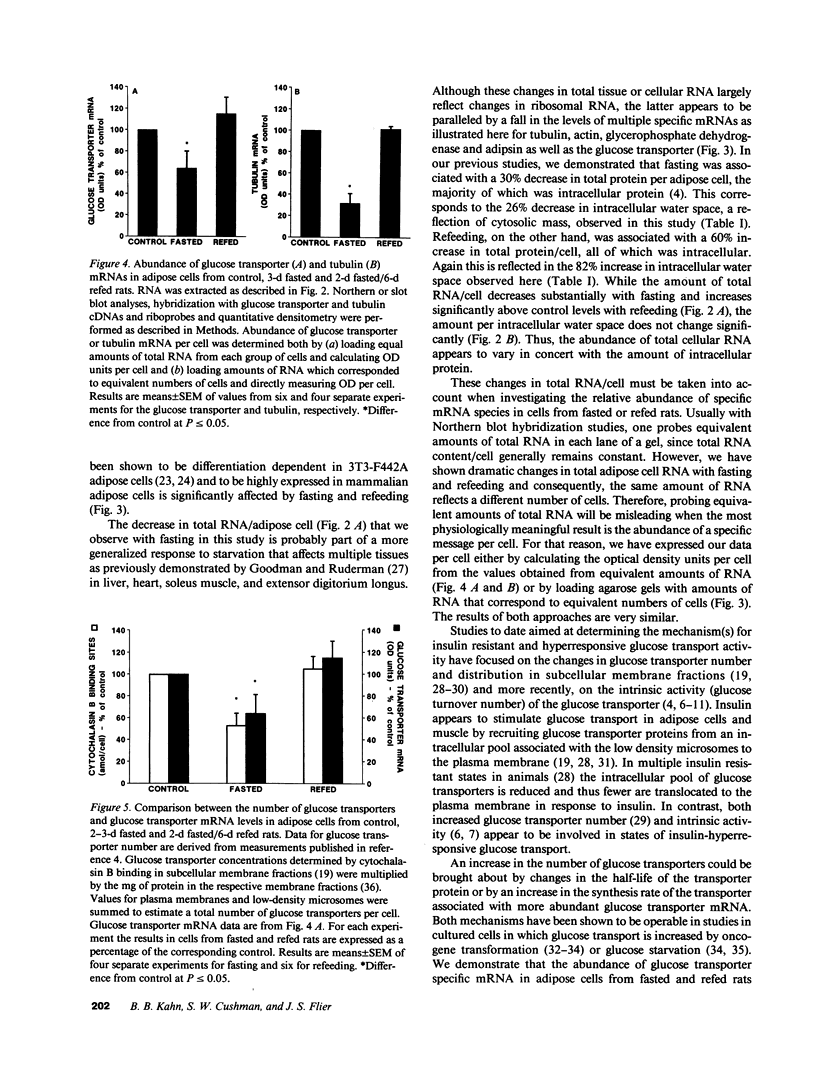
Abstract
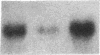
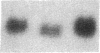
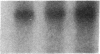
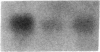
Fasting in the rat is associated with a rapid and progressive decrease in insulin-stimulated glucose transport activity in adipose cells, which is not only restored to normal, but increased transiently to supranormal levels by refeeding. The mechanisms for these changes in glucose transport activity appear to involve alterations in both glucose transporter number and intrinsic activity (glucose turnover number). In this study, we use the human hepatoma Hep G2 glucose transporter complementary DNA clone to examine the molecular basis for these alterations. Extractable RNA per adipose cell is decreased 35% with 3 d of fasting and increased to 182% of control with 6 d of refeeding after 2 d of fasting. This parallels changes in adipose cell intracellular water, so that total RNA/water space remains relatively constant. When the changes in total RNA/cell are taken into account, Northern and slot blot analyses with quantitative densitometry reveal a 36% decrease in specific glucose transporter mRNA level in cells from the fasted rats. The mRNA level in cells from the fasted/refed rats is restored to normal. These observations correlate closely with previous measurements of glucose transporter number in adipose cell membrane fractions using cytochalasin B binding and Western blotting. The levels of specific mRNAs for tubulin and actin on a per cell basis show similar but more dramatic changes and mRNAs encoding several differentiation-dependent adipose cell proteins are also significantly affected. Thus, the levels of mRNA for multiple adipose cell genes are affected by fasting and refeeding. In particular, this demonstrates that in vivo metabolic alterations can influence the level of a glucose transporter mRNA in adipose cells. This may have implications for the regulation of glucose transporter number and glucose transport activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cook K. S., Groves D. L., Min H. Y., Spiegelman B. M. A developmentally regulated mRNA from 3T3 adipocytes encodes a novel serine protease homologue. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6480–6484. doi: 10.1073/pnas.82.19.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Salans L. B. Determinations of adipose cell size and number in suspensions of isolated rat and human adipose cells. J Lipid Res. 1978 Feb;19(2):269–273. [PubMed] [Google Scholar]
- Cushman S. W. Structure-function relationships in the adipose cell. I. Ultrastructure of the isolated adipose cell. J Cell Biol. 1970 Aug;46(2):326–341. doi: 10.1083/jcb.46.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B. Starvation in the rat. I. Effect of age and obesity on organ weights, RNA, DNA, and protein. Am J Physiol. 1980 Oct;239(4):E269–E276. doi: 10.1152/ajpendo.1980.239.4.E269. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Horuk R., Matthaei S., Olefsky J. M., Baly D. L., Cushman S. W., Simpson I. A. Biochemical and functional heterogeneity of rat adipocyte glucose transporters. J Biol Chem. 1986 Feb 5;261(4):1823–1828. [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W. Qualitative and quantitative comparison of glucose transport activity and glucose transporter concentration in plasma membranes from basal and insulin-stimulated rat adipose cells. Biochem J. 1988 Jan 1;249(1):155–161. doi: 10.1042/bj2490155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W., Simpson I. A. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986 Aug 5;261(22):10033–10036. [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Mechanism for markedly hyperresponsive insulin-stimulated glucose transport activity in adipose cells from insulin-treated streptozotocin diabetic rats. Evidence for increased glucose transporter intrinsic activity. J Biol Chem. 1987 Apr 15;262(11):5118–5124. [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Subcellular translocation of glucose transporters: role in insulin action and its perturbation in altered metabolic states. Diabetes Metab Rev. 1985;1(3):203–227. doi: 10.1002/dmr.5610010301. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Simpson I. A., Cushman S. W. Divergent mechanisms for the insulin resistant and hyperresponsive glucose transport in adipose cells from fasted and refed rats. Alterations in both glucose transporter number and intrinsic activity. J Clin Invest. 1988 Aug;82(2):691–699. doi: 10.1172/JCI113649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Armoni M., Cohen P., Kanter Y., Rafaeloff R. Reversal of insulin resistance in diabetic rat adipocytes by insulin therapy. Restoration of pool of glucose transporters and enhancement of glucose-transport activity. Diabetes. 1987 Aug;36(8):925–931. doi: 10.2337/diab.36.8.925. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Barzilai A., Rafaeloff R., Armoni M. Distribution of glucose transporters in membrane fractions isolated from human adipose cells. Relation to cell size. J Clin Invest. 1986 Oct;78(4):1051–1055. doi: 10.1172/JCI112660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kasuga M., Akanuma Y., Iwamoto Y., Kosaka K. Effects of fasting and refeeding of insulin receptors and glucose metabolism in rat adipocytes. Endocrinology. 1977 May;100(5):1384–1390. doi: 10.1210/endo-100-5-1384. [DOI] [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Kuroda M., Honnor R. C., Cushman S. W., Londos C., Simpson I. A. Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem. 1987 Jan 5;262(1):245–253. [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Kasuga M., Kanazawa Y., Takaku F. Studies with antipeptide antibody suggest the presence of at least two types of glucose transporter in rat brain and adipocyte. J Biol Chem. 1988 Sep 15;263(26):13432–13439. [PubMed] [Google Scholar]
- Olefsky J. M. Effects of fasting on insulin binding, glucose transport, and glucose oxidation in isolated rat adipocytes: relationships between insulin receptors and insulin action. J Clin Invest. 1976 Dec;58(6):1450–1460. doi: 10.1172/JCI108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. L., Thompson D., Shah N., DiGirolamo M. Effects of fasting and refeeding in the rat on adipocyte metabolic functions and response to insulin. J Nutr. 1979 Sep;109(9):1584–1591. doi: 10.1093/jn/109.9.1584. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Sørensen N. S. Insulin receptor binding and insulin action in human fat cells: effects of obesity and fasting. Metabolism. 1982 Sep;31(9):884–895. doi: 10.1016/0026-0495(82)90177-9. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Shawver L. K., Olson S. A., White M. K., Weber M. J. Degradation and biosynthesis of the glucose transporter protein in chicken embryo fibroblasts transformed by the src oncogene. Mol Cell Biol. 1987 Jun;7(6):2112–2118. doi: 10.1128/mcb.7.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Smith U., Kuroda M., Simpson I. A. Counter-regulation of insulin-stimulated glucose transport by catecholamines in the isolated rat adipose cell. J Biol Chem. 1984 Jul 25;259(14):8758–8763. [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Wheeler T. J., Simpson I. A., Sogin D. C., Hinkle P. C., Cushman S. W. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem Biophys Res Commun. 1982 Mar 15;105(1):89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]