Abstract
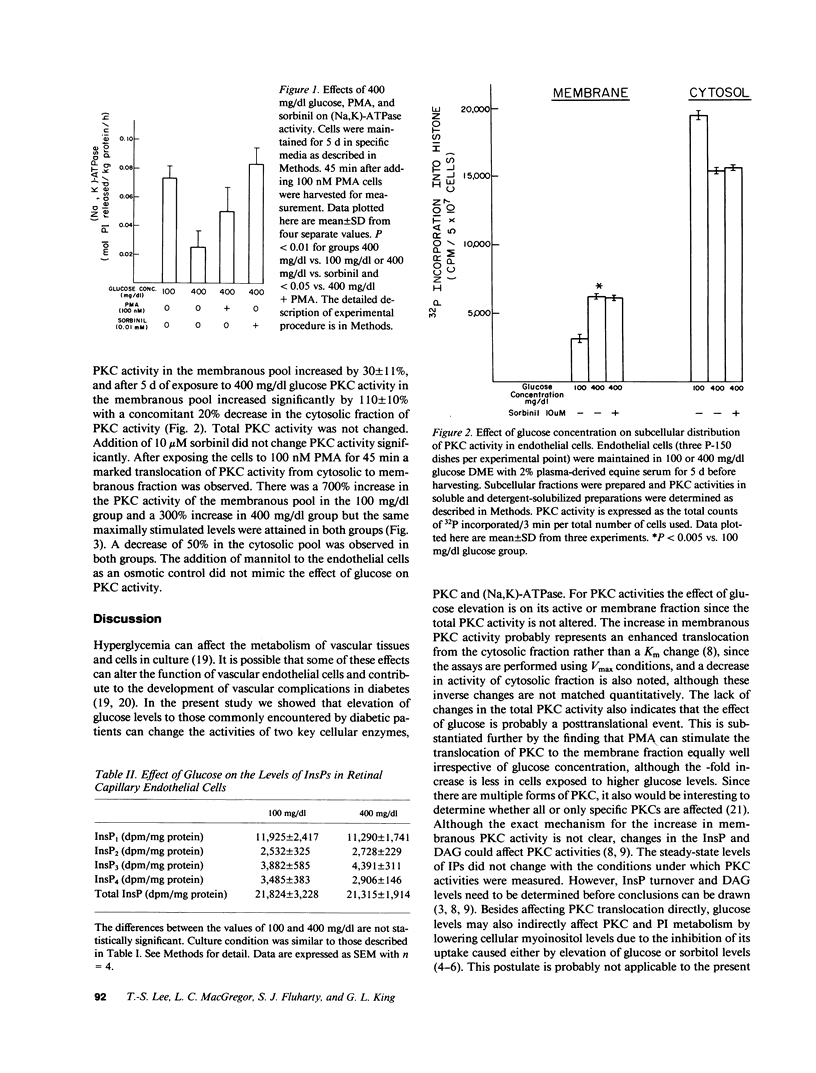
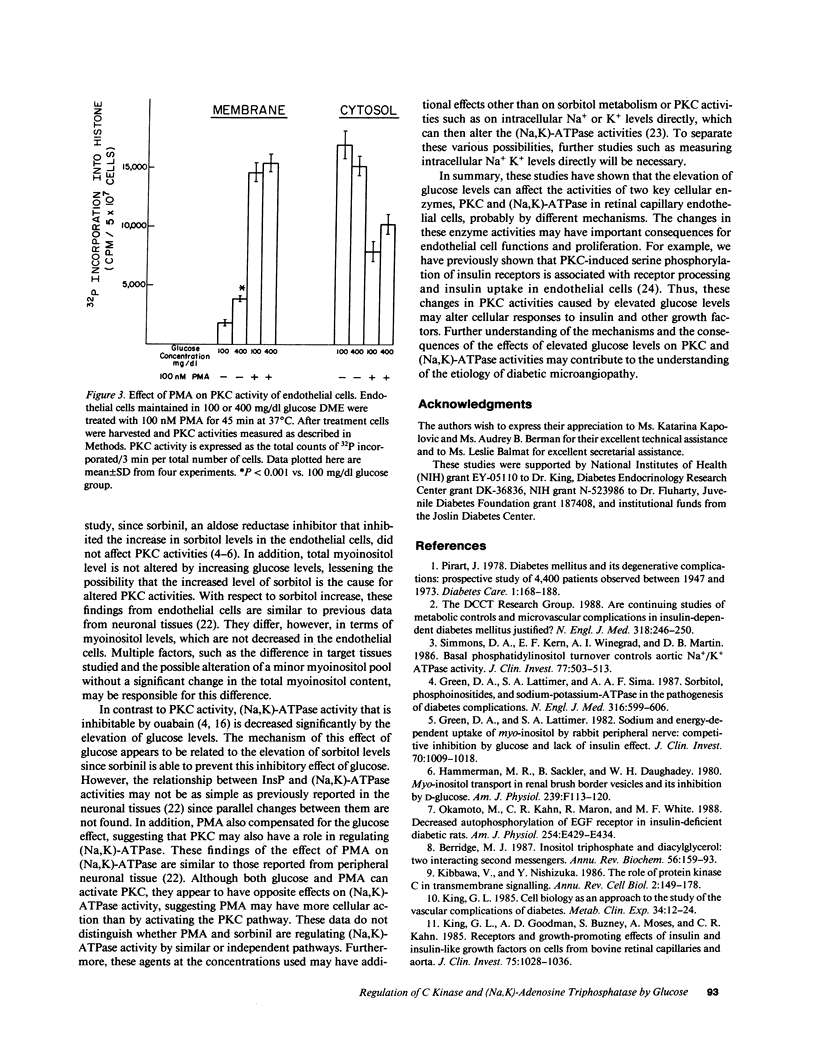
Elevated cellular sorbitol levels resulting from conversion of increased glucose by aldose reductase might deplete cellular myoinositol content, which could then lower inositol phosphates (InsPs) and diacylglycerol levels, key regulators of protein kinase C (PKC). Secondary to altered PKC activity, other cellular enzymes such as (Na,K)-ATPase could be affected. To test this hypothesis we examined the association between PKC activity, (Na,K)-ATPase activity, and sorbitol, myoinositol, and InsP levels in cultured bovine retinal capillary endothelial cells, a cell type prominently involved in diabetic retinopathy. Elevating glucose concentration in culture media from 100 to 400 mg/dl led to a 100% increase in sorbitol levels, which could be inhibited completely by sorbinil, an aldose reductase inhibitor. In contrast, no changes were observed in myoinositol or InsP levels. Subfractionated PKC activities showed a 100% increase in the membranous pool with a parallel decrease in the cytosolic fraction. Adding sorbinil did not affect PKC activity, whereas the PKC agonist, phorbol myristate acetate (PMA), stimulated translocation of PKC. Ouabain-inhibitable (Na,K)-ATPase activity was decreased 70% by elevated glucose levels. This decrease could be prevented by adding either PMA or sorbinil. Thus, in retinal capillary endothelial cells elevated glucose concentration can affect PKC and (Na,K)-ATPase activities, probably via different mechanisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Protein kinase C agonists acutely normalize decreased ouabain-inhibitable respiration in diabetic rabbit nerve. Implications for (Na,K)-ATPase regulation and diabetic complications. Diabetes. 1986 Feb;35(2):242–245. doi: 10.2337/diab.35.2.242. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya H. L., Takayama S., White M. F., King G. L. Regulation of insulin receptor internalization in vascular endothelial cells by insulin and phorbol ester. J Biol Chem. 1987 May 5;262(13):6417–6424. [PubMed] [Google Scholar]
- Harris R. C., Seifter J. L., Lechene C. Coupling of Na-H exchange and Na-K pump activity in cultured rat proximal tubule cells. Am J Physiol. 1986 Nov;251(5 Pt 1):C815–C824. doi: 10.1152/ajpcell.1986.251.5.C815. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Ono Y., Ogita K., Fujii T., Asaoka Y., Sekiguchi K., Kosaka Y., Igarashi K., Nishizuka Y. Identification of the structures of multiple subspecies of protein kinase C expressed in rat brain. FEBS Lett. 1987 Jun 15;217(2):227–231. doi: 10.1016/0014-5793(87)80668-3. [DOI] [PubMed] [Google Scholar]
- King G. L., Goodman A. D., Buzney S., Moses A., Kahn C. R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. 1985 Mar;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li W., Shen S., Khatami M., Rockey J. H. Stimulation of retinal capillary pericyte protein and collagen synthesis in culture by high-glucose concentration. Diabetes. 1984 Aug;33(8):785–789. doi: 10.2337/diab.33.8.785. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Montisano D. F., Toledo S., Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):322–325. doi: 10.1172/JCI112295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. Altered retinal metabolism in diabetes. II. Measurement of sodium-potassium ATPase and total sodium and potassium in individual retinal layers. J Biol Chem. 1986 Mar 25;261(9):4052–4058. [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. An enzymatic fluorimetric assay for myo-inositol. Anal Biochem. 1984 Sep;141(2):382–389. doi: 10.1016/0003-2697(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Malone J. I., Knox G., Benford S., Tedesco T. A. Red cell sorbitol: an indicator of diabetic control. Diabetes. 1980 Nov;29(11):861–864. doi: 10.2337/diab.29.11.861. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Kahn C. R., Maron R., White M. F. Decreased autophosphorylation of EGF receptor in insulin-deficient diabetic rats. Am J Physiol. 1988 Apr;254(4 Pt 1):E429–E434. doi: 10.1152/ajpendo.1988.254.4.E429. [DOI] [PubMed] [Google Scholar]
- Simmons D. A., Kern E. F., Winegrad A. I., Martin D. B. Basal phosphatidylinositol turnover controls aortic Na+/K+ ATPase activity. J Clin Invest. 1986 Feb;77(2):503–513. doi: 10.1172/JCI112330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]



