-C-tail fusions of GM130 and Fas were prepared by inserting annealed oligonucleotides encoding the relevant coding regions downstream of the GST open reading frame.15 Cytokeratin 8-GFP was a gift from Professor Viki Allan (Manchester, UK). mCherry–Rab7a and Rab9a were gifts from Professor Pete Cullen (Bristol, UK). All constructs were verified by sequencing. Transient transfections were carried out using Fugene 6 (Roche, Lewes, UK) or GeneJuice (Novagen, Nottingham, UK) according to the manufacturers' instructions.
Fluorescence microscopy and live cell imaging
Wide-field fluorescence images were obtained using an Olympus (Southend-on-Sea, UK) IX-71 inverted microscope (60 × Uplan Fluorite objective 0.65–1.25 NA, at maximum aperture) fitted with a CoolSNAP HQ CCD camera (Photometrics, Tucson, AZ, USA) driven by MetaMorph software (Universal Imaging Corporation, Downington, PA, USA). Confocal images were obtained using a Leica (Milton Keynes, UK) AOBS SP2 microscope (63 × PLAPO objective 1.4 NA) at 0.2 μm z-steps. For immunofluorescence, cells were fixed in 2% formaldehyde (methanol free, EM grade; TAAB, Aldermaston, UK) followed by permeabilisation with 0.1% Triton X-100, or in −20°C methanol. Cells were routinely stained with DAPI and mounted in mowiol containing 25 mg/ml DABCO anti-fade.
Live-cell imaging was carried out using the Olympus IX-71 system. Halogen lamp illumination was used for both transmitted light and for epifluorescence to extend cell viability (e.g., Moss et al.38). Cells were maintained in CO2-independent DMEM (Invitrogen), at 37°C in 3 cm cell imaging dishes (MatTek Co, Ashland, MA, USA). Calculations of the intensity of Golgi-associated GRASP65 signal was carried out by image thresholding GRASP65-GFP fluorescence using MetaMorph software. Fluorescence levels were then plotted as a function of the maximal Golgi fluorescence value (pre-cell rounding) from the thresholded image data. FRAP investigations of Golgi enzyme lateral mobility were carried out in cells stably expressing wild-type or N320 GRASP65–mCherry, transiently transfected with NA–GFP, using a Leica AOBS SP2 confocal microscope (as described in Moss et al.37).
siRNA-mediated silencing
For siRNA silencing, the following oligonucleotides were used: for GRASP65, G651 – AGGCACUACUGAAAGCCAAAUTT and UTR1 – GGAAUGCAGCAAGUAGAACAGAAUCGC directed at the translated and untranslated regions of GRASP65, respectively; for Bid, GAAGACAUCAUCCGGAAUATT and GAAUAGAGGCAGAUUCUGATT. Oligonucleotides were transfected using Oligofectamine (Invitrogen; manufacturer's instructions) or by calcium phosphate at final concentrations of 100 and 20 nM, respectively. For calcium phosphate transfections, oligonucleotides (100 nM final) were added directly to 250 mM CaCl2, and supplemented with an equal volume of BBS (50 mM BES pH 6.95, 280 mM NaCl, 1.5 mM Na2HPO4). The mixture was incubated at room temperature for 20 min then added drop-wise to ∼30% confluent cells in DMEM. These were incubated overnight in 3% CO2 at 37°C, before washing, addition of standard DMEM and incubation at 5% CO2, 37°C for 72 h.
Antibody array and in vitro caspase assays
The apoptosis antibody array was from Hypromatrix (Worcester, MA, USA), and was used according to the manufacturer's instructions. HRP-tagged anti-GRASP65 was prepared by covalently attaching HRP to the sheep GRASP65 polyclonal according to the manufacturer's instructions (Thermo Scientific). In vitro caspase cleavage of GRASP65 was carried out using 35S-labelled, in vitro translated GRASP65 and recombinant caspase-3 and caspase-8 (Alexis Biochemicals) as described in Lane et al.11 Products were incubated in caspase buffer (0.1% CHAPS, 10% sucrose, 5 mM DTT, 2 mM EDTA, 50 mM Hepes, pH 7.4) for 90 min at 30°C and run on SDS-PAGE gels for autoradiography.
Acknowledgments
We thank the various colleagues who have provided us with reagents and feedback for this research, particularly Professors David Stephens and Pete Cullen. We are grateful to the University of Bristol, MRC and Wolfson Foundation for providing generous support to establish and develop the Wolfson Bioimaging Facility. This work was supported by a Wellcome Trust Research Career Development Fellowship to JDL (no. 067358), a Wellcome Trust Project Grant (no. 074208) and a BBSRC studentship (to JPXC). HW was supported by a Nuffield student summer bursary (ref: URB/34325).
Glossary
- GRASP65
Golgi reassembly and stacking protein of 65 kDa
- TNT
tumour necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- GFP
green fluorescent protein
- BFA
Brefeldin A
- TGN
trans-Golgi network
- HMGB1
high mobility group box 1
- CHX
cycloheximide
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Supplementary Material
References
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Betin VMS, Lane JD. The cytoskeleton and the control of organelle dynamics in the apoptotic execution phase. SEB Symp Series. 2007;59:267–289. [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Hicks SW, Machamer CE. Golgi structure in stress sensing and apoptosis. Biochim Biophys Acta. 2005;1744:406–414. doi: 10.1016/j.bbamcr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Maag RS, Hicks SW, Machamer CE. Death from within: apoptosis and the secretory pathway. Curr Opin Cell Biol. 2003;15:456–461. doi: 10.1016/s0955-0674(03)00075-9. [DOI] [PubMed] [Google Scholar]
- Lane JD, Allan VJ, Woodman PG. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J Cell Sci. 2005;118 (Part 17:4059–4071. doi: 10.1242/jcs.02529. [DOI] [PubMed] [Google Scholar]
- Cosulich SC, Horiuchi H, Zerial M, Clarke PR, Woodman PG. Cleavage of rabaptin-5 blocks endosome fusion during apoptosis. EMBO J. 1997;16:6182–6191. doi: 10.1093/emboj/16.20.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M, Lane JD, Woodman PG, Allan VJ. Caspase-mediated cleavage of syntaxin 5 and giantin accompanies inhibition of secretory traffic during apoptosis. J Cell Sci. 2004;117 (Part 7:1139–1150. doi: 10.1242/jcs.00950. [DOI] [PubMed] [Google Scholar]
- Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, et al. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol. 2000;149:603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R, Novikov L, Mukherjee S, Shields D. A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol. 2002;159:637–648. doi: 10.1083/jcb.200208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ, et al. Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol. 2002;156:495–509. doi: 10.1083/jcb.200110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maag RS, Mancini M, Rosen A, Machamer CE. Caspase-resistant Golgin-160 disrupts apoptosis induced by secretory pathway stress and ligation of death receptors. Mol Biol Cell. 2005;16:3019–3027. doi: 10.1091/mbc.E04-11-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8:238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- Barr FA, Preisinger C, Kopajtich R, Korner R. Golgi matrix proteins interact with p24 cargo receptors and aid their efficient retention in the Golgi apparatus. J Cell Biol. 2001;155:885–891. doi: 10.1083/jcb.200108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G, Prencipe L, Iodice L, Beznoussenko G, Savarese M, Marra P, et al. GRASP65 and GRASP55 sequentially promote the transport of C-terminal valine bearing cargoes to and through the golgi complex. J Biol Chem. 2009;284:34849–34860. doi: 10.1074/jbc.M109.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Zhong C, Lane WS, Derynck R. Transmembrane transforming growth factor-alpha tethers to the PDZ domain-containing, Golgi membrane-associated protein p59/GRASP55. Embo J. 2000;19:6427–6439. doi: 10.1093/emboj/19.23.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Yoshioka K, Barr FA, Lowe M, Nakayama K, Ohkuma S, et al. Convergence of cell cycle regulation and growth factor signals on GRASP65. J Biol Chem. 2005;280:23048–23056. doi: 10.1074/jbc.M502442200. [DOI] [PubMed] [Google Scholar]
- Bisel B, Wang Y, Wei JH, Xiang Y, Tang D, Miron-Mendoza M, et al. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol. 2008;182:837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger C, Korner R, Wind M, Lehmann WD, Kopajtich R, Barr FA. Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling. EMBO J. 2005;24:753–765. doi: 10.1038/sj.emboj.7600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Madsen ML, Yarm FR, Jang YJ, Liu X, Erikson RL. Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2. Proc Natl Acad Sci USA. 2000;97:12589–12594. doi: 10.1073/pnas.220423497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C, Hsu P, Mallabiabarrena A, Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 2002;109:359–369. doi: 10.1016/s0092-8674(02)00720-1. [DOI] [PubMed] [Google Scholar]
- Sutterlin C, Polishchuk R, Pecot M, Malhotra V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol Biol Cell. 2005;16:3211–3222. doi: 10.1091/mbc.E04-12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Seemann J, Pypaert M, Shorter J, Warren G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 2003;22:3279–3290. doi: 10.1093/emboj/cdg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M, Barr FA. Inheritance and biogenesis of organelles in the secretory pathway. Nat Rev Mol Cell Biol. 2007;8:429–439. doi: 10.1038/nrm2179. [DOI] [PubMed] [Google Scholar]
- Walker A, Ward C, Sheldrake TA, Dransfield I, Rossi AG, Pryde JG, et al. Golgi fragmentation during Fas-mediated apoptosis is associated with the rapid loss of GM130. Biochem Biophys Res Commun. 2004;316:6–11. doi: 10.1016/j.bbrc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Hicks SW, Machamer CE. The NH2-terminal domain of Golgin-160 contains both Golgi and nuclear targeting information. J Biol Chem. 2002;277:35833–35839. doi: 10.1074/jbc.M206280200. [DOI] [PubMed] [Google Scholar]
- Sbodio JI, Hicks SW, Simon D, Machamer CE. GCP60 preferentially interacts with a caspase-generated Golgin-160 fragment. J Biol Chem. 2006;281:27924–27931. doi: 10.1074/jbc.M603276200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Satoh A, Warren G. Mapping the functional domains of the Golgi stacking factor GRASP65. J Biol Chem. 2005;280:4921–4928. doi: 10.1074/jbc.M412407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler OT, Peter ME, Hinderlich S, Moldenhauer G, Stehling P, Schmitz I, et al. Differential sialylation of cell surface glycoconjugates in a human B lymphoma cell line regulates susceptibility for CD95 (APO-1/Fas)-mediated apoptosis and for infection by a lymphotropic virus. Glycobiology. 1999;9:557–569. doi: 10.1093/glycob/9.6.557. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188:237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- Ivanov VN, Lopez Bergami P, Maulit G, Sato TA, Sassoon D, Ronai Z. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–3635. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang J, Kim HP, Wang Y, Choi AM, Ryter SW. Bcl-XL disrupts death-inducing signal complex formation in plasma membrane induced by hypoxia/reoxygenation. Faseb J. 2004;18:1826–1833. doi: 10.1096/fj.04-2047com. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L, Boulon S, Lam YW, Urcia R, Boisvert FM, Vandermoere F, et al. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. J Cell Biol. 2008;183:223–239. doi: 10.1083/jcb.200805092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DK, Betin VM, Malesinski SD, Lane JD. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci. 2006;119 (Part 11:2362–2374. doi: 10.1242/jcs.02959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DK, Wilde A, Lane JD. Dynamic release of nuclear RanGTP triggers TPX2-dependent microtubule assembly during the apoptotic execution phase. J Cell Sci. 2009;122:656–666. doi: 10.1242/jcs.037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Marra P, Maffucci T, Daniele T, Tullio GD, Ikehara Y, Chan EK, et al. The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat Cell Biol. 2001;3:1101–1113. doi: 10.1038/ncb1201-1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.