Abstract
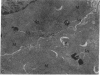
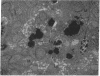
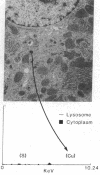
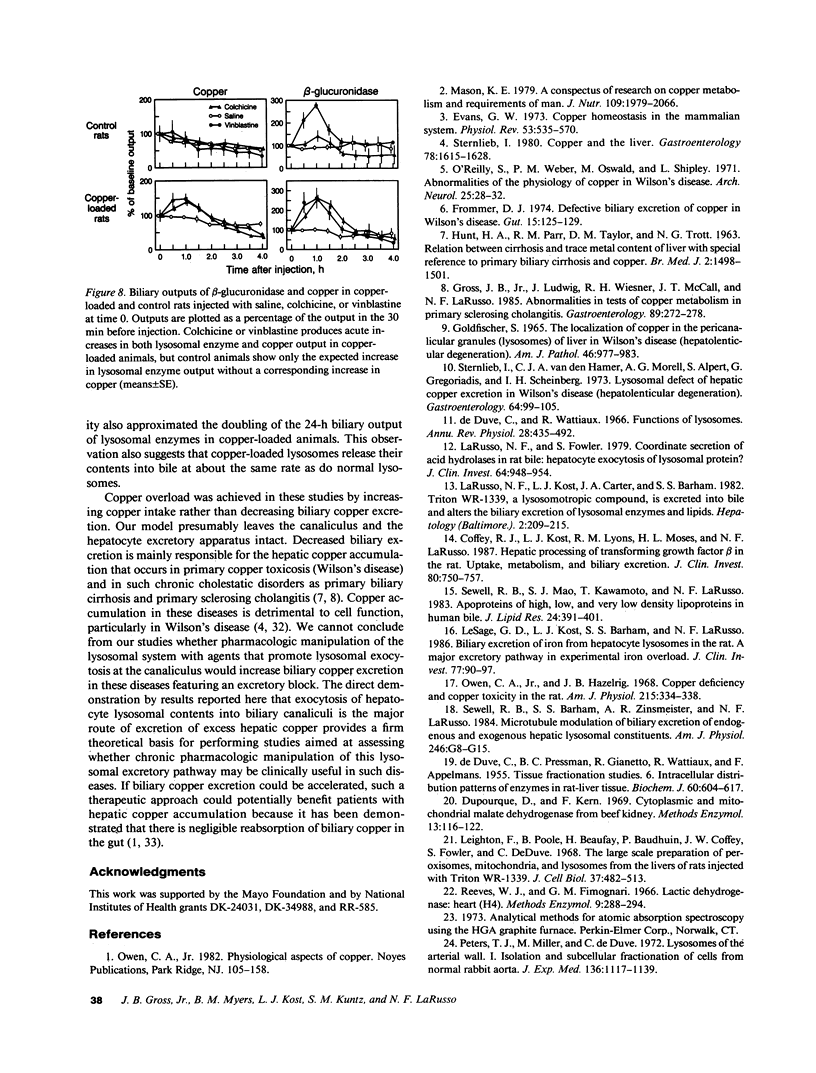
We investigated the hypothesis that lysosomes are the main source of biliary copper in conditions of hepatic copper overload. We used a rat model of oral copper loading and studied the relationship between the biliary output of copper and lysosomal hydrolases. Male Sprague-Dawley rats were given tap water with or without 0.125% copper acetate for up to 36 wk. Copper loading produced a 23-fold increase in the hepatic copper concentration and a 30-65% increase in hepatic lysosomal enzyme activity. Acid phosphatase histochemistry showed that copper-loaded livers contained an increased number of hepatocyte lysosomes; increased copper concentration of these organelles was confirmed directly by both x ray microanalysis and tissue fractionation. The copper-loaded rats showed a 16-fold increase in biliary copper output and a 50-300% increase in biliary lysosomal enzyme output. In the basal state, excretory profiles over time were similar for biliary outputs of lysosomal enzymes and copper in the copper-loaded animals but not in controls. After pharmacologic stimulation of lysosomal exocytosis, biliary outputs of copper and lysosomal hydrolases in the copper-loaded animals remained coupled: injection of colchicine or vinblastine produced an acute rise in the biliary output of both lysosomal enzymes and copper to 150-250% of baseline rates. After these same drugs, control animals showed only the expected increase in lysosomal enzyme output without a corresponding increase in copper output. We conclude that the hepatocyte responds to an increased copper load by sequestering excess copper in an increased number of lysosomes that then empty their contents directly into bile. The results provide direct evidence that exocytosis of lysosomal contents into biliary canaliculi is the major mechanism for biliary copper excretion in hepatic copper overload.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKA T., SCHEUER P. J., SCHAFFNER F., POPPER H. STRUCTURAL CHANGES OF LIVER CELLS IN COPPER INTOXICATION. Arch Pathol. 1964 Oct;78:331–349. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Kost L. J., Lyons R. M., Moses H. L., LaRusso N. F. Hepatic processing of transforming growth factor beta in the rat. Uptake, metabolism, and biliary excretion. J Clin Invest. 1987 Sep;80(3):750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Evans G. W. Copper homeostasis in the mammalian system. Physiol Rev. 1973 Jul;53(3):535–570. doi: 10.1152/physrev.1973.53.3.535. [DOI] [PubMed] [Google Scholar]
- Farrer P., Mistilis S. P. Absorption of exogenous and endogenous biliary copper in the rat. Nature. 1967 Jan 21;213(5073):291–292. doi: 10.1038/213291b0. [DOI] [PubMed] [Google Scholar]
- Frommer D. J. Defective biliary excretion of copper in Wilson's disease. Gut. 1974 Feb;15(2):125–129. doi: 10.1136/gut.15.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
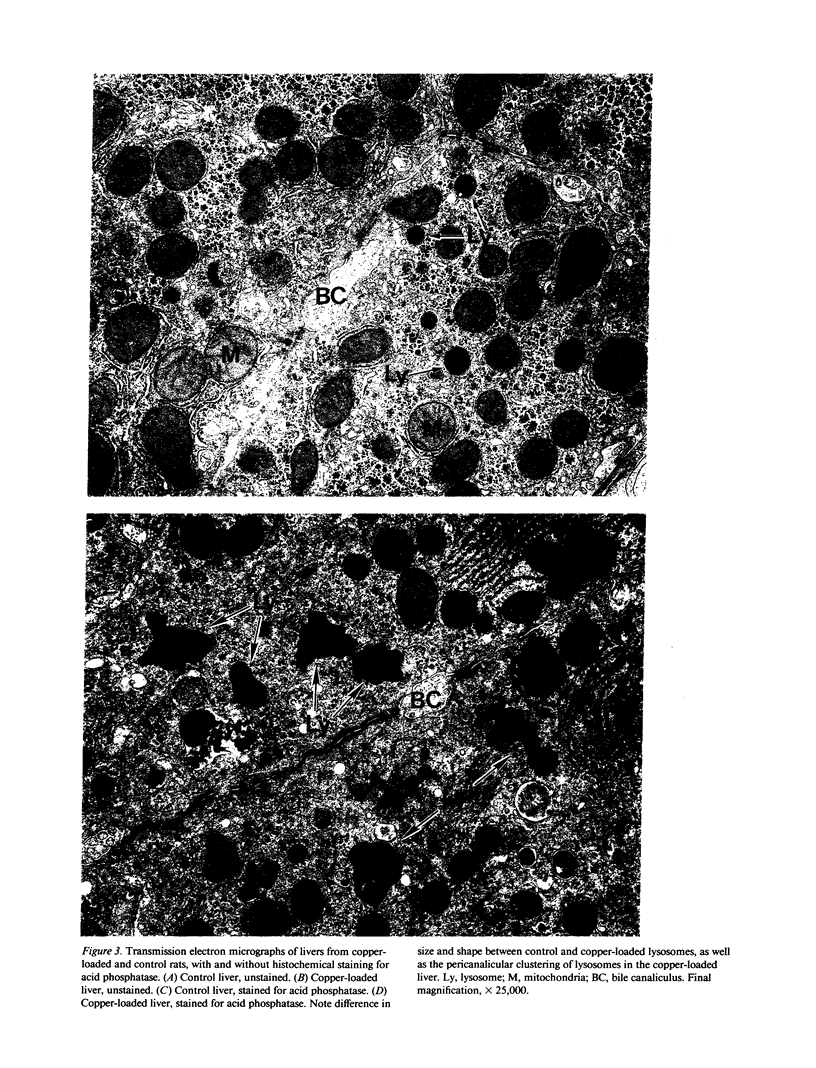
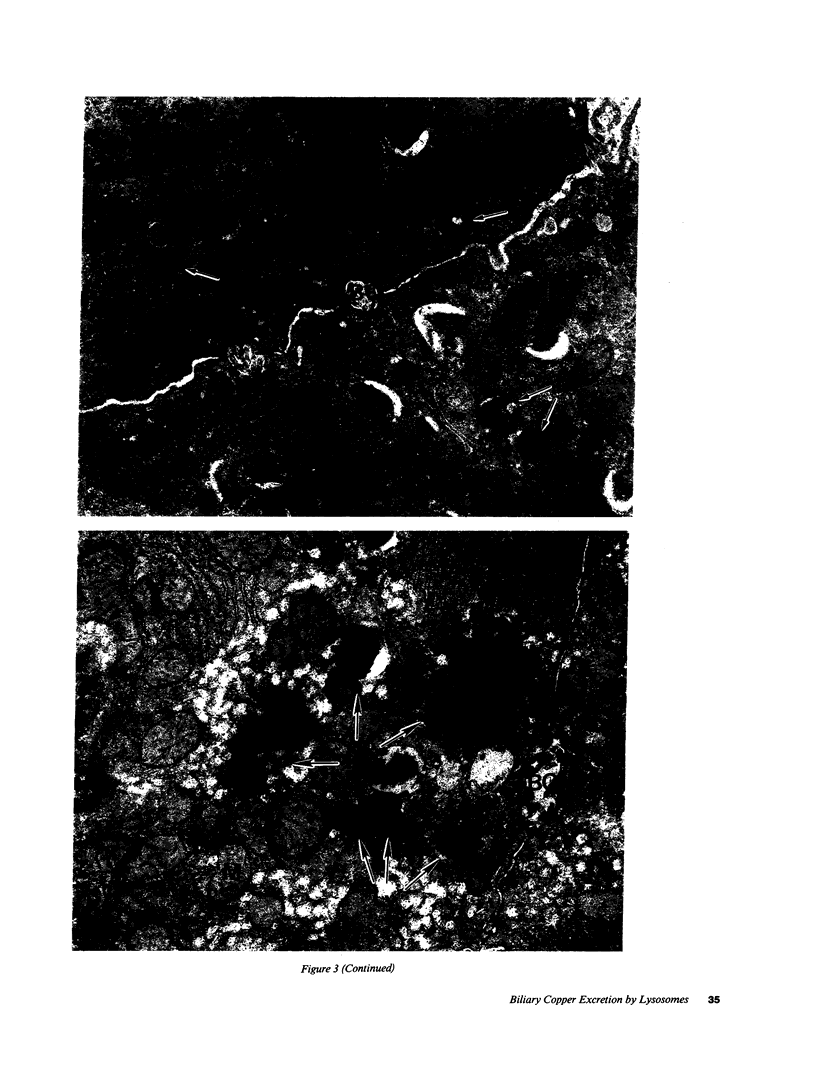
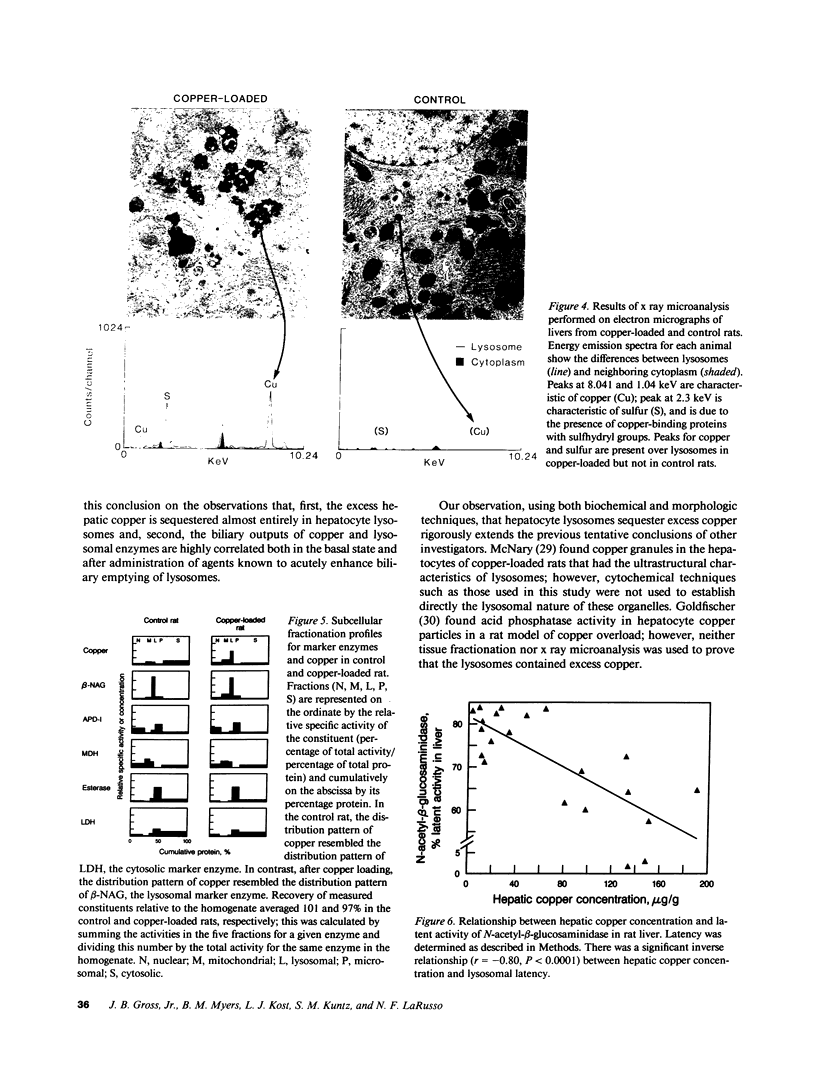
- GOLDFISCHER S. THE LOCALIZATION OF COPPER IN THE PERICANALICULAR GRANULES (LYSOSOMES) OF LIVER IN WILSON'S DISEASE (HEPATOLENTICULAR DEGENERATION) . Am J Pathol. 1965 Jun;46:977–983. [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S. Demonstration of copper and acid phosphatase activity in hepatocyte lysosomes in experimental copper toxicity. Nature. 1967 Jul 1;215(5096):74–75. doi: 10.1038/215074a0. [DOI] [PubMed] [Google Scholar]
- Gross J. B., Jr, Ludwig J., Wiesner R. H., McCall J. T., LaRusso N. F. Abnormalities in tests of copper metabolism in primary sclerosing cholangitis. Gastroenterology. 1985 Aug;89(2):272–278. doi: 10.1016/0016-5085(85)90326-9. [DOI] [PubMed] [Google Scholar]
- HUNT A. H., PARR R. M., TAYLOR D. M., TROTT N. G. RELATION BETWEEN CIRRHOSIS AND TRACE METAL CONTENT OF LIVER WITH SPECIAL REFERENCE TO PRIMARY BILIARY CIRRHOSIS AND COPPER. Br Med J. 1963 Dec 14;2(5371):1498–1501. doi: 10.1136/bmj.2.5371.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrig J. B., Owen C. A., Jr, Ackerman E. A mathematical model for copper metabolism and its relation to Wilson's disease. Am J Physiol. 1966 Nov;211(5):1075–1081. doi: 10.1152/ajplegacy.1966.211.5.1075. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
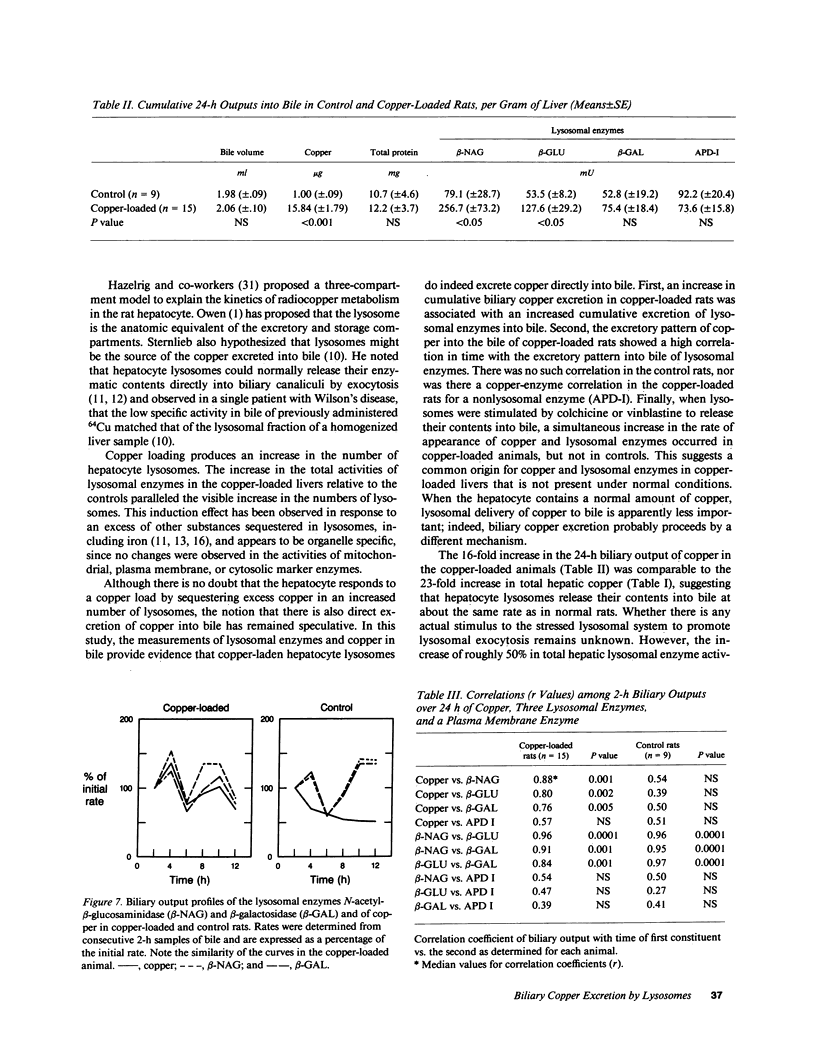
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRusso N. F., Kost L. J., Carter J. A., Barham S. S. Triton WR-1339, a lysosomotropic compound, is excreted into bile and alters the biliary excretion of lysosomal enzymes and lipids. Hepatology. 1982 Mar-Apr;2(2):209–215. doi: 10.1002/hep.1840020204. [DOI] [PubMed] [Google Scholar]
- LeSage G. D., Kost L. J., Barham S. S., LaRusso N. F. Biliary excretion of iron from hepatocyte lysosomes in the rat. A major excretory pathway in experimental iron overload. J Clin Invest. 1986 Jan;77(1):90–97. doi: 10.1172/JCI112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger P. M., Novikoff A. B. Studies on microperoxisomes. VII. Pigment epithelial cells and other cell types in the retina of rodents. J Cell Biol. 1975 May;65(2):324–334. doi: 10.1083/jcb.65.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNARY W. F., Jr THE INTRAHEPATIC AND INTRACELLULAR DISTRIBUTION OF COPPER FOLLOWING CHRONIC ADMINISTRATION OF THE METAL IN THE DIET. Anat Rec. 1963 Jul;146:193–199. doi: 10.1002/ar.1091460303. [DOI] [PubMed] [Google Scholar]
- Mason K. E. A conspectus of research on copper metabolism and requirements of man. J Nutr. 1979 Nov;109(11):1979–2066. doi: 10.1093/jn/109.11.1979. [DOI] [PubMed] [Google Scholar]
- O'Reilly S., Weber P. M., Oswald M., Shipley L. Abnormalities of the physiology of copper in Wilson's disease. 3. The excretion of copper. Arch Neurol. 1971 Jul;25(1):28–32. doi: 10.1001/archneur.1971.00490010038005. [DOI] [PubMed] [Google Scholar]
- Owen C. A., Jr, Hazelrig J. B. Copper deficiency and copper toxicity in the rat. Am J Physiol. 1968 Aug;215(2):334–338. doi: 10.1152/ajplegacy.1968.215.2.334. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell R. B., Barham S. S., Zinsmeister A. R., LaRusso N. F. Microtubule modulation of biliary excretion of endogenous and exogenous hepatic lysosomal constituents. Am J Physiol. 1984 Jan;246(1 Pt 1):G8–15. doi: 10.1152/ajpgi.1984.246.1.G8. [DOI] [PubMed] [Google Scholar]
- Sewell R. B., Mao S. J., Kawamoto T., LaRusso N. F. Apolipoproteins of high, low, and very low density lipoproteins in human bile. J Lipid Res. 1983 Apr;24(4):391–401. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sternlieb I. Copper and the liver. Gastroenterology. 1980 Jun;78(6):1615–1628. [PubMed] [Google Scholar]
- Sternlieb I., Van den Hamer C. J., Morell A. G., Alpert S., Gregoriadis G., Scheinberg I. H. Lysosomal defect of hepatic copper excretion in Wilson's disease (hepatolenticular degeneration). Gastroenterology. 1973 Jan;64(1):99–105. [PubMed] [Google Scholar]