Abstract
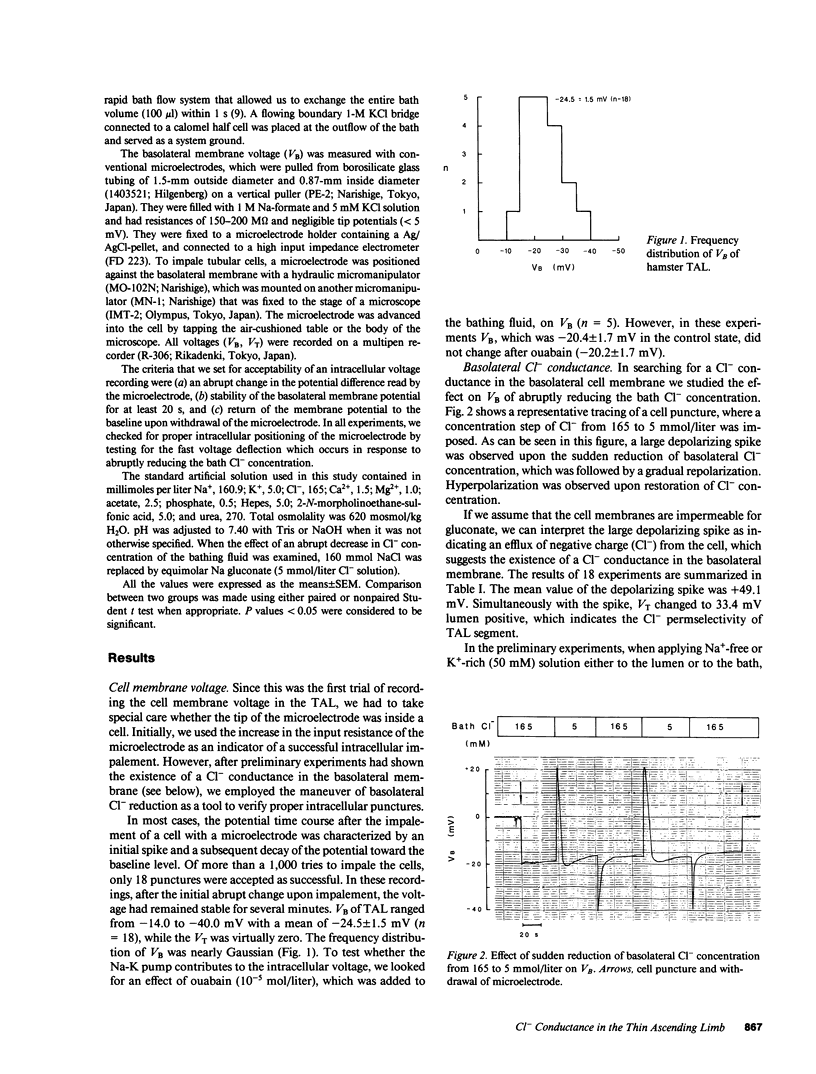
To examine whether Cl- is transported via transcellular pathways in the thin ascending limb of Henle's loop (TAL), conventional microelectrode technique was applied in isolated TAL segments of hamsters perfused in vitro. The average basolateral membrane voltage (VB) was -24.5 +/- 1.5 mV (n = 18). Ouabain (10(-4) M) had no effect on VB. Sudden reduction of basolateral Cl- concentration from 165 to 5 mmol/liter caused a large depolarizing spike (+49.1 +/- 2.7 mV, n = 18), while the transepithelial potential (VT) showed lumen positive deflection by 33.4 +/- 1.2 mV, which indicates that a large Cl- conductance exists in the basolateral membrane. Reduction of luminal Cl- concentration caused sustained depolarization of luminal cell membrane from +24.5 +/- 2.1 to -9.7 +/- 3.4 mV (n = 6), which indicates that there is also a Cl- conductance in the luminal membrane. Since we have previously shown that acidification of ambient solution suppresses the transmural Cl- permeability, we tested whether acid pH also inhibits the Cl- conductance of the basolateral membrane. When pH of the bathing fluid was lowered to 5.8, the depolarizing spike of VB and the change of VT upon sudden reduction of basolateral Cl- were almost completely abolished. From these results we conclude: (a) both the luminal and the basolateral membrane of hamster TAL segments have Cl- conductances, and (b) Cl- transport in the TAL takes place, at least in part, via a transcellular route when a transepithelial Cl- gradient is present.
Full text
PDF
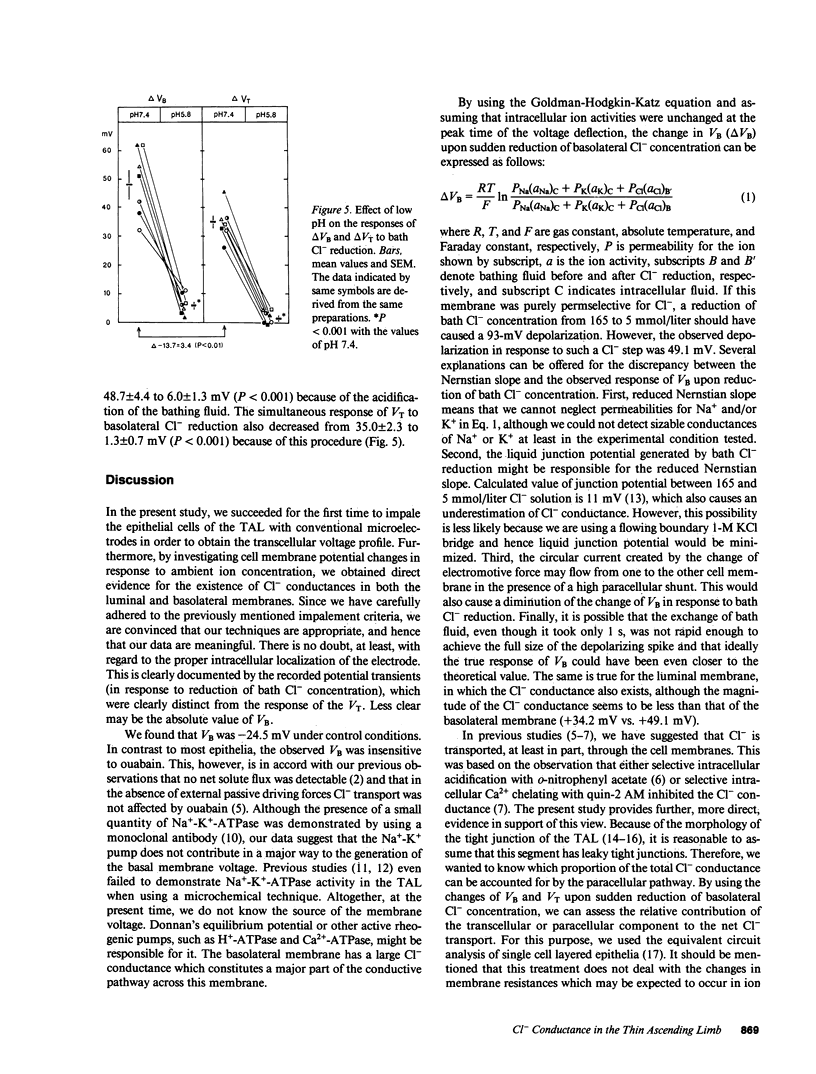


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann S., Kriz W. Histotopography and ultrastructure of the thin limbs of the loop of Henle in the hamster. Cell Tissue Res. 1982;225(1):111–127. doi: 10.1007/BF00216222. [DOI] [PubMed] [Google Scholar]
- Barrett J. M., Kriz W., Kaissling B., de Rouffignac C. The ultrastructure of the nephrons of the desert rodent (Psammomys obesus) kidney. II. Thin limbs of Henle of long-looped nephrons. Am J Anat. 1978 Apr;151(4):499–514. doi: 10.1002/aja.1001510405. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L., Sackin H. Equivalent electrical circuit analysis and rheogenic pumps in epithelia. Fed Proc. 1979 May;38(6):2030–2036. [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Frömter E. The electrophysiological analysis of tubular transport. Kidney Int. 1986 Aug;30(2):216–228. doi: 10.1038/ki.1986.174. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Knepper M. A., Burg M. B. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol. 1981 Jun;240(6):F536–F544. doi: 10.1152/ajprenal.1981.240.6.F536. [DOI] [PubMed] [Google Scholar]
- Gelbart D. R., Battilana C. A., Bhattacharya J., Lacy F. B., Jamison R. L. Transepithelial gradient and fractional delivery of chloride in thin loop of Henle. Am J Physiol. 1978 Sep;235(3):F192–F198. doi: 10.1152/ajprenal.1978.235.3.F192. [DOI] [PubMed] [Google Scholar]
- Hogg R. J., Kokko J. P. Comparison between the electrical potential profile and the chloride gradients in the thin limbs of Henle's loop in rats. Kidney Int. 1978 Nov;14(5):428–436. doi: 10.1038/ki.1978.147. [DOI] [PubMed] [Google Scholar]
- Imai M. Function of the thin ascending limb of Henle of rats and hamsters perfused in vitro. Am J Physiol. 1977 Mar;232(3):F201–F209. doi: 10.1152/ajprenal.1977.232.3.F201. [DOI] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest. 1974 Feb;53(2):393–402. doi: 10.1172/JCI107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Taniguchi J., Tabei K. Function of thin loops of Henle. Kidney Int. 1987 Feb;31(2):565–579. doi: 10.1038/ki.1987.37. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Doucet A., Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979 Aug;237(2):F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Yoshitomi K., Imai M. Effect of Ca2+ on Cl- transport in thin ascending limb of Henle's loop. Am J Physiol. 1988 Feb;254(2 Pt 2):F232–F239. doi: 10.1152/ajprenal.1988.254.2.F232. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Yoshitomi K., Imai M. Effect of pH on Cl- transport in TAL of Henle's loop. Am J Physiol. 1987 Dec;253(6 Pt 2):F1216–F1222. doi: 10.1152/ajprenal.1987.253.6.F1216. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Yoshitomi K., Imai M. Effects of anion transport inhibitors and ion substitution on Cl- transport in TAL of Henle's loop. Am J Physiol. 1987 Dec;253(6 Pt 2):F1206–F1215. doi: 10.1152/ajprenal.1987.253.6.F1206. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. M., Venkatachalam M. A. Structural differences in thin limbs of Henle: physiological implications. Kidney Int. 1974 Oct;6(4):193–208. doi: 10.1038/ki.1974.101. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Koseki C., Taniguchi J., Imai M. Functional heterogeneity in the hamster medullary thick ascending limb of Henle's loop. Pflugers Arch. 1987 May;408(6):600–608. doi: 10.1007/BF00581162. [DOI] [PubMed] [Google Scholar]


