Abstract
Environmental complexity and season both influence brain cell proliferation in adult vertebrates, but their relative importance and interaction have not been directly assessed. We examined brain cell proliferation during both the breeding and non-breeding seasons in adult male electric fish, Brachyhypopomus gauderio, exposed to three environments that differed in complexity: (1) a complex natural habitat in northern Uruguay, (2) an enriched captive environment where fish were housed socially and (3) a simple laboratory setting where fish were isolated. We injected fish with BrdU 2.5 h before sacrifice to label newborn cells. We examined the hindbrain and midbrain and quantified the density of BrdU+ cells in whole transverse sections, proliferative zones and two brain nuclei in the electrocommunication circuitry (the pacemaker nucleus and the electrosensory lateral line lobe). Season had the largest effect on cell proliferation, with fish during the breeding season having three to seven times more BrdU+ cells than those during the non-breeding season. Although the effect was smaller, fish from a natural environment had greater rates of cell proliferation than fish in social or isolated captive environments. For most brain regions, fish in social and isolated captive environments had equivalent levels of cell proliferation. However, for brain regions in the electrocommunication circuitry, group-housed fish had more cell proliferation than isolated fish, but only during the breeding season (season × environment interaction). The regionally and seasonally specific effect of social environment on cell proliferation suggests that addition of new cells to these nuclei may contribute to seasonal changes in electrocommunication behavior.
Keywords: brain cell proliferation, season, environmental enrichment, electrocommunication, electric fish, Brachyhypopomus
INTRODUCTION
The brains of animals living in complex environments differ structurally from those of animals living in simplified laboratory environments. Beginning in the 1960s, researchers showed that environmental stimuli modify many features of brain structure, including cortical size, neuronal architecture and synapse formation (Diamond et al., 1966). More recent studies demonstrate that environmental complexity also influences cell proliferation and neurogenesis in the adult brain. For example, rodents housed with conspecifics and enriched physical environments produce more neurons in the hippocampus than those living alone in standard laboratory housing (Kempermann et al., 1997). These studies have greatly contributed to our understanding of specific stimuli that can enhance neurogenesis (Fabel et al., 2009; Kozorovitskiy and Gould, 2004; Lu et al., 2003; Stranahan et al., 2006). However, because they were conducted in laboratory conditions, their conclusions may not hold true for animals in nature, where environments are much more complex.
When examined in animals living in natural habitats, brain cell proliferation or survival is greatly enhanced by the complex physical and social stimuli in the wild (Amerin et al., 2008; Barnea, 2010; Barnea and Nottebohm, 1996; Boonstra et al., 2001; Delgado-Gonzalez et al., 2008; Hansen and Schmidt, 2004; LaDage et al., 2010). Most free-living animals face seasonal changes in the physical and social environment and undergo corresponding drastic changes in physiology and behavior (e.g. breeding and food caching). Such natural seasonal changes affect brain cell proliferation (or survival) in some but not all species (Amerin et al., 2008; Galea and McEwen, 1999; Hoshooley and Sherry, 2004; Lavenex et al., 2000; Ormerod and Galea, 2003; Sampedro et al., 2008; Thompson and Brenowitz, 2005).
Despite many studies showing that enriched captive environments and complex natural environments can influence brain cell proliferation, the relative importance of season and environmental complexity has not been addressed in depth in tetrapods and not at all in fish. We examined brain cell proliferation in a weakly electric fish, Brachyhypopomus gauderio, in three environments (natural habitat, enriched laboratory environment and isolated laboratory housing) across two seasons to estimate the relative importance of seasonal, social and physical features of the environment.
Weakly electric fish, such as B. gauderio, are particularly suitable for addressing this issue because specific brain regions – those used for electrogenesis and electroreception – are directly linked to the ways that the fish sense and interact with the physical and social environment. Fish navigate through the environment by sensing how nearby objects distort a self-generated electric signal, the electric organ discharge (EOD). The electric signal originates in a hindbrain nucleus, the pacemaker nucleus (Pn), which stimulates the discharge of the electric organ, located primarily in the tail. Electroreceptors distributed all over the skin of the fish detect spatial and temporal variation in electric fields and pass information for primary sensory processing to the electrosensory lateral line lobe (ELL) in the hindbrain.
The EOD also is used as the primary means of social communication. EOD rate and waveform are sexually dimorphic, hormone sensitive and seasonally variable and thereby convey information about the sex and reproductive status of the fish (Salazar and Stoddard, 2009; Silva et al., 2002; Silva et al., 2007; Silva et al., 2008; Stoddard et al., 2006). Fish can also make rapid EOD modulations that function in aggression and courtship. Several EOD modulations are generated by diencephalic nuclei, such as the prepacemaker nucleus (PPn), that transiently modify the activity of the Pn (Perrone et al., 2009; Zakon et al., 2002; Zupanc, 2002). Because these three brain regions – Pn, ELL and PPn – all function directly in interactions with the environment, we were particularly interested in their response to environmental change. We examined whether these specific brain regions or proliferative zones that contribute cells to these regions showed particularly high levels of cell proliferation when fish were exposed to environments that varied in physical and social complexity.
A second advantage of studying environmental influences on brain cell proliferation in electric fish is that a number of laboratory studies on one species (Apteronotus leptorhynchus) have previously detailed patterns of cell proliferation and social influences on cell addition. In A. leptorhynchus, cell proliferation is abundant throughout the brain. In a 2 h period, approximately 0.2% of all brain cells undergo mitosis, a rate ∼10–100 times that found in proliferative zones in the adult rodent brain (Zupanc, 2008). Cell birth is particularly concentrated in proliferation zones in the cerebellum and the periventricular zone (PVZ) but is also scattered at lower densities throughout the brain (Zupanc and Horschke, 1995). Cell addition in the PVZ that contributes cells to the PPn is enhanced by experimental manipulation of social interactions in the laboratory (Dunlap et al., 2006). Thus, there is good reason to believe that natural changes in the social environment may affect cell proliferation, especially in brain regions associated with electrocommunication.
Among electric fish, B. gauderio is particularly appropriate for assessing the role of natural stimuli and seasonal change on brain cell proliferation because of the extensive previous field and laboratory work documenting seasonal, hormonal and social influences on their electrocommunication behavior and the key brain nuclei used in electrocommunication (Quintana et al., 2004; Salazar and Stoddard, 2009; Silva et al., 2007). Indeed, more is known about the seasonality, natural behavior and ecology of B. gauderio than any other South American electric fish. In Uruguay, these fish inhabit shallow freshwater lakes and streams that are commonly covered by floating vegetation (Silva et al., 2003). During the breeding season (October–February), they live in dense concentrations, with fish usually separated by less than 1 m (Miranda et al., 2008). In the non-breeding season (March–September), fish continue to aggregate, but their overall density is lower than in the breeding season (A.C.S., personal observations).
In our study of B. gauderio, we found that season had a large effect on cell proliferation rates, with environmental complexity (natural habitat vs enriched captivity vs isolated captivity) having a secondary but still significant effect. Fish during the breeding season and those living in a natural habitat had much greater rates of cell proliferation throughout the brain than fish during the non-breeding season and fish in captivity. In addition, fish living in enriched captive environments that allowed for abundant social interaction had higher rates of brain cell proliferation than fish in isolation. This effect was specific to the breeding season and present only in brain regions that are associated with electrogenesis and electrosensation. These results suggest that environmentally induced changes in brain cell proliferation may contribute to changes in social signaling associated with reproduction.
MATERIALS AND METHODS
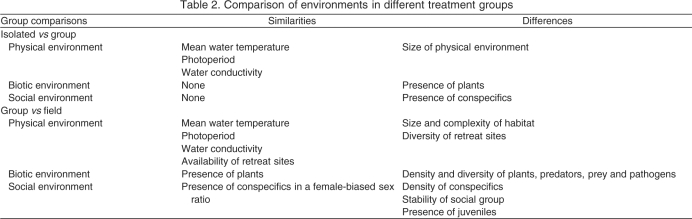
We measured brain cell proliferation and plasma 11-ketotestosterone (11kT) levels in male electric fish, Brachyhypopomus gauderio (Giora & Balabarba 2009), in three different treatment groups: (1) fish collected directly from the natural habitat (‘field’); (2) fish housed in groups held in large, semi-natural outdoor pools (‘group’); and (3) fish housed in isolation in simple indoor aquaria (‘isolated’). We conducted this experiment during both the breeding season (mid-January to February) and the non-breeding season (late May to mid-June). The similarities and differences across seasons and environments are presented in Tables 1 and 2. We examined the brains of six to eight male fish from each subject group. The length of fish ranged from 14 to 19 cm, and all subject groups had equivalent mean (±s.e.m.) body length (16.1±0.4 cm). A previous study has reliably shown that fish greater than 12 cm in length are adult animals (Franchina, 1997) and thus all fish in our study were well within the size range for adults.
Table 1.
Comparison of environments in different seasons in each treatment group
Table 2.
Comparison of environments in different treatment groups
Animals and field site
All fish in the study were collected from a lake, Laguna Lavalle, in northern Uruguay (Department of Tacuarembo, 31°48′S, 55°13′W). The ecology and social behavior of this population have been described previously (Miranda et al., 2008), and the reproductive cycle and behavior of a neighboring population of B. gauderio have been studied extensively (Perrone et al., 2009; Quintana et al., 2004; Silva et al., 2003).
We collected fish in mid-January 2009, during the breeding season, and in late May 2009, during the non-breeding season. At the time of sampling, the water temperature was 25–27°C in January and 17–18°C in May. We located fish using a detector that transduces the electric signal into an audio signal, and captured fish with scoop nets. We placed fish individually in inflated plastic bags partially filled with water and floated them on the lake before bringing them to the shore. At the shore, approximately one-third of the fish were injected with bromodeoxyuridine (BrdU) and bled (see below) to measure cell proliferation and androgen levels in the field; the remaining fish were packaged for transport to the laboratory.
Captive conditions
During both the breeding and non-breeding seasons, fish were housed in captivity under one of two conditions: social groups in semi-natural outdoor pools or isolated in a controlled environmental chamber. During each season, we created four pools (250 l, 80×80×40 cm length×width×diameter) on the roof of the laboratory at Instituto Investigaciones Biologicas Clemente Estable in Montevideo, Uruguay (Perrone et al., 2009). Each pool housed 12–16 adult fish. The social composition was three to four males and nine to 12 females per group to maintain a constant sex ratio of 1:3, which mimicked the adult sex ratio in the natural breeding population (Miranda et al., 2008). Each pool contained floating plants (Eichhornia crassipes, Pistia stratiotes) native to the natural habitat of the fish and six to eight PVC tubes for shelter. During the breeding season, the mean water temperature of the pools was 27–30°C; during the non-breeding season, it was 16–17°C. In previous experiments, fish living in these conditions showed seasonal changes in reproductive physiology and behavior that parallel the seasonal changes in the wild (Silva et al., 2007; Quintana et al., 2004).
Isolated fish were housed in individual 10–12 l tanks with a shelter tube and kept at a constant light cycle and temperature that mimicked field conditions during each season. During the breeding season, the light cycle was 14 h:10 h light:dark and the temperature was a constant 28°C. During the non-breeding season, the light cycle was 10 h:14 h light:dark and the temperature was a constant 17°C. All fish were fed with Tubifex tubifex worms three times per week. Fish were kept under these conditions for 2–4 weeks before they were terminally anesthetized in late February and mid-June.
BrdU injection, and processing of brains and plasma samples
To label newborn brain cells from fish in the field, we injected fish with BrdU (Sigma B-9285; 20 μg g–1 body weight) 15–60 min after capture and held them in plastic boxes filled with lake water (1–2 l) and placed in the shade. Water temperature was 24–27°C during the period of BrdU incorporation. Approximately 2.5 h (135–165 min) following BrdU injection, fish were killed with 2-phenoxyethanol (0.075%). Brains were dissected and placed on ice in buffered paraformaldehyde (4%, 90–120 min) followed by a series of washes in PBS (3×30 min). Brains were then placed in sucrose (30%, 4–8 h) and a 1:1 mixture of sucrose and mounting medium for cryoprotection (4–8 h), placed in mounting medium alone and quickly frozen by covering them with pulverized dry ice.
For plasma androgen measurements, fish were bled from the caudal vein using a heparinized syringe and needle (25 G) within 3 h after capture and 3 min after anesthesia. Blood samples were placed on ice for 4–8 h and then centrifuged. All brain and plasma samples were stored on dry ice in the field for 1–2 d until they were shipped to the laboratory, where they were stored at –80°C.
Fish were measured for body length and dissected to determine sex and gonadal state. During the non-breeding season, when the sex of fish was sometimes ambiguous, gonads were preserved in paraformaldehyde and later examined under a dissecting scope. Testes were scored according to three phases in the annual cycle of this species: 1=regressing or recovering gonads, 2=maturing gonads and 3=mature gonads (Quintana et al., 2004). Visual inspection did not allow us to discriminate between regressing and recovering gonads, so they were pooled in a single group.
For captive fish, the procedures for BrdU injection, brain processing and plasma sampling were identical to those for the field fish, except for the following: in the laboratory, fish were injected with BrdU within 15–20 min after capture, water temperature during the period of BrdU incorporation was 23–24°C and brains were frozen in 2-methylbutane (–80°C) after sucrose cryoprotection.
BrdU immunohistochemistry
To identify newborn cells, we used BrdU immunohistochemistry with a protocol identical to that used in a similar study (Dunlap et al., 2006). In brief, sections were treated with citrate buffer (5 mmol l–1, 10 min, 95°C) and pepsin solution (2.5% pepsin in 0.1 mol l–1 HCl, 3 min, 37°C) to expose the BrdU epitope. Sections were treated sequentially with sheep anti-BrdU (1:200, overnight, 4°C; Capralogics Inc., Hardwick, MA, USA), biotin-conjugated donkey anti-sheep IgG (1:800, 2 h, room temperature; Chemicon International, Temecula, CA, USA) and streptavidin conjugated to Alexa 488 (1:800, 1 h, room temperature; Molecular Probes, Eugene, OR, USA).
Quantifying the distribution of BrdU+ cells
In quantifying our observations, we addressed three general questions: (1) what is the overall, global rate of cell proliferation; (2) what fraction of newborn cells are generated in specific proliferation zones; and (3) what is the rate of cell proliferation in brain regions specifically associated with electrogenesis and electrosensation? We examined transverse sections of the brain using a Nikon Eclipse E600 epifluorescence microscope and used the brain atlas of a closely related electric fish, Apteronotus leptorhynchus (Maler et al., 1991), as a reference to identify particular brain nuclei. We estimated the density of BrdU labeling at four axial levels, two regions of the hindbrain (sections –9 to –7 and sections –7 to –5 of the Maler atlas) and two regions of the midbrain (sections 14–16 and sections 17–19, which we term the posterior and anterior midbrain, respectively). Each region spanned ∼500 μm in axial length.
To estimate the overall cell proliferation, we counted all the BrdU+ cells in one lateral half of each transverse section and doubled this value to estimate the number of cells born in the whole section. To estimate the number of newborn cells in two proliferative zones, we counted all the BrdU cells in a 100 μm band surrounding the ventricle (the PVZ) in the hindbrain and midbrain and a 100 μm band along the dorsal border of the hindbrain cistern, which contains the eminentia granularis pars medialis (EGm). To estimate the number of cells born outside of these proliferative zones, the ‘background’ area, we subtracted the number of BrdU+ cells in the PVZ and EGm from those in the whole section. To quantify the relative importance of proliferative zones as sites of cell proliferation, we divided the number of BrdU+ cells in the PVZ and EGm by the total number of cells in each transverse section. To quantify cell proliferation in the electrocommunication system, we counted BrdU+ cells unilaterally in the ELL in atlas sections –9 to –6 and bilaterally in the Pn in atlas sections –8 to –5. Cell counts in the ELL were doubled to estimate the total number in the whole section. We did not directly assess cell proliferation in the PPn. Instead, we used BrdU+ cell density in anterior midbrain PVZ as an indirect measure of cell addition to the PPn because, in the closely related electric fish A. leptorhynchus, the majority of cells added to the PPn during adulthood arise from the adjacent PVZ in the anterior midbrain (Zupanc and Zupanc, 1992).
The area of each whole transverse section and brain region was quantified by capturing an image of the sections and then measuring the area using NIH Image J software (Bethesda, MD, USA). The background area was calculated by subtracting the area of the PVZ and EGm from the area of the whole section. All values were multiplied by the section thickness (0.030 mm) to calculate the volume of the sample of each region examined, and the density was expressed as BrdU+ cells mm–3. For each individual brain, we averaged the values of two to five randomly chosen sections in each axial level.
Measurement of plasma 11-ketotestoterone
We measured 11kT in plasma samples (5–10 μl) of male fish (N=5–6) using an enzyme linked immunoassay kit (Cayman Chemical Co., Ann Arbor, MI, USA). The detection limit for this assay is 6 pg ml–1, and the cross-reactivity of the 11kT antibody is <0.05% with other androgens. We assayed plasma samples in duplicate in a single run of the assay. The intra-assay variation was 3.3%.
Statistical analysis
We examined variation in BrdU+ cell density and plasma 11kT using multivariate ANOVA, with region, season and environment as factors, followed by Bonferroni post hoc tests to identify differences among groups. In our initial analysis, we found that the two axial regions of the hindbrain (sections –10 to –8 and –7 to –5) showed no differences in any subject group (F=3.0, P>0.05), and so these axial regions were combined in subsequent analysis. All data are presented as means ± s.e.m., and P<0.05 was considered statistically significant.
RESULTS
Our quantitative analysis of BrdU labeling allowed us to estimate the overall rates of cell proliferation across the hindbrain and midbrain, the relative importance of proliferation zones as sites of cell birth and the density of newborn cells in specific brain nuclei associated with electrogenesis (Pn) and electrosensation (ELL). In addition, our experimental design allowed us to partition variance in BrdU+ cell density and plasma 11kT levels to season (breeding vs non-breeding), environment (isolated vs group housed vs field) and their interaction.
General patterns of cell proliferation
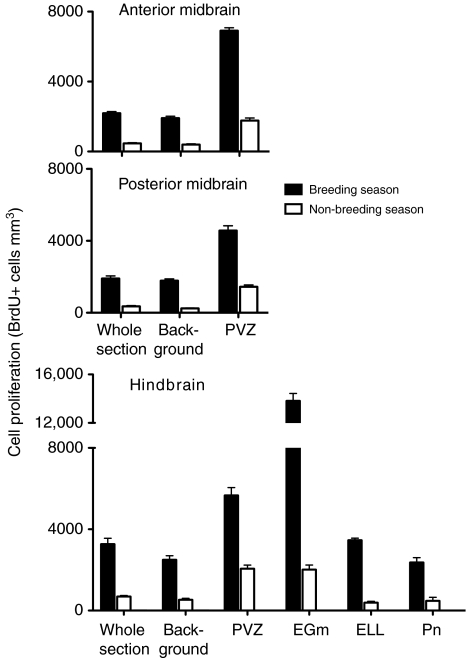
Averaged over all brain regions, environments and seasons, transverse sections had a mean of 135±32 BrdU+ cells, a mean volume of 0.124±0.002 mm3 and a mean density of 1088±293 BrdU+ cells mm–3. In all subject groups, BrdU+ cell density was greater in the hindbrain than in the midbrain (F=6.9, P<0.005; for field active animals, see Fig. 3). The overall distribution of BrdU+ cells was similar to that reported for A. leptorhynchus (Zupanc and Horschke, 1995), with the exception that the cerebellum of B. gauderio appeared to have relatively fewer BrdU+ cells than that of A. leptorhynchus.
Fig. 3.
Effect of season on cell proliferation in different brain regions of field active Brachyhypopomus gauderio. The anterior midbrain comprises sections 17–19 and the posterior midbrain comprises sections 14–16, according the Maler brain atlas (Maler et al., 1991). EGm, eminentia granularis pars medialis; ELL, electrosensory lateral line lobe; Pn, pacemaker; PVZ, periventricular zone. Whole section is the density of BrdU+ cells in the whole transverse section. Background is the density of BrdU+ cells in the transverse section outside of proliferative zones (PVZ and EGm). Cell proliferation was significantly higher (P<0.0001) during the breeding season than during the non-breeding season in all brain regions. N=6–8 fish per sample.
Hindbrain
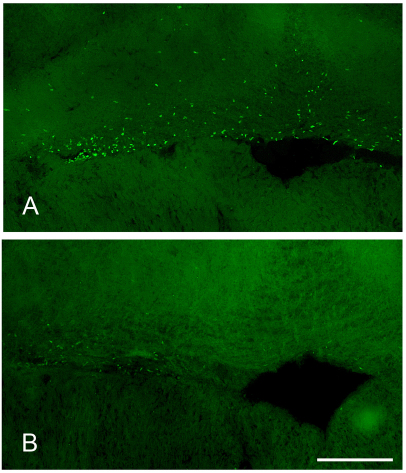
In the hindbrain, the EGm was the primary site of cell proliferation, containing ∼20–55% of the newborn cells in the transverse section and a BrdU+ cell density three to five times greater than that of the background area. Although newborn cells were found surrounding the entire cistern, the vast majority were located in the dorsal border of the cistern in the EGm (Fig. 1). The PVZ of the hindbrain was also a proliferative zone, but was quantitatively less important than the EGm. The PVZ contained ∼5–10% of all the newborn cells in the transverse section and a BrdU+ cell density two to three times greater than that of the background area.
Fig. 1.
Seasonal variation in brain cell proliferation in the eminentia granularis pars medialis, the major proliferative zone of the hindbrain, of adult Brachyhypopomus gauderio. (A) Breeding season; (B) non-breeding season. The cavity in the lower right of each photograph is the cerebromedullary cistern. Scale bar, 100 μm.
Midbrain
The anterior and posterior midbrain differed according to season and environment (F=32.2, P<0.0001), and thus these regions were kept separate in our analysis. Cell proliferation was concentrated in the PVZ (Fig. 2A), which contained 15–40% of all labeled cells in the transverse section and a BrdU+ cell density three to six times greater than background. BrdU+ cells were scattered throughout the torus semicircularis and along the medial edge of optic tectum and were in particularly high density in the ventral hypothalamus.
Fig. 2.
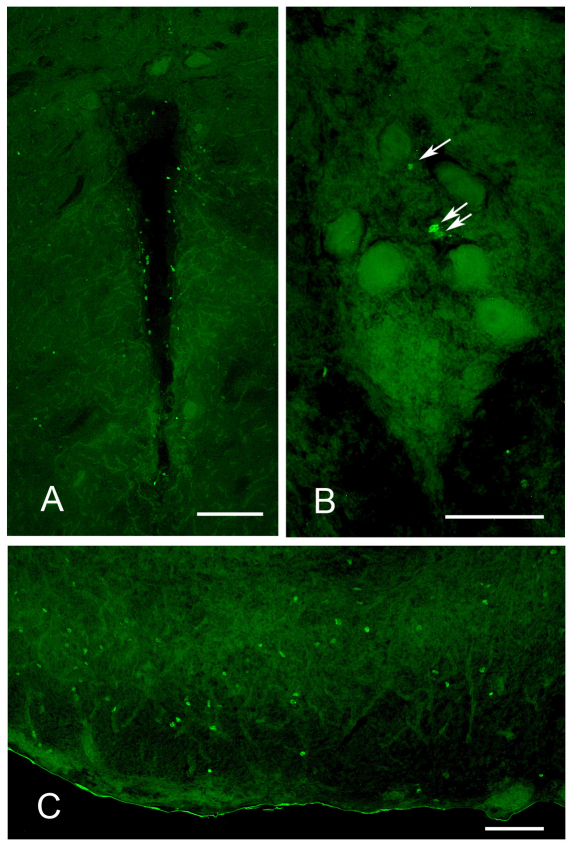
BrdU labeling in the brain of adult Brachyhypopomus gauderio. In all photographs, dorsal is towards the top of the figure. (A) Periventricular zone of the anterior midbrain. The cavity at the center is the ventricle. Scale bar, 100 μm. (B) Pacemaker nucleus. Arrows point to BrdU+ cells. The large cells are relay cells, which only show background labeling. Scale bar, 50 μm. (C) Electrosensory lateral line lobe (ELL). Figure shows one lateral half of the ELL, with lateral toward the left. Scale bar, 50 μm.
Pn and ELL
In the Pn and ELL, BrdU+ cells were found at approximately the same density as the background areas, and thus these nuclei cannot be considered areas of concentrated mitosis. Within the Pn (Fig. 2B), labeled cells were located at all axial regions and tended to be more abundant in the ventral half of the Pn. In field fish during the breeding season (the subject group with the highest Pn cell proliferation), each 30 μm section of the Pn contained a mean of 4.1±1.1 BrdU+ cells per section. Given that the Pn is ∼500 μm in axial length (Quintana et al., 2010), we estimate that the entire nucleus would generate 60–70 new cells in a 2.5 h period. This is a rate approximately half that reported from the Pn in Apteronotus (Zupanc and Horschke, 1995). Labeled cells were found throughout the ELL, although they were in much greater density in the pyramidal and granule layers than in the molecular layers (Fig. 2C). Similar to another electric fish, Gymnotus (Castello et al., 2008), BrdU+ cells also tended to be more concentrated in the lateral segment than the central segments, although this difference was not quantified. This apparent concentration of newborn cells in the lateral segment deserves future research because, in other electric fish species, this is the subsection of the ELL that is specifically involved with processing social electric signals (Metzner and Juranek, 1997; Marsat et al., 2009).
Effect of season
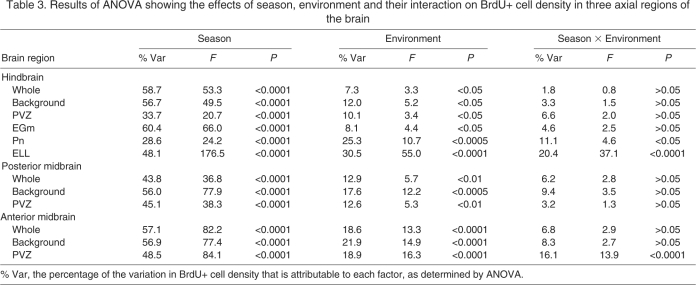
There was a strong overall effect of season on BrdU+ cell density for all regions of the brain (Table 3, Figs 1, 3), with season accounting for ∼30–60% of the overall variation in BrdU+ cell density. Across all treatment groups and brain regions, fish during the breeding season had BrdU+ cell densities three to seven times greater than fish during the non-breeding season.
Table 3.
Results of ANOVA showing the effects of season, environment and their interaction on BrdU+ cell density in three axial regions of the brain
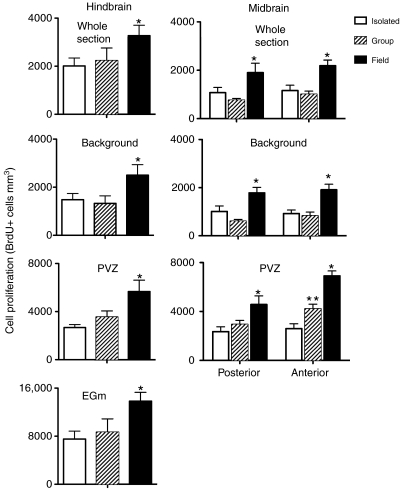
Effect of environmental complexity
For all brain regions, there was a significant effect of environment (isolation vs group housing vs field) on cell proliferation (Table 3, Fig. 4), with environment accounting for ∼10–30% of the overall variation in BrdU+ cell density. In both seasons and in all brain regions, field active fish had greater (approximately two to five times) BrdU+ cell density than the two captive groups (isolation and group housed). One of the only methodological differences in sampling field and captive fish was that, for logistical reasons, fish sampled in the field spent approximately 30 min longer in holding boxes before they were injected with BrdU than captive fish. Such capture stress inhibits cell proliferation in some species (Amerin et al., 2008), so it is likely that our report underestimates the true difference between field and captive fish.
Fig. 4.
Effect of environmental complexity on cell proliferation in whole brain, background regions and proliferation zones [eminentia granularis pars medialis (EGm) and periventricular zone (PVZ)] of Brachyhypopomus gauderio. Data are presented only from the breeding season. Note the difference in y-axis scale. *, significant difference from both isolated and group fish; **, significant difference from isolated fish and field fish. N=6–8 fish per sample.
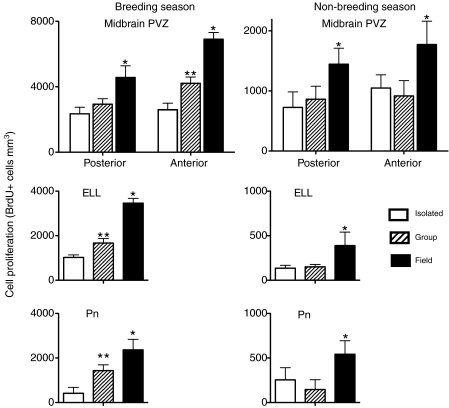
Season × environment interaction
The effect of environment varied by season in some brain regions (Table 3, Fig. 5). During the breeding season, group-housed fish had greater BrdU+ cell density in the Pn, ELL and the anterior midbrain PVZ than isolated fish, but lower BrdU+ cell density than field active fish. However, during the non-breeding season, group-housed and isolated fish had equivalent BrdU+ cell densities (P>0.05; Fig. 5). Thus group housing elevated cell proliferation in these regions to an intermediate level during the breeding season, but had no effect during the non-breeding season. There were no significant environment × season interactive effects on BrdU cell density in the whole transverse sections and background regions in any axial region, the hindbrain PVZ and EGm, or the posterior midbrain PVZ (Table 3). Thus these regions were enhanced by field conditions but not group housing during both seasons.
Fig. 5.
Season × environment interactive effect on brain cell proliferation in brain regions of Brachyhypopomus gauderio associated with electrogenesis and electrosensation. Note the difference in y-axis scale. The pacemaker nucleus (Pn) initiates the production of the electric organ discharge, the electrosensory lateral line lobe (ELL) is the primary electrosensory processing region and the anterior midbrain periventricular zone (PVZ) likely contributes cells to the prepacemaker nucleus, which regulates the production of social electric signals. *, significant difference from both isolated and group fish; **, significant different from isolated fish and field fish. N=6–8 fish per sample.
Relative importance of proliferation zones as a function of season and environment
For almost all comparisons, season and environment had no significant effect on the fraction of BrdU+ cells located in proliferative zones (PVZ and EGm). Thus, overall, it appears that environmental stimuli affect cell proliferation uniformly in proliferative and background regions. There is one exception to this generality. In the hindbrain, the relative importance of the PVZ as a site of new cell birth was affected by season: the percentage of BrdU+ cells in the PVZ compared with the whole transverse section at the same axial level was significantly lower during the breeding season (5.3±0.7%) than during the non-breeding season (12.8±1.3%) (F=15.9; P<0.001). The hindbrain PVZ appears less responsive to seasonal change than other hindbrain regions: the PVZ only showed an approximately threefold increase in BrdU+ cell density from the non-breeding to the breeding season whereas the whole brain section, background, EGm, ELL and Pn showed an approximately five- to seven-fold increase.
Testis maturity and plasma 11-ketotestosterone levels
In our January sampling, most fish captured from the field (five of eight) and those in group housing (six of nine) had fully mature testes (gonadal score=3) and the remaining fish had testes of intermediate maturity (gonadal score=2). The mean gonadal maturity scores were 2.63±0.24 and 2.66±0.21 for the field and group fish, respectively. All isolated fish during the breeding season and all fish during the non-breeding season had fully regressed gonads (gonadal score=1).
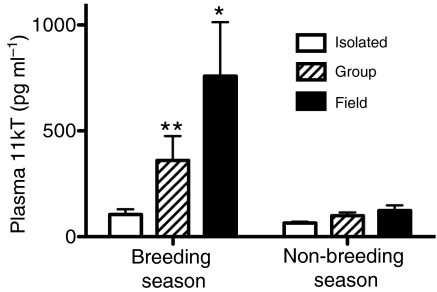
Plasma 11kT concentrations varied significant by season (F=7.58, P<0.01), environment (F=3.64, P<0.05) and their interaction (F=2.82, P<0.05). Concentrations of 11kT were higher during the breeding season than during the non-breeding season (Fig. 4). During the breeding season, field fish had significantly higher 11kT levels than fish in either captive group, and group-housed fish had significantly higher levels than isolated fish. During the non-breeding season, fish in all environment groups had equivalent values (P>0.05). Fish were held in holding boxes for ∼3 h following capture (during the period of BrdU incorporation), and because such acute stress commonly inhibits androgen secretion, the levels for 11kT that we report may be less than those experienced by undisturbed fish. Nevertheless, our values are well within the range of those reported in other studies of B. gauderio, in which fish were bled immediately after capture (Gavassa et al., 2010; Salazar and Stoddard, 2009). More importantly, fish were treated equivalently across subject groups, allowing us to make comparisons of androgen concentration among groups.
DISCUSSION
We found that environmental complexity enhanced the proliferation of brain cells in the weakly electric fish, B. gauderio. Fish in the natural environment had greater rates of cell proliferation than captive fish in all the brain regions we examined. This result corroborates the findings of other studies (Amerin et al., 2008; Barnea, 2010; Boonstra et al., 2001; LaDage et al., 2010), indicating that examining animals in captivity tends to underestimate the proliferative capacity of the adult vertebrate brain. We also found that group-housed captive fish had greater rates of cell proliferation than isolated fish, but only in brain regions associated with electrosensation and electrogenesis. Thus the degree of environmental complexity is correlated with the specificity of the effect: the highly complex natural environment had a broad, global effect on the brain whereas the limited enhancement of the captive environment had a more regionalized, specific effect.
Although the effect of environmental complexity was significant, it was quantitatively surpassed by the effect of season. The overall effect of season on the brain was likely attributable to seasonal changes in temperature or photoperiod whereas regionally specific changes were likely due to changes in reproductive physiology and behavior. Thus the role of external stimuli in promoting brain plasticity must be understood in the context of annual cycles of the environment and reproduction.
Seasonality in cell proliferation, reproductive state and physical environment
Across all brain regions and treatment groups, fish produced approximately three to seven times more new cells during the breeding season than during the non-breeding season. Seasonality in brain cell proliferation has been documented in other vertebrate classes, such as cyclostomes (Vidal Pizarro et al., 2004), amphibians (Dawley et al., 2000), reptiles (Delgado-Gonzalez et al., 2008; Ramirez et al., 1997), birds (Barnea and Nottebohm, 1994; Goldman, 2008) and mammals (Amerin et al., 2008; Galea and McEwen, 1999; Lavenex et al., 2000), but our study is the first report of seasonality in brain cell proliferation in a teleost fish. Such seasonal variation in cell proliferation could be attributable to physiological and/or behavioral changes in the annual reproductive cycle of the animal or to changes in physical environment, such as temperature and photoperiod. In considering both possibilities, we conclude that, although reproductive physiology and behavior may influence seasonal changes in brain cell proliferation, annual cycles of temperature or photoperiod explain most of the seasonal variation in cell proliferation.
To assess the reproductive state of fish during each season, we examined testis maturity and plasma levels of the primary teleost androgen, 11kT. In our breeding season sample, most field active and group-housed fish had fully mature testes, but some had testes of intermediate maturity. Plasma levels of 11kT were also elevated in field active and group-housed fish, although group-housed fish had significantly lower 11kT levels than field active fish. The presence of some fish with partially regressed testes and low 11kT levels combined with other data from this same population (Gavassa et al., 2010) indicate that our sampling period was toward the end of the breeding season.
In many vertebrates, brain cell proliferation peaks during or just prior to the breeding season (Chetverukhin and Polenov, 1993; Dawley et al., 2000; Delgado-Gonzalez et al., 2008; Vidal Pizarro et al., 2004), and some of our results are consistent with the idea that high levels of cell proliferation are causally related to seasonal changes in reproductive physiology and behavior. For example, isolation during the breeding season caused the regression of testes, inhibition of plasma 11kT levels and a corresponding decrease in cell proliferation. Conversely, 11kT levels were highest in breeding fish from the field, which also had the highest levels of cell proliferation.
However, a closer look at our results indicates that cell proliferation is only partially tied to reproductive physiology and behavior. The fact that season affected cell proliferation rates even in isolated fish – which had regressed gonads, low 11kT levels and no social interaction during both seasons – demonstrates that seasonal changes in reproductive physiology and social signaling are not the sole factors driving the seasonal change in cell proliferation. Similarly, the fact that, during the non-breeding season, field active fish had higher rates of cell proliferation than captive fish yet all fish had equivalently low levels of 11kT indicates that cell proliferation is not strictly attributable to seasonal changes in androgens. Finally, during the breeding season, field active fish had higher rates of cell proliferation than group-housed fish even though their testes were at equivalent stages of maturity, and thus proliferation rates are not strictly correlated to testes maturity. Thus, some factor beyond reproductive state must play a large role in seasonal changes in cell proliferation rate.
For temperate species, the breeding season typically has high temperatures and a long photoperiod. These features of the physical environment commonly stimulate changes in reproductive physiology and behavior. Indeed, for B. gauderio, seasonal changes in temperature are likely the crucial zeitgeber stimulating reproductive activity (Quintana et al., 2004). However, changes in the thermal and photic environment can also have direct effects on cellular processes. For example, mitotic rate increases dramatically with temperature (Rieder and Cole, 2002) and, for many ectotherms, mean body temperature is higher during the breeding season. Thus, the seasonal changes in brain cell proliferation we found are likely influenced directly by temperature.
When using BrdU to assess cell proliferation, temperature could influence the basal mitotic rates and/or the transport and metabolism of BrdU during the period following BrdU injection. In our sampling, the temperature of the water that fish inhabited varied from approximately 27°C during the breeding season to 17°C during the non-breeding season. We kept water temperature during the period of BrdU incorporation within a narrower range, but it nonetheless varied from 23 to 27°C. To estimate the effect of this temperature variation during the period of BrdU incorporation, we conducted an experiment on A. leptorhynchus in which all fish were housed at the same temperature (27°C) but were then shifted to 23 or 27°C for 2.5 h during the period of BrdU incorporation. We found no effect of temperature on BrdU+ cell density in the midbrain PVZ (4858±1367 BrdU+ cells mm–3 at 23°C vs 4539±1359 at 27°C; t=0.16, P>0.05). Thus it is likely that any effect of temperature on BrdU labeling in our B. gauderio study is related to mitotic rate in the environment where they were living, rather than to the dynamics of BrdU uptake and metabolism.
The effect of the thermal environment and photoperiod on brain cell proliferation has received little attention, but several studies indicate they have a potent influence. Radmilovich et al. examined cell proliferation in the brains of turtles acclimated to warm and cold temperatures (Radmilovich et al., 2003). Turtles held at 20°C produced approximately six times more cells in brain proliferative zones than those held at 11°C. Similarly, Peñafiel et al. found that lizards (Psammodromus algirus) maintained at 20°C had approximately three to 10 times more BrdU-labeled cells than lizards maintained at 10°C (Peñafiel et al., 2001). Ramirez et al. found no effect of temperature (10 vs 25°C) on brain cell proliferation in another lizard species (Podarcis hispanica), but long daylength (16 vs 8 h) increased brain cell proliferation by approximately threefold (Ramirez et al., 1997). Our results from isolated laboratory conditions showed that fish during the breeding season housed at 28°C with long daylengths (14 h) produced approximately three to eight times more newborn cells than those held at 17°C at short daylengths (10 h) during the non-breeding season. For most brain regions (those except Pn, ELL and anterior midbrain PVZ), fish in all three environments had similar magnitudes of seasonal change (i.e. no season × environment interaction). Because temperature and photoperiod were the only common features shared by these environments, we believe that seasonal changes in temperature and photoperiod are sufficient to explain seasonal differences in cell proliferation in most brain regions. However, as discussed below, season likely affects cell proliferation in the Pn, ELL and anterior midbrain PVZ through seasonal changes in reproductive physiology and/or associated changes in electrocommunication behavior.
Cell proliferation and environmental complexity
Simple vs enriched captive environment
Compared with isolated fish, group-housed fish had both abundant opportunity for social interaction and larger, more complex physical environments (Table 2). During the breeding season, they were also in a more advanced reproductive state, as evidenced by testis maturity and androgen levels (Fig. 6). Yet, for most brain regions, cell proliferation rates were equivalent between these two captive groups. This indicates that, for most of the brain, the size and complexity of the physical environment, the presence of plants and conspecifics, and the reproductive status of the fish do not affect brain cell proliferation. However, for the Pn, ELL and anterior midbrain PVZ, group housing enhanced cell proliferation. This effect only occurred during the breeding season, when the reproductive system is active and most of the social signaling occurs (Fig. 5). The structure of the physical environment did not change from the breeding to the non-breeding seasons (Table 1) and cannot account for this season × environment interaction. Thus, the higher rates of cell proliferation in group-housed fish than in isolated fish during the breeding season is most closely associated with differences in social environment and/or reproductive physiology.
Fig. 6.
Plasma 11-ketotesterone (11kT) levels in Brachyhypopomus gauderio according to season and environment. *, significant difference from both isolated and group fish; **, significant different from isolated fish and field fish. N=5–6 fish per sample.
Our study does not enable us to determine whether elevated rates of cell proliferation are the cause or effect of socially induced changes in behavior and reproductive physiology. Moreover, our study only examined the birth of cells, and we do not know the long-term fate of these cells. While recognizing these limitations, we hypothesize that social stimuli and/or activation of the reproductive system enhance the production of cells in brain regions involved with electrocommunication. Addition of cells to these nuclei and their differentiation into neurons may then contribute to socially induced changes in electrocommunication behavior.
The Pn drives the production of electric signals for electrocommunication and reproductive signaling and, in B. gauderio, its activity is modulated by social interactions and breeding conditions (Pouso et al., 2010; Quintana et al., 2010; Silva et al., 2007). For example, the nocturnal EOD rate of males is greater during the breeding season than during the non-breeding season and is further elevated when males are housed with females (Silva et al., 2007). The Pn expresses androgen receptors, which likely play a role in reproductive and social changes in Pn activity (Pouso et al., 2010). One possibility is that the cells born under the influence of social interactions and breeding conditions differentiate into neurons and incorporate into the Pn circuitry. These new neurons could then modify Pn activity either by providing sites for novel inputs to the Pn or by directly changing its intrinsic firing rate. Such incorporation of new neurons could be mediated by androgen binding to receptors on these new cells or on adjacent cells of the Pn.
The ELL also shows elevated cell proliferation in response to group housing and is crucial for electrocommunication. However, less is known about how the ELL changes functionally in response to social interaction and reproductive state. In other electric fish (Sternopygus), electroreceptors in the periphery, which send inputs directly to the ELL, change their tuning properties in response to androgens (Keller et al., 1986). In addition, the ELL of Gymnotus shows accelerated cell proliferation as fish enter adulthood (Castello et al., 2008). These observations indicate that examining reproductive influences on ELL function and cell proliferation may be a fruitful area for future research.
Group housing also enhances cell proliferation in the anterior midbrain PVZ. The fate of cells born in this proliferation zone is unknown in B. gauderio. In the closely related fish, Eigenmannia sp., some of these cells migrate laterally and become neurons in the PPn, which controls an important electrocommunication signal termed chirping (Zupanc and Zupanc, 1992). In another electric fish, A. leptorhynchus, approximately two-thirds of the cells born in this region differentiate into neurons (Dunlap et al., 2008). Cell addition to the PVZ adjacent to the PPn increases in response to social housing and correlates with socially induced changes in chirping behavior (Dunlap et al., 2002; Dunlap et al., 2006). Male B. gauderio also exhibit chirping behavior (Perrone et al., 2009), and in other members of the genus, chirping is controlled by the PPn (Kawasaki and Heiligenberg, 1989). If the anterior midbrain PVZ also contributes cells to the PPn in B. gauderio, then social enhancement of cell addition to the PPn may also participate in socially induced changes in chirping behavior. However, it is important to recognize that the anterior midbrain PVZ may also contribute cells to other nearby regions of the brain (e.g. the hypothalamus), so, without further study, it is difficult to assess whether the social effect on the PVZ is restricted to the electrocommunication system.
The regionally specific effect of social interaction on brain cell proliferation in B. gauderio and A. leptorhynchus is also found in a frog, Hyla cinerea. In this frog, exposure to conspecific acoustic stimuli elevates cell proliferation rates in brain regions involved with calling behavior, but not in control regions that are unrelated to calling behavior (Almli and Wilczynski, 2009). Together, these results indicate that cell proliferation in brain regions involved with communication behavior is particularly responsive to social stimuli and may contribute to adaptive changes in social behavior.
Enriched captive environment vs complex natural environment
Fish from the field had higher rates of cell proliferation in all brain regions and during both seasons than captive fish. Even in brain regions that are stimulated by group housing (ELL, Pn and anterior midbrain PVZ), cell proliferation is further enhanced by field conditions during the breeding season.
There are many physical, social and biotic differences between the enriched laboratory environment and the natural environment that could contribute to differences in brain cell proliferation (Table 2). Compared with group-housed fish, field active fish experienced a more complex structural environment. Enhancing the structural complexity of the environment stimulates cell proliferation in the telencephalon of zebrafish (von Krogh et al., 2010), and the greater number and diversity of objects in the natural habitat of B. gauderio (compared with the pools in captivity) may broadly stimulate brain activity and promote brain cell proliferation in B. gauderio. In addition, the natural environment is simply larger than the artificial pools of group-housed fish. At Laguna Lavalle, males during the reproductive season move a span of ∼4 m over a 3–4 d period (Miranda et al., 2008). This is approximately three times farther than the maximum dimension in the pools in our study. Considering that locomotor behavior stimulates brain cell proliferation in some vertebrates (van Praag, 2008), it is possible that field active fish have higher rates of cell proliferation because they swim more than group-housed fish. However, the only test of this idea in fish indicates that, in laboratory environments that promote swimming, fish actually have lower rates of brain cell proliferation (von Krogh et al., 2010).
The social environment was also more complex in the field than in the group laboratory housing. Certain features of the natural social environment, such as the presence of fish of both sexes in a realistic sex ratio, were preserved in group housing, but the composition of the social environment was fixed and limited to only adults. The more dynamic and age-diverse social stimuli fish receive in the wild might contribute to higher rates of proliferation throughout the brain and/or the particular enhancement of proliferation in the Pn, ELL and anterior midbrain PVZ. In zebra finches, social complexity significantly influences cell recruitment into brain regions subserving vocal communication (Lipkind et al., 2002) and, in B. gauderio, the history and complexity of social interactions affect electrocommunication (Salazar and Stoddard, 2009). Thus, it seems plausible that the greater social complexity of the natural environment contributes to the elevated rates of cell proliferation in the electrocommunication circuity of B. gauderio.
Finally, fish in the field were exposed to more biotic complexity, with greater numbers and diversity of plants, prey, predators and pathogens. Sensory cues from predators inhibit brain cell proliferation in rats (Tanapat et al., 2001) but, otherwise, nothing is known about how the biotic environment affects brain cell dynamics, and it is difficult to evaluate its influence on B. gauderio.
Relative importance of proliferation zones as sites of cell proliferation
In many vertebrate species, most cells added to the adult brain originate from discrete proliferation zones (Chapouton et al., 2007). Although fish clearly possess such proliferation zones, some of which are likely homologous to those of other vertebrates (Zupanc, 2008; Zupanc and Horschke, 1995), they also generate a substantial number of new cells scattered throughout the brain, a phenomenon we term ‘background proliferation’. In B. gauderio, we found that ∼15–55% of new cells were born in proliferation zones. This proportion was greater in the hindbrain than in the midbrain. However, in almost all cases, the relative importance of the proliferative zones as sites of cell birth was independent of season and environment. Thus field conditions and breeding season uniformly increase cell proliferation in proliferative and background zones.
The only exception to this generality was that the hindbrain PVZ was relatively less important as a site of cell proliferation during the breeding season compared with the non-breeding season. The overall quantitative effect of this seasonal difference is small, because only ∼10% of all newborns cells in the hindbrain originate in the PVZ. Nevertheless, this result shows that, in some cases, the environment can influence the distribution of newborn cells in and out of proliferative zones.
CONCLUSIONS
We found that the natural environment and the breeding season had a large positive effect on the overall proliferative activity of the brain. We are uncertain about the precise features that stimulate cell proliferation, but we suggest that the structural and social complexity of the natural environment and the warm temperatures and long daylengths of the breeding season globally enhance brain cell proliferation.
Our study also highlights the importance of regional and seasonal specificity in the effect of the environment of cell proliferation. Experimental manipulation of the social environment only affects specific brain regions – the Pn, ELL and anterior midbrain PVZ – and only during the breeding season. These brain regions are important components of the electrocommunication circuitry that contribute to seasonal changes in social signaling. This correlation between regionally and seasonally specific brain cell proliferation in the electrocommunication nuclei and changes in electrocommunication behavior support the idea that selective addition of new cells may be a mechanism contributing to behavioral responses to environmental change.
LIST OF ABBREVIATIONS
- 11kT
11-ketotestosterone
- BrdU
bromodeoxyuridine
- EGm
eminentia granularis pars medialis
- ELL
electrosensory lateral line lobe
- EOD
electric organ discharge
- Pn
pacemaker nucleus
- PPn
prepacemaker nucleus
Footnotes
We thank R. Perrone, G. Batista, L. Zubizarreta, L. Quintana and T. de los Campos for their generous and invaluable assistance in the field; T. Williams for comments on the manuscript; and O. Macadar for inspiration. This work was supported by fellowships from the US Fulbright Scholar program and the Charles Dana Foundation to K.D.D. M.C. was supported by grants to K.D.D. from the NIH (R15H080731-01) and Trinity College Faculty Research Committee. Deposited in PMC for release after 12 months.
REFERENCES
- Almli L. M., Wilczynski W. (2009). Sex-specific modulation of cell proliferation by socially relevant stimuli in the adult green treefrog brain (Hyla cinerea). Brain Behav. Evol. 74, 143-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerin I., Lipp H. P., Boonstra R., Wojtowicz J. (2008). Adult hippocampal neurogenesis in natural populations. In Adult Neurogenesis (ed. Gage F. H., Kempermann G., Song H.), pp. 645-660 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Barnea A. (2010). Wild neurogenesis. Brain Behav. Evol. 75, 86-87 [DOI] [PubMed] [Google Scholar]
- Barnea A., Nottebohm F. (1994). Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl. Acad. Sci. USA 91, 11217-11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A., Nottebohm F. (1996). Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal learning. Proc. Natl. Acad. Sci. USA 93, 714-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra R., Galea L., Matthews S., Wojtowicz J. M. (2001). Adult neurogenesis in natural populations. Can. J. Physiol. Pharmacol. 79, 297-302 [PubMed] [Google Scholar]
- Castello M. E., Iribarne L., Caputi A. A. (2008). Postnatal brain development of Gymnotus omari. Abstr. Soc. Neurosci. 38 [Google Scholar]
- Chapouton P., Jagasia R., Bally-Cuif L. (2007). Adult neurogenesis in non-mammalian vertebrates. BioEssays 29, 745-757 [DOI] [PubMed] [Google Scholar]
- Chetverukhin V. K., Polenov A. L. (1993). Ultrastructural radioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Rana temporaria, with special reference to physiological regeneration of the preoptic nucleus. I. Ventricular zone cell proliferation. Cell Tissue Res. 271, 341-350 [DOI] [PubMed] [Google Scholar]
- Dawley E. M., Fingerlin A., Hwang D., John S. S., Stankiewicz C. A. (2000). Seasonal cell proliferation in the chemosensory epithelium and brain of red-backed salamanders, Plethodon cinereus. Brain Behav. Evol. 56, 1-13 [DOI] [PubMed] [Google Scholar]
- Delgado-Gonzalez F. J., Alonso-Fuentes A., Delgado-Fumero A., Garcia-Verdugo J. M., Gonzalez-Granero S., Trujillo-Trujillo C. M., Damas-Hernandez M. C. (2008). Seasonal differences in ventricular proliferation of adult Gallotia galloti lizards. Brain Res. 1191, 39-46 [DOI] [PubMed] [Google Scholar]
- Diamond M. C., Law F., Rhodes H., Lindner B., Rosenzweig M. R., Krech D., Bennett E. L. (1966). Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 128, 117-126 [DOI] [PubMed] [Google Scholar]
- Dunlap K. D., Pelczar P. L., Knapp R. (2002). Social interactions and cortisol treatment increase the production of aggressive electrocommunication signals in male electric fish, Apteronotus leptorhynchus. Horm. Behav. 42, 97-108 [DOI] [PubMed] [Google Scholar]
- Dunlap K. D., Castellano J. F., Prendaj E. (2006). Social interaction and cortisol treatment increase cell addition and radial glia fiber density in the diencephalic periventricular zone of adult electric fish, Apteronotus leptorhynchus. Horm. Behav. 50, 10-17 [DOI] [PubMed] [Google Scholar]
- Dunlap K. D., McCarthy E., Jashari D. (2008). Electrocommunication signals alone are sufficient to increase neurogenesis in the brain of adult electric fish, Apteronotus leptorhynchus. Dev. Neurobiol. 68, 1420-1428 [DOI] [PubMed] [Google Scholar]
- Fabel K., Wolf S. A., Ehninger D., Babu H., Leal-Galicia P., Kempermann G. (2009). Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front. Neurosci. 3, 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchina C. R. (1997). Ontogeny of the electric organ discharge and the electric organ in the weakly electric pulse fish Brachyhypopomus pinnicaudatus (Hypopomidae, Gymnotiformes). J. Comp. Physiol. A 181, 111-119 [DOI] [PubMed] [Google Scholar]
- Galea L. A., McEwen B. S. (1999). Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience 89, 955-964 [DOI] [PubMed] [Google Scholar]
- Gavassa S., Silva A., Stoddard P. K. (2010). Reproductive output of Brachyhypopomus gauderio decreases throughout the breeding season. Abstracts Int. Cong. Neuroethol, vol. 11 Salamanca, Spain: [Google Scholar]
- Goldman S. A. (2008). Neurogenesis in the adult songbird: a model for inducible striatal neuronal addition. In Adult Neurogenesis (ed. Gage F. H., Kempermann G., Song H.), pp. 593-617 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Hansen A., Schmidt M. (2004). Influence of season and environment on adult neurogenesis in the central olfactory pathway of the shore crab, Carcinus maenas. Brain Res. 1025, 85-97 [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Sherry D. F. (2004). Neuron production, neuron number, and structure size are seasonally stable in the hippocampus of the food-storing black-capped chickadee (Poecile atricapillus). Behav. Neurosci. 118, 345-355 [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Heiligenberg W. (1989). Distinct mechanisms of modulation in a neuronal oscillator generate different social signals in the electric fish Hypopomus. J. Comp. Physiol. A 165, 731-741 [DOI] [PubMed] [Google Scholar]
- Keller C. H., Zakon H. H., Sanchez D. Y. (1986). Evidence for a direct effect of androgens upon electroreceptor tuning. J. Comp. Physiol. A 158, 301-310 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493-495 [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y., Gould E. (2004). Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J. Neurosci. 24, 6755-6759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDage L. D., Roth T. C., 2nd, Fox R. A., Pravosudov V. V. (2010). Ecologically relevant spatial memory use modulates hippocampal neurogenesis. Proc. Biol. Sci. 277, 1071-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P., Steele M. A., Jacobs L. F. (2000). Seasonal pattern of cell proliferation and neuron number in the dentate gyrus of wild adult eastern grey squirrels. Eur. J. Neurosci. 12, 643-648 [DOI] [PubMed] [Google Scholar]
- Lipkind D., Nottebohm F., Rado R., Barnea A. (2002). Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav. Brain Res. 133, 31-43 [DOI] [PubMed] [Google Scholar]
- Lu L., Bao G., Chen H., Xia P., Fan X., Zhang J., Pei G., Ma L. (2003). Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp. Neurol. 183, 600-609 [DOI] [PubMed] [Google Scholar]
- Maler L., Sas E., Johnston S., Ellis W. (1991). An atlas of the brain of the electric fish Apteronotus leptorhynchus. J. Chem. Neuroanat. 4, 1-38 [DOI] [PubMed] [Google Scholar]
- Marsat G., Proville R. D., Maler L. (2009). Transient signals trigger synchronous bursts in an identified population of neurons. J. Neurophysiol. 102, 714-723 [DOI] [PubMed] [Google Scholar]
- Metzner W., Juranek J. (1997). A sensory brain map for each behavior? Proc. Natl. Acad. Sci. USA 94, 14798-14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M., Silva A. C., Stoddard P. K. (2008). Use of space as an indicator of social behavior and breeding systems in the gymnotiform electric fish Brachyhypopomus pinnicaudatus. Environ. Biol. Fishes 83, 379-389 [Google Scholar]
- Ormerod B. K., Galea L. A. (2003). Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci. Lett. 346, 25-28 [DOI] [PubMed] [Google Scholar]
- Peñafiel A., Rivera A., Gutiérrez A., Trías S., De La Calle A. (2001). Temperature affects adult neurogenesis in the lizard brain. Int. J. Dev. Biol. 45, 83-84 11291874 [Google Scholar]
- Perrone R., Macadar O., Silva A. (2009). Social electric signals in freely moving dyads of Brachyhypopomus pinnicaudatus. J. Comp. Physiol. A 195, 501-514 [DOI] [PubMed] [Google Scholar]
- Pouso P., Quintana L., Bolatto C., Silva A. C. (2010). Brain androgen receptor expression correlates with seasonal changes in the behavior of a weakly electric fish, Brachyhypopomus gauderio. Horm. Behav. 58, 729-736 [DOI] [PubMed] [Google Scholar]
- Quintana L., Silva A., Berois N., Macadar O. (2004). Temperature induces gonadal maturation and affects electrophysiological sexual maturity indicators in Brachyhypopomus pinnicaudatus from a temperate climate. J. Exp. Biol. 207, 1843-1853 [DOI] [PubMed] [Google Scholar]
- Quintana L., Pouso P., Fabbiani G., Macadar O. (2010). A central pacemaker that underlies the production of seasonal and sexually dimorphic social signals: anatomical and electrophysiological aspects. J. Comp. Physiol. A 197, 75-88 [DOI] [PubMed] [Google Scholar]
- Radmilovich M., Fernandez A., Trujillo-Cenoz O. (2003). Environment temperature affects cell proliferation in the spinal cord and brain of juvenile turtles. J. Exp. Biol. 206, 3085-3093 [DOI] [PubMed] [Google Scholar]
- Ramirez C., Nacher J., Molowny A., Sanchez-Sanchez F., Irurzun A., Lopez-Garcia C. (1997). Photoperiod-temperature and neuroblast proliferation-migration in the adult lizard cortex. NeuroReport 8, 2337-2342 [DOI] [PubMed] [Google Scholar]
- Rieder C. L., Cole R. W. (2002). Cold-shock and the mammalian cell cycle. Cell Cycle 1, 169-175 [PubMed] [Google Scholar]
- Salazar V. L., Stoddard P. K. (2009). Social competition affects electric signal plasticity and steroid levels in the gymnotiform fish Brachyhypopomus gauderio. Horm. Behav. 56, 399-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro C., Font E., Desfilis E. (2008). Size variation and cell proliferation in chemosensory brain areas of a lizard (Podarcis hispanica): effects of sex and season. Eur. J. Neurosci. 28, 87-98 [DOI] [PubMed] [Google Scholar]
- Silva A., Quintana L., Ardanaz J. L., Macadar O. (2002). Environmental and hormonal influences upon EOD waveform in gymnotiform pulse fish. J. Physiol. Paris 96, 473-484 [DOI] [PubMed] [Google Scholar]
- Silva A., Quintana L., Galeano M. (2003). Biogeography and breeding in gymnotiformes from Uruguay. Environ. Biol. Fishes 66, 329-338 [Google Scholar]
- Silva A., Perrone R., Macadar O. (2007). Environmental, seasonal, and social modulations of basal activity in a weakly electric fish. Physiol. Behav. 90, 525-536 [DOI] [PubMed] [Google Scholar]
- Silva A., Quintana L., Perrone R., Sierra F. (2008). Sexual and seasonal plasticity in the emission of social electric signals. Behavioral approach and neural bases. J. Physiol. Paris 102, 272-278 [DOI] [PubMed] [Google Scholar]
- Stoddard P. K., Zakon H. H., Markham M. R., McAnelly L. (2006). Regulation and modulation of electric waveforms in gymnotiform electric fish. J. Comp. Physiol. A 192, 613-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan A. M., Khalil D., Gould E. (2006). Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 9, 526-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P., Hastings N. B., Rydel T. A., Galea L. A., Gould E. (2001). Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. 437, 496-504 [DOI] [PubMed] [Google Scholar]
- Thompson C. K., Brenowitz E. A. (2005). Seasonal change in neuron size and spacing but not neuronal recruitment in a basal ganglia nucleus in the avian song control system. J. Comp. Neurol. 481, 276-283 [DOI] [PubMed] [Google Scholar]
- van Praag H. (2008). Neurogenesis and exercise: past and future directions. Neuromolecular Med. 10, 128-140 [DOI] [PubMed] [Google Scholar]
- Vidal Pizarro I., Swain G. P., Selzer M. E. (2004). Cell proliferation in the lamprey central nervous system. J. Comp. Neurol. 469, 298-310 [DOI] [PubMed] [Google Scholar]
- von Krogh K., Sorensen C., Nilsson G. E., Overli O. (2010). Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol. Behav. 101, 32-39 [DOI] [PubMed] [Google Scholar]
- Zakon H., Oestreich J., Tallarovic S., Triefenbach F. (2002). EOD modulations of brown ghost electric fish: JARs, chirps, rises, and dips. J. Physiol. Paris 96, 451-458 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K. (2002). From oscillators to modulators: behavioral and neural control of modulations of the electric organ discharge in the gymnotiform fish, Apteronotus leptorhynchus. J. Physiol. Paris 96, 459-472 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K. H. (2008). Adult neurogenesis in teleost fish. In Adult Neurogenesis (ed. Gage F. H., Kempermann G., Song H.), pp. 571-592 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Zupanc G. K., Horschke I. (1995). Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. J. Comp. Neurol. 353, 213-233 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K., Zupanc M. M. (1992). Birth and migration of neurons in the central posterior/prepacemaker nucleus during adulthood in weakly electric knifefish (Eigenmannia sp.). Proc. Natl. Acad. Sci. USA 89, 9539-9543 [DOI] [PMC free article] [PubMed] [Google Scholar]