Abstract
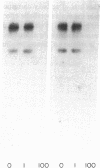
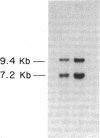
Insulin actions and receptors were studied in capillary endothelial cells cultured from diabetic BB rats and their nondiabetic colony mates. The endothelial cells from diabetic rats of 2 mo duration had persistent biological and biochemical defects in culture. Compared with normal rats, endothelial cells from diabetic rats grew 44% more slowly. Binding studies of insulin and insulin-like growth factor I (IGF-I) showed that cells from diabetic rats had 50% decrease of insulin receptor binding (nondiabetic: 4.6 +/- 0.7; diabetic: 2.6 +/- 0.4% per milligram protein, P less than 0.01), which was caused by a 50% decrease in the number of binding sites per milligram protein, whereas IGF-I binding was not changed. Insulin stimulation of 2-deoxy-glucose uptake and alpha-aminoisobutyric acid uptake were also severely impaired with a 80-90% decrease in maximal stimulation, in parallel with a 62% decrease in insulin-stimulated autophosphorylation (P less than 0.05). 125I-insulin cross-linking revealed an 140-kD alpha subunit of the insulin receptor similar to that in cells from nondiabetic rats, although bands at greater than 200 kD were also detected. The molecular weight of the insulin receptor beta subunit (by SDS-PAGE) was smaller in cells from diabetic than from normal rats (88-90 vs. 95 kD). Neuraminadase treatment of the partially purified insulin receptors decreased the molecular weight of the insulin receptors from nondiabetic rats to a greater degree than its diabetic counterpart. In contrast, Northern blot analysis of insulin receptor mRNAs using human cDNA probes revealed two species of 9.4 and 7.2 kb with no difference in mRNA abundance between cells from diabetic and nondiabetic rats. We conclude that the exposure of capillary endothelial cells to a diabetic milieu in vivo can cause specific and persistent changes in the insulin receptor and insulin action.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banskota N. K., Carpentier J. L., King G. L. Processing and release of insulin and insulin-like growth factor I by macro- and microvascular endothelial cells. Endocrinology. 1986 Nov;119(5):1904–1913. doi: 10.1210/endo-119-5-1904. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Frank H. J., Karasic D., Capdeville M. Lipoprotein-induced insulin resistance in aortic endothelium. Diabetes. 1984 Nov;33(11):1039–1044. doi: 10.2337/diab.33.11.1039. [DOI] [PubMed] [Google Scholar]
- Bowersox J. C., Sorgente N. Altered growth kinetics of dermal fibroblasts and arterial smooth muscle cells from spontaneously diabetic BB rats. Diabetes. 1985 Jul;34(7):628–633. doi: 10.2337/diab.34.7.628. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Steffes M. W., Thibert P., Azar S., Mauer S. M. Glomerular manifestations of diabetes in the BB rat. Metabolism. 1983 Jul;32(7 Suppl 1):131–135. doi: 10.1016/s0026-0495(83)80026-2. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. P., Surma M. L., Wu V. Y. In vivo biosynthesis and turnover of glomerular basement membrane in diabetic rats. Am J Physiol. 1982 Apr;242(4):F385–F389. doi: 10.1152/ajprenal.1982.242.4.F385. [DOI] [PubMed] [Google Scholar]
- Comi R. J., Grunberger G., Gorden P. Relationship of insulin binding and insulin-stimulated tyrosine kinase activity is altered in type II diabetes. J Clin Invest. 1987 Feb;79(2):453–462. doi: 10.1172/JCI112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkey R. F., Corkey B. E., Gimbrone M. A., Jr Hexose transport in normal and SV40-transformed human endothelial cells in culture. J Cell Physiol. 1981 Mar;106(3):425–434. doi: 10.1002/jcp.1041060312. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Goldstein B. J., Muller-Wieland D., Kahn C. R. Variation in insulin receptor messenger ribonucleic acid expression in human and rodent tissues. Mol Endocrinol. 1987 Nov;1(11):759–766. doi: 10.1210/mend-1-11-759. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Littlefield J. W., Soeldner J. S. Diabetes mellitus and aging: diminished planting efficiency of cultured human fibroblasts. Proc Natl Acad Sci U S A. 1969 Sep;64(1):155–160. doi: 10.1073/pnas.64.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Moerman E. J., Soeldner J. S., Gleason R. E., Barnett D. M. Diabetes mellitus and genetic prediabetes. Decreased replicative capacity of cultured skin fibroblasts. J Clin Invest. 1979 Mar;63(3):358–370. doi: 10.1172/JCI109311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya H. L., Carpentier J. L., King G. L. Comparative studies on insulin-like growth factor II and insulin processing by vascular endothelial cells. Diabetes. 1986 Oct;35(10):1065–1072. doi: 10.2337/diab.35.10.1065. [DOI] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Howard B. V., Fields R. M., Mott D. M., Savage P. J., Nagulesparan M., Bennett P. H. Diabetes and cell growth--lack of differences in growth characteristics of fibroblasts from diabetic and nondiabetic Pima Indians. Diabetes. 1980 Feb;29(2):119–124. doi: 10.2337/diab.29.2.119. [DOI] [PubMed] [Google Scholar]
- Ishibashi F., Hidaka H., Howard B. V. Glucose enhancement of insulin action: elevated glucose levels increase insulin stimulation of 2-deoxyglucose uptake in cultured human fibroblasts. J Clin Endocrinol Metab. 1982 Jan;54(1):34–39. doi: 10.1210/jcem-54-1-34. [DOI] [PubMed] [Google Scholar]
- Jialal I., Crettaz M., Hachiya H. L., Kahn C. R., Moses A. C., Buzney S. M., King G. L. Characterization of the receptors for insulin and the insulin-like growth factors on micro- and macrovascular tissues. Endocrinology. 1985 Sep;117(3):1222–1229. doi: 10.1210/endo-117-3-1222. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Kasuga M., White M. F., Kahn C. R. Phosphorylation of the insulin receptor in cultured hepatoma cells and a solubilized system. Methods Enzymol. 1985;109:609–621. doi: 10.1016/0076-6879(85)09118-2. [DOI] [PubMed] [Google Scholar]
- King G. L., Buzney S. M., Kahn C. R., Hetu N., Buchwald S., Macdonald S. G., Rand L. I. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. J Clin Invest. 1983 Apr;71(4):974–979. doi: 10.1172/JCI110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Goodman A. D., Buzney S., Moses A., Kahn C. R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. 1985 Mar;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Johnson S. M. Receptor-mediated transport of insulin across endothelial cells. Science. 1985 Mar 29;227(4694):1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohner E. M. The problems of retinal blood flow in diabetes. Diabetes. 1976;25(2 Suppl):839–844. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Like A. A., Butler L., Williams R. M., Appel M. C., Weringer E. J., Rossini A. A. Spontaneous autoimmune diabetes mellitus in the BB rat. Diabetes. 1982;31(Suppl 1 Pt 2):7–13. doi: 10.2337/diab.31.1.s7. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Montisano D. F., Toledo S., Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):322–325. doi: 10.1172/JCI112295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. S., Pohl S. L. Insulin-induced insulin resistance of alpha-aminoisobutyric acid transport in cultured human skin fibroblasts. J Biol Chem. 1979 Oct 25;254(20):9976–9978. [PubMed] [Google Scholar]
- Megyesi K., Kahn C. R., Roth J., Neville D. M., Jr, Nissley S. P., Humbel R. E., Froesch E. R. The NSILA-s receptor in liver plasma membranes. Characterization and comparison with the insulin receptor. J Biol Chem. 1975 Dec 10;250(23):8990–8996. [PubMed] [Google Scholar]
- Mogensen C. E. Glomerular filtration rate and renal plasma flow in short-term and long-term juvenile diabetes mellitus. Scand J Clin Lab Invest. 1971 Sep;28(1):91–100. doi: 10.3109/00365517109090667. [DOI] [PubMed] [Google Scholar]
- Okamoto M., White M. F., Maron R., Kahn C. R. Autophosphorylation and kinase activity of insulin receptor in diabetic rats. Am J Physiol. 1986 Nov;251(5 Pt 1):E542–E550. doi: 10.1152/ajpendo.1986.251.5.E542. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Tan G., Fellows R. E. Fibroblastic cultures from the diabetic db/db mouse. Demonstration of decreased insulin receptors and impaired responses to insulin. J Biol Chem. 1980 Oct 10;255(19):9149–9155. [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M., Goldfine I. D., Wells C. A. DNA synthesis in human fibroblasts: stimulation by insulin and by nonsuppressible insulin-like activity (NSILA-S). J Clin Endocrinol Metab. 1974 Sep;39(3):512–521. doi: 10.1210/jcem-39-3-512. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. L., Silverstein J. H., Lezotte D. C., Richardson K., McCallum M. Limited joint mobility in childhood diabetes mellitus indicates increased risk for microvascular disease. N Engl J Med. 1981 Jul 23;305(4):191–194. doi: 10.1056/NEJM198107233050403. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Chakrabarti S., Garcia-Salinas R., Basu P. K. The BB-rat--an authentic model of human diabetic retinopathy. Curr Eye Res. 1985 Oct;4(10):1087–1092. doi: 10.3109/02713688509003353. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Viberti G., Pickup J. C., Bilous R. W., Keen H., Mackintosh D. Correction of exercise-induced microalbuminuria in insulin-dependent diabetics after 3 weeks of subcutaneous insulin infusion. Diabetes. 1981 Oct;30(10):818–823. doi: 10.2337/diab.30.10.818. [DOI] [PubMed] [Google Scholar]
- Vracko R., Benditt E. P. Restricted replicative life-span of diabetic fibroblasts in vitro: its relation to microangiopathy. Fed Proc. 1975 Jan;34(1):68–70. [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Froesch E. R. Insulin-like growth factors I and II: some biological actions and receptor binding characteristics of two purified constituents of nonsuppressible insulin-like activity of human serum. Eur J Biochem. 1978 Jun 15;87(2):285–296. doi: 10.1111/j.1432-1033.1978.tb12377.x. [DOI] [PubMed] [Google Scholar]