Abstract
Background
DNA sequencing techniques used to estimate biodiversity, such as DNA barcoding, may reveal cryptic species. However, disagreements between barcoding and morphological data have already led to controversy. Species delimitation should therefore not be based on mtDNA alone. Here, we explore the use of nDNA and bioclimatic modelling in a new species of aquatic beetle revealed by mtDNA sequence data.
Methodology/Principal Findings
The aquatic beetle fauna of Australia is characterised by high degrees of endemism, including local radiations such as the genus Antiporus. Antiporus femoralis was previously considered to exist in two disjunct, but morphologically indistinguishable populations in south-western and south-eastern Australia. We constructed a phylogeny of Antiporus and detected a deep split between these populations. Diagnostic characters from the highly variable nuclear protein encoding arginine kinase gene confirmed the presence of two isolated populations. We then used ecological niche modelling to examine the climatic niche characteristics of the two populations. All results support the status of the two populations as distinct species. We describe the south-western species as Antiporus occidentalis sp.n.
Conclusion/Significance
In addition to nDNA sequence data and extended use of mitochondrial sequences, ecological niche modelling has great potential for delineating morphologically cryptic species.
Introduction
DNA sequencing is an increasingly popular and important tool for the assessment of global species diversity. At present, the mitochondrial cytochrome c oxidase 1 is a standard marker, and “DNA barcoding” or “barcoding” is the name coined for this approach of DNA-based species identification [1]–[8]. Barcoding is an especially valuable tool for conservation planning, as it provides rapidly releasable quantitative biodiversity data and a glimpse of a level of diversity that extends beyond morphologically delineated entities. Barcoding uses short, standardised sequence segments of the genome, and has proven highly useful when researchers are confronted with high expected species numbers and morphologically cryptic groups ([9]–[12], see also [13]). As argued in Burns et al. [14], there exist cases in which morphologically and ecologically well distinguishable species exhibit only minimal divergence in their barcodes, and species delimitation by barcoding should not depend on arbitrarily chosen levels of divergence. Similarly, it remains unclear how to deal taxonomically with cases in which morphologically identical populations exhibit certain amounts of divergence in the mitochondrial genome [9], [10]. Apparently, the conflict between mtDNA sequence data and morphology requires consideration of other character sources in order to delimit species.
The species concept and the delimitation of species have been a matter of controversy since the early days of systematic biology. Efforts have been made to find a concept which encompasses different approaches to the species problem. DeQueiroz [15] suggested the “Unified Species Concept”, which relies on the single definition of species as “existence as a separately evolving metapopulation lineage”. Traditional species concepts like the biological [16], ecological [17], [18] or genotypic cluster [19] species concepts are “secondary species criteria” or “operational criteria”, meaning that not every single criterion must fit every species, but on the other hand more than one of these criteria may be appropriate to a species. They rather act as tools to delimit species.
Among many other possible characters, ecological factors should help delimit species, assuming that each species has formed its own particular niche. However, even for sister-species pairs having detectably distinct niches, the collection of life history data is usually problematic. Ecological Niche Modelling (ENM) is one possible approach to this problem, using widely available environmental data and universally available georeferenced distributional records as a proxy for species ecology. Van Valen [17] and Andersson [18] argued that species can be understood as groups of individuals occupying the same niche or adaptive zone. Explicit models can be based on species locality data and a raster representing various environmental, mainly climatic, variables (bioclimatic modelling). They have demonstrated their ability in circumscribing species' ecological niches and assessing their potential distributions [20]. Such models always remain restricted to a small selection of environmental variables, but nevertheless have been shown to be capable of predicting potential distributions of species and estimating the impact of the ecological variables studied [21]. Particularly when integrated with phylogenetic studies, ENMs have also proven to be a powerful tool in species delimitation [22]–[27]. To our knowledge no such studies have yet been conducted for beetles, a group for which generally relatively few analyses using ENM approaches exist [28]–[30].
Modelling approaches can aid in species delimitation only if the species studied actually diverge in their response to the environmental variables incorporated in the analysis [31]. Evidence suggests that niche conservatism, i.e., the stability of ecological niches over time, is a common pattern in closely related species and that it is a major force driving allopatric speciation [32]–[37]. Kozak and Wiens [35] postulated that certain North American salamander species are allopatric because of their inability to tolerate the climatic conditions in the lowland areas between their highland habitats, even if these differences appear relatively minor. However, most of these case studies concerned sibling species inhabiting climatically similar areas. Other studies present evidence for niche divergence between sibling species, for distributions of closely related species on environmental axes and for niche divergence as a speciation mechanism [23], [38]–[40]. This apparent contradiction suggests that neither assumption is valid for all groups of organisms and that both cases can occur in closely related species and may contribute to speciation.
We conducted a molecular biodiversity assessment of Australian diving beetles, using 3′ cytochrome c oxidase 1 sequences [41] and found divergence between geographically separated populations of one species, Antiporus femoralis (Boheman, 1958). In the absence of morphological differences, we evaluated other data sources and suggest that ecological niche modelling and nDNA characters provide evidence for the presence of a new, cryptic species which we will describe below.
Materials and Methods
Study group: Australian diving beetles
Australia's diverse and highly characteristic diving beetle (Dytiscidae) fauna offers many opportunities to study speciation and radiation events. To date, almost 300 dytiscid species are known, of which approximately 90% are endemic to the continent, belonging to 18 or 19 exclusively Australian radiations [42]–[47]. Many endemic species of diving beetle are not widespread, but rather restricted to certain climatic regions, river drainage systems or other geographical features. In southern Australia, the arid Nullarbor Plain with a West-East-extent of more than 1200 kilometres acts as a very potent geographical barrier for freshwater organisms due to its arheic conditions and its virtual lack of surface water [48]. Many groups, including the diving beetles, show patterns of disjunct distributions in south-western and south-eastern Australia, excluding the Nullarbor Plain. Geological evidence shows that this situation is a result of rather recent events [49]–[52]. During the Miocene, vast stretches of southern Australia, including the Nullarbor Plain, were covered by seas during marine intrusions, with lush tropical forests growing in the humid climate along its coast. Only after regression of sea levels, from about 10 to 6.6 million years before present, did the area fall dry and the humid conditions make way for today's arid climate.
The genus Antiporus Sharp, 1882 (tribe Hydroporini Aubé, 1836), with 16 described species to date [53]–[57], is distributed in still or slow-flowing water, mainly in south-eastern and south-western Australia, along the east coast of the continent and with one species in the Northern Territory, north-western Australia and northern Queensland. An additional species is distributed widely across New Zealand in different habitats. Watts [54] described two additional species from Western Australia, A. pembertoni Watts, 1997 and A. hollingsworthi Watts, 1997. Four additional species (A. mcraeae Watts and Pinder, 2000, A. pennifoldae Watts and Pinder, 2000, A. gottwaldi Hendrich, 2001 and A. kalbarriensis Hendrich and Watts, 2010) have been described recently. Most Antiporus species are restricted to the southwest, to the eastern coast or to south-eastern Australia, and some show remarkable regional endemism. However, two disjunct populations of A. femoralis (Boheman, 1958) have been reported from south-western and south-eastern Australia and were considered conspecific because of the lack of morphological differences (e.g. Watts [53], Brancucci [58]). In this study, we focus on these two A. femoralis populations.
DNA sequencing and data analysis
We preserved a part of our collections in pure ethanol in the field and later extracted DNA for sequencing, employing methods explained in detail in Balke et al. [59] and Hendrich and Balke [60].
For a population-level screening of all Australian diving beetles, we sequenced the 3′ end of cytochrome c oxidase 1 (cox1) [41]. In a second step, we sequenced additional genes to infer phylogenetic relations within the present focal clade, Antiporus. Genes and primers used for sequencing are given in Table S1. After detection of a possible cryptic species, we sequenced a fragment of the nuclear protein coding gene arginine kinase (ARK).
Sequences were submitted to GenBank and are publicly available under accession numbers FR727264 to FR727325 and as part of a general cox1 dataset of Australian Dytiscidae (FR 732513 to FR 733591). Individual beetles from which we extracted and sequenced DNA all bear a green cardboard label that indicates the DNA extraction number of M. Balke (e.g. “DNA 2000 M.Balke”). This number links the DNA sample, the dry mounted voucher specimen and GenBank entries.
We ran analyses for two separate datasets. Dataset one included 24 specimens from all available Antiporus species and four outgroup taxa, and 2953 characters over all five DNA loci. Dataset 2 included 70 specimens: all available A. femoralis specimens (n = 30) and other Antiporus species as outgroups. We used 799 characters of cytochrome c oxidase 1 only. The following analyses were all performed on the CIPRES portal 2.2 [61] unless stated otherwise. Both datasets were aligned using the program MUSCLE 3.7 [62]. We used jModeltest 0.1.1 [63] to choose appropriate substitution models.
We ran maximum likelihood analyses using the program GARLI [64] until 10,000 generations revealed no significant improvement of likelihood scores of the topology. We then ran resampling with 250 bootstrap replicates.
We also used Bayesian analyses with the program MrBayes 3.0 [65]. Each of two runs consisted of 4 chains which ran for 1,000,000 generations, with samplefreq = 1,000 and 25% burnin fraction. Convergence between runs and posterior probabilities of the estimates was determined by plotting the log likelihoods in Excel.
Finally, we used parsimony searches to infer phylogenetic relations as implemented in the program TNT version 1.1 (on a local desktop computer), which we also used to run 500 jackknife (removal 36%) replications to assess node stability [66] (hit best tree 5 times, keep 10,000 in memory).
Pairwise distances were computed using the Kimura 2-parameter model in MEGA 4.0 [67].We used the sequence editor Se-Al v2.0a11 (http://tree.bio.ed.ac.uk/software/seal/) to detect diagnostic characters.
Ecological niche modelling
We used the Maxent 3.3.2 [68] software for modelling the potential distribution of the two major clades of A. femoralis detected in the phylogenetic analyses. Maxent follows the Maximum Entropy principle [69] and combines presence-only data and environmental layers to create a gridded model of the potential distribution of the target species. Several studies have shown that Maxent produces better results than comparable methods [70], [71] and have confirmed its ability to predict a species' distribution outside its known range [72]–[75]. It has also been frequently used in phylogeographic studies [76], [77], some having taxonomic implications [24], [27], [78]. We obtained a total of 80 distribution points of A. femoralis (61 from eastern Australia and 19 from western Australia, Table S2) from our own databases (Hendrich unpublished) and from the ANIC database (http://anic.ento.csiro.au/database/biota_details.aspx). We excluded a single doubtful New Zealand locality that might refer to A. uncifer Sharp, 1882. Climate data was obtained from the worldclim database ([79], http://www.worldclim.org). We used the bioclimatic variables at a resolution of 2.5 arc-minutes. These 19 variables likely summarise dimensions of climate of special importance for determining species distributions [80].
As proposed by several authors [81], [82], inclusion of too many of these climate variables may cause “over-fitting” problems, as many represent similar and highly correlated dimensions of climate. Furthermore, a specific selection of predictors according to natural history properties of the target species may significantly enhance the reliability of ENMs [37]. Rödder and Lötters [83] also showed that transferability of models across space requires careful attention. To avoid misleading results, Environmental variables should be chosen with special care when models are used to predict species' distributions outside their native range.
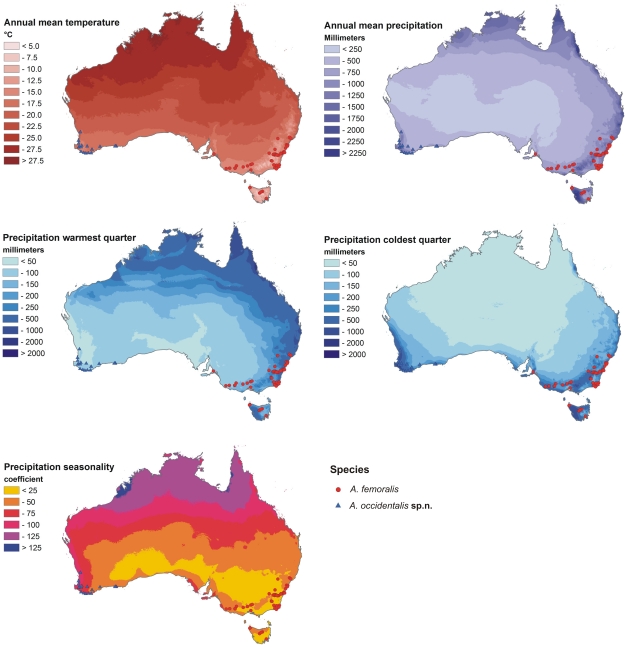
A. femoralis inhabits summer-dry wetlands and rest pools of small rivers and creeks having a high seasonal variation in water volume, many of which fall almost completely dry during the dry season (November to March). Thus, precipitation and its seasonal variation is the climatic factor assumed to have the highest impact on the long-term persistence of A. femoralis populations. Temperature may also be important as higher insolation and thus higher temperature causes drought. Therefore, aside from “annual mean temperature” and “annual precipitation”, we chose factors representing the interaction of precipitation and temperature and the seasonality of these factors, i.e., “precipitation warmest quarter”, “precipitation coldest quarter” and “precipitation seasonality”. This latter factor gives a direct measurement of the strength of the seasonality, whereas values of precipitation of the warmest and coolest quarters indicate its direction.
We used the default Maxent settings with a random test percentage of 25% of the input localities set aside for model testing. We chose the logistic output format, displaying suitability values from 0 (unsuitable) to 1 (optimal) [84]. Jackknifing was performed to measure the importance of the variables. Model validation was conducted by calculating the area under the curve (AUC), which reflects the model's ability to distinguish between presence records and random background points [68], [85]. AUC values range from 0.5 for models without any predictive ability to 1.0 for models with perfect predictive ability. According to Swets [86], AUC values >0.9 are considered to have ‘very good’, >0.8 ‘good’ and >0.7 ‘useful’ discrimination abilities.
We performed ENMs using locality data of A. femoralis from eastern Australia and of A. femoralis from Western Australia, restricting background data to areas likely to be colonizable for the species as recommended by Phillips et al. [87] Therefore, we manually delimited areas encompassing the known localities, separating them from completely arid areas away from the coast (Fig. S1). We also performed an ENM using data from all A. femoralis individuals pooled together. All runs were performed with 100 bootstrap repeats. Test localities were randomly selected anew for each repeat, and mean output values were used as final results.
For further statistical analysis of the modelling results, we used the ENMtools software [88]. We measured niche overlap of A. femoralis from eastern Australia and of A. femoralis from Western Australia using Schoener's D [89] and the I statistic, modified from the Hellinger distance [90].
We also used two hypothesis tests included in ENMtools. First, we used the niche identity test to determine whether the ENMs generated for the two species are identical or exhibit statistically significant difference. The test combines the samples of both species into a common pool. Under the assumption that the species behave interchangeably in their use of ecological niche space, their identities are randomized, and two new samples with the same sizes as the original samples are extracted. By repeating this process, a set of pseudoreplicates is generated. The results are compared with the true calculated niche overlap (see above). The lower the true niche overlap in comparison to the scores created by the pseudoreplicates of the pooled samples is, the more significant the niche difference between the two species compared.
Second, we used the background test to evaluate the null hypothesis that all divergence in the ecological niches of two taxa, given that the niches are represented by two sets of localities, can be explained by the differences in their environmental feature spaces. Specifically, we use it to ascertain whether ENMs of A. femoralis from eastern Australia and of A. femoralis from western Australia are more or less similar than expected based on the environmental differences in their completely disjunct ranges. This test is particularly appropriate for allopatric species because in many cases, distinct geographic spaces provide a different set of environmental conditions. That is, differences in ENMs may result from niche space availability rather than from niche diversification [30]. The test places random occurrence points within the range of one of the two species to be compared and measures niche similarity between these points and the original localities of the second species. If the true measured overlap values are significantly higher (or lower) than the values generated by the background test, the null hypothesis that ENMs are more similar (or divergent) than based on habitat availability is rejected. This test is conducted in both directions, and different directions may yield different results.We performed the identity test, as well as background tests in both directions, with 500 iterations.
Morphology and taxonomy
Specimen depositories:
ANICAustralian Insect Collection, Canberra, Australia
CFPCollection Fernando Pederzani, Ravenna, Italy
CLHCollection Lars Hendrich, Berlin, Germany, property of NMW
CSRCollection Saverio Rocchi, Firenze, Italy
NMWNaturhistorisches Museum Wien, Austria
SAMASouth Australian Museum, Adelaide, South Australia, Australia
WAMWestern Australian Museum, Perth, Western Australia, Australia
ZSMZoological State Collection, Munich, Germany
Beetles were examined using a Leica MZ 12.5 dissecting scope at 10–100x. Male genitalia were studied and figured in wet conditions. Images of male genitalia were made using incident light and a digital photo imaging system, composed of a Leica DM 2500 M microscope and a Tucsen 5.0 MP camera. The microscope was fitted with Leica HCX PL “Fluotar” 5x and 10x metallurgical grade lenses [91]. Habitus images were taken with a Nikon D700, equipped with a bellows and Leica Photar 2.8/25 mm lens. Image stacks were aligned and assembled in Helicon Focus 4.77TM.
The terminology to denote the orientation of the genitalia follows Miller and Nilsson [92]. Coordinates are given in decimal notation unless cited verbatim from labels. To determine the position of these localities, we used various Australian road maps and Google Earth (http://earth.google.com).
Nomenclatural acts
The electronic version of this document does not represent a published work according to the International Code of Zoological Nomenclature (ICZN), and hence, the nomenclatural acts contained in the electronic version are not available under that Code from the electronic edition. Therefore, a separate edition of this document was produced by a method that ensures numerous identical and durable copies, and those copies were simultaneously obtainable (from the publication date noted on the first page of this article) for the purpose of providing a public and permanent scientific record, in accordance with Article 8.1 of the Code. The separate print-only edition is available on request from PLoS by sending a request to PLoS ONE, 185 Berry Street, Suite 3100, San Francisco, CA 94107, USA along with a cheque for US $10 (to cover printing and postage) payable to “Public Library of Science”.
In addition, this published work and the nomenclatural acts it contains have been registered in ZooBank (http://zoobank.org/), the proposed online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved, and the associated information can be viewed, through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:445E5E19-C6A5-46C5-84AF-B35986BB7AAE.
We deposit printed copies of the work in the libraries of:
CSIRO Entomology (Canberra, urn:lsid:biocol.org:col:32981),
Natural History Museum London (urn:lsid:biocol.org:col:1009),
Naturhistorisches Museum Wien (Vienna, urn:lsid:biocol.org:col:34043),
Queensland Museum (Brisbane, urn:lsid:biocol.org:col:34161),
South Australian Museum (Adelaide, urn:lsid:biocol.org:col:34244) and
Zoologische Staatssammlung (Munich, urn:lsid:biocol.org:col:34660).
Results
Molecular phylogenetics
jModeltest selected the GTR+G model for all gene regions but cytochrome c oxidase 1 and histone 3, for which the GTR+I+G model was selected. These models were used for all further analyses. Where partitioning was not possible, the GTR+G model was used.
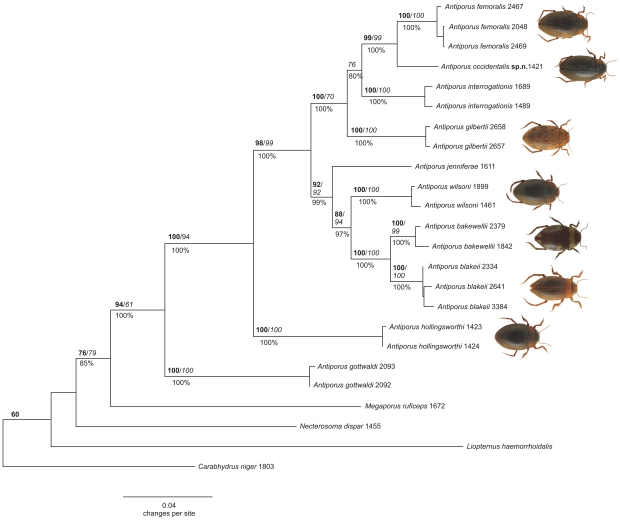
Maximum likelihood, Bayesian and parsimony analysis of a multigene dataset of Australian Antiporus all yielded very similar topologies with generally significant node support values (Fig. 1). Four specimens that we initially identified as Antiporus femoralis always formed a monophyletic group, but the single Western Australian specimen diverged from the remaining three specimens, all from the eastern part of Australia, by 6.5%. The sister species of that clade is either A. interrogationis or A. gilbertii. The Bayesian analysis supported A. interrogationis as sister taxon to the A. femoralis clade. Maximum likelihood and parsimony analyses yielded a clade comprising A. interrogationis and A. gilbertii as sister group to the A. femoralis clade, albeit with support values of less than 60 in both cases (not shown).
Figure 1. Phylogram of the genus Antiporus.
The phylogram is based on a maximum likelihood tree with 5 gene loci and 2953 characters made in GARLI. Branch values are: GARLI bootstrap (bold/above branch), TNT jackknife (italic/above branch), and MrBayes posterior probability (below branch). Each tip represents one specimen. Specimen collection numbers are given after the species name.
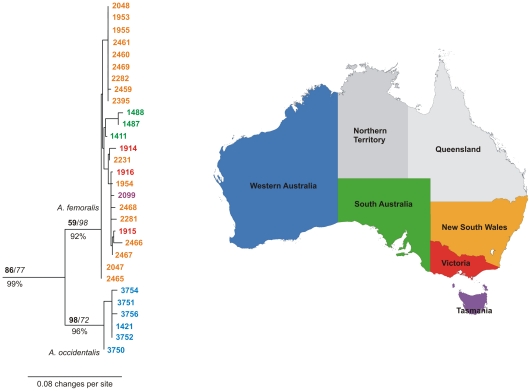
Analysis of cox1 for 30 specimens from the A. femoralis clade clearly confirmed a subdivision into a western and an eastern clade (Fig. 2). Within the eastern and western groups of A. femoralis, pairwise distances were 0.0% to 2.9% (mean 0.7%±0.7%) for eastern and 0.0% to 1.0% (mean 0.5%±0.3%) for western A. femoralis. The divergence between the two clades was 3.5% to 6.6% (mean 4.46%±0.6%).
Figure 2. Phylogram of Antiporus femoralis and Antiporus occidentalis sp.n.
Tree based on a cytochrome c oxidase 1 tree with 799 characters made in GARLI. Branch values are: GARLI bootstrap (bold), TNT jackknife (italic), and MrBayes posterior probability (below branch). Each tip represents one specimen. Outgroups (A. interrogationis, A. jenniferae, A. wilsoni, A. bakewellii, A. blakeii, A. gilbertii, A. hollingsworthi, A. gottwaldi and Sternopriscus eikei) are not shown. Colours of specimen numbers represent their state of origin, see map of Australia on the right.
Within eastern A. femoralis, only specimens from South Australia seem to form a monophyletic group, but this clade of three individuals is not significantly supported. The only morphologically divergent specimen, which is larger and darker and originates from Tasmania (“DNA M. Balke 2099”), is nested in a clade comprising specimens from New South Wales and Victoria.
A 510-bp fragment of the nuclear protein coding gene arginine kinase was successfully amplified for specimens from both clades. The sequence divergence was 1.39%, and six parsimony-informative sites were identified.
Ecological Niche Modelling
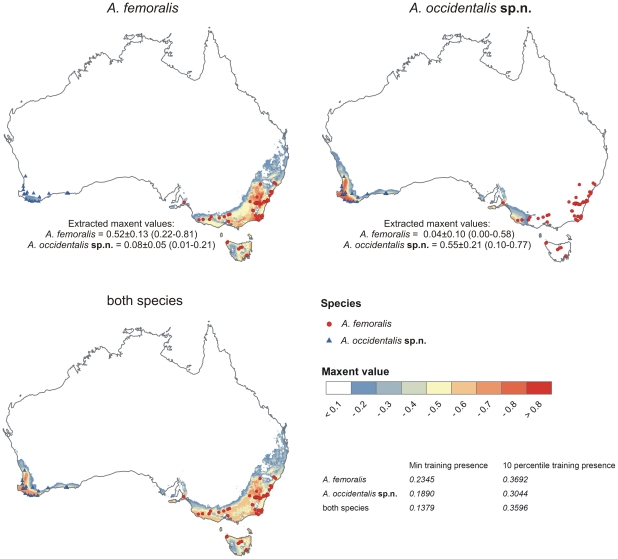
Ecological niche models are visualised in Fig. 3. According to their AUC values, the ability to distinguish presence from random background points of all models was larger than 0.9 and thus considered ‘very good’ according to the classification of Swets [86]. AUC values were 0.982 for the ENM of eastern and 0.993 for the ENM of western A. femoralis. The ENMs of both species together had a slightly lower AUC of 0.977.
Figure 3. Ecological niche models.
Localities of Antiporus femoralis (blue triangles) and Antiporus occidentalis sp.n. (red circles), displayed on the backgrounds of Maxent-created ecological niche models. Higher Maxent values (yellow and red colours) represent areas more suitable for the species according to the Maxent models, lower values (green and blue or white colours) represent areas less suitable.
Analysis of the environmental variable contribution showed that for the distributions of eastern as well as western A. femoralis, “precipitation coldest quarter” was the variable of highest importance (Table S3). “Annual mean temperature” and “annual precipitation” were the second and third most important predictors in the models of eastern A. femoralis and of both groups together. “Annual mean temperature” also provided the highest training gain when used in isolation. For western A. femoralis, “precipitation warmest quarter” and “precipitation seasonality” were the second and third most important variables. For the model that included both species, variable importance was similar to that found for eastern A. femoralis.
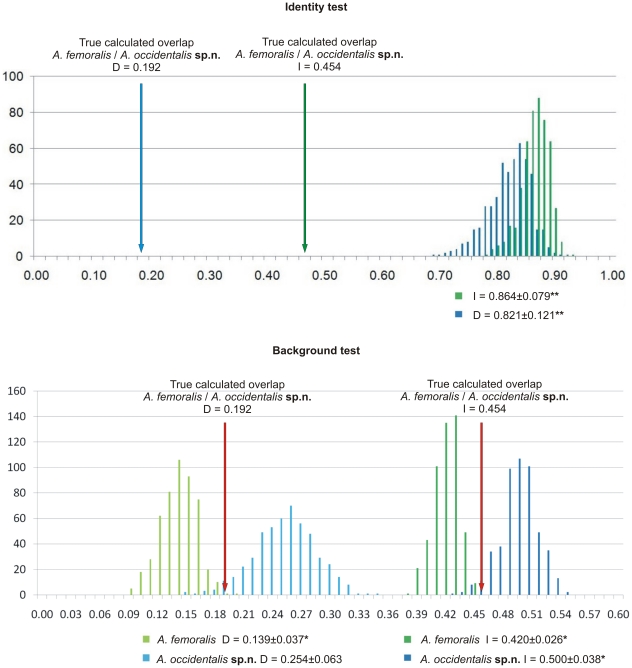
The measured niche overlap between eastern and western A. femoralis was I = 0.454 and D = 0.192. Values close to 0 describe little overlap in ecological niches and values close to 1 describe high similarity. The overlap between the niches of eastern and western A. femoralis can therefore be considered low, judging from these values alone. Note that values of D are generally lower than of I.
The results of the identity and background tests are shown in Fig. 4. According to the identity test, the null hypothesis of niche identity is rejected, meaning that the climate envelopes of eastern and western A. femoralis, as modelled here, are highly significantly distinct. In the background test, the null hypothesis that differences in the ecological niches can be explained by environmental differences in their areas of occupancy alone is rejected. The niches are significantly (I and D) more similar than expected based on the distribution of eastern A. femoralis and significantly (I only) more different based on the distribution of western A. femoralis.
Figure 4. Results of the identity and background tests.
Arrows indicate the results of ENMtools' niche overlap test representing the true calculated niche overlap. Columns represent the niche overlap values created in the replicates of the identity and background tests. The true calculated overlap values (I and D) are far outside the 99.9% confidence intervals of the identity test results and thus highly significant (indicated by two asterisks **). For the background tests, results are given for A. femoralis (compared to the background of A. occidentalis sp.n.) and for A. occidentalis sp.n. (compared to the background of A. femoralis). If marked with an asterisk *, the true calculated niche overlaps are outside the 95% confidence intervals but not outside the 99.9% confidence intervals of the background test results and are therefore significant.
Taxonomic treatment
Evidence from mtDNA and nDNA sequences, combined with results of ecological niche modelling, suggests presence of two species. Antiporus femoralis was described from New South Wales: Sydney, within the geographical range of the eastern clade. Thus, we assign the new species name A. occidentalis sp.n. to the western clade.
Antiporus occidentalis sp.n
Figure 5. Habitus photographs.
Habitus of a) Antiporus femoralis (male, SE Australia), b) A. occidentalis sp.n. (male, SW Australia) (scale bar 1 mm). Ventral views of median lobes of aedeagi of c) A. femoralis and d) A. occidentalis sp.n. (scale bar 0.4 mm). Minor differences between median lobi of A. femoralis and A. occidentalis sp.n. (c, d) are attributed to individual variability. Photos: L. Hendrich.
http://www.species-id.net/w/index.php?title=Antiporus_occidentalis&oldid=2012
urn:lsid:zoobank.org:act:481E4A90-4127-4B33-B878-A35F33A0A35F
Type locality
Australia: Western Australia, Lane Poole Conservation Reserve, Nalyerin Lake.
Type material
Holotype: Male: “AUSTRALIA/WA: Lane Poole Conservation Reserve, Nalyerin Lake, 300 m, 29. & 30.12.1999, Hendrich leg. (loc.4/151)”, “DNA M.Balke 3757”, [green printed label], “HOLOTYPE Antiporus occidentalis sp.n. des. 2010” [red label, printed] (WAM).
Paratypes. 8 specimens with same locality data as holotype (7 specimens with “DNA M.Balke 3750”, “3751”, “3752”, “3753”, “3754”, “3755”, “3756” [green labels, printed]) and “PARATYPE Antiporus occidentalis sp.n. Hawlitschek, Hendrich & Balke des. 2010” [red label, printed] (SAMA, CLH, ZSM); 2 exs., “AUSTRALIA, WA, 10 Km S Cataby, Brand Highway, Nammegarra Road, 9.9.2002, 30°53′S 115°36′E, Hendrich leg./Loc. 29/193” (1 specimen with “DNA M. Balke 1421” [green label, printed]) (CLH); 1 ex., “Australia, WA/North of Bunbury, Yalgorup N.P., east of Preston Beach, 0 m, 24.11.1996, L. Hendrich leg./Coll. Lok. 30” (CLH); 3 exs., “Australia,WA/Nannup, “Wildflower Walk” n. Nannup 100 m, 25.11.1996, L. Hendrich leg./Coll. Lok. 32” (CLH); 6 exs., “Australia, WA/Nannup, Balingup-Nannup Road, Revelly Bridge, 130 m, 25.11.1996, L. Hendrich leg./Coll. Lok. 33” (CLH); 1 ex., “Australia, WA/5 km S Northcliffe, 10 m, 27.11.1996, L. Hendrich leg./Coll. Lok. 37” (CLH); 1 ex., “Australia, WA/20 km NW Walpole, Interstate Hwy. No. 1, 27.11.1996, L. Hendrich leg./Coll. Lok. 38” (CLH); 2 exs., “Australia, WA/Walepole-Nornalup N.P., Peaceful Bay, 0 m, 28.11.1996, L. Hendrich leg./Coll. Lok. 39” (CLH); 3 exs., “Australia, WA/Stirling Range N.P., Stirling Range Drive in Richtung Red Gum Pass, 450 m, 29.11.1996, L. Hendrich leg./Coll. Lok. 41” (CLH); 1 ex., “Australia (WA), Nannup envir., roadside creeks, 1.12.95 Pederzani” (CFP); 16 exs., “Australia (WA), Pemberton, pond, Della Franca farm, 3.12.98 Pederzani” (CFP, CSR); 2 exs., “AUSTRALIA/WA: Nannup, Balingup-Nannup Road, Revelly Bridge, 130 m, 31.12.1999, Hendrich leg. (loc.6/153)” (CLH); 3 exs., “AUSTRALIA/WA: 5 km S Northcliffe, 50 m, 2.1.2000, Hendrich leg. (loc.10a/156)” (CLH); 2 exs., “AUSTRALIA/WA: D′Entrecasteaux N.P., 15 km S Northcliffe, Windy Harbour Road, 50 m, 3.1.2000, Hendrich leg. (loc. 10c/156)” (CLH); 1 ex., “AUSTRALIA/WA: Albany Hwy, Muir Lakes Nature Reserve, SW part of Byenup Lagoon, 4.&5.1.2000, Hendrich leg. (loc. 11/157)” (CLH). 1 ex., “WA Cannington 14/08/1924/32°01′00“S 115°57′00“E L. Glauert leg.” [40086] (WAM); 1 ex., “WA Cokatea Creek Tenindewa 8/01/1926” [40708] (WAM); 1 ex., “WA Wanneroo Melaleuca Park, 14/08/1976 31°40′25”S 115°53′23”E Southwell-Keely leg.” [42685] (WAM); 3 exs., “WA Banksiadale 01/05/1969 32°38′S 116°06′E D.S. Adair leg.” [42736, 42737, 42738] (WAM); 3 exs., “WA Bullsbrook Tortoise Reserve 10/1963 31°39′S 115°59′E Zoological Honours Class leg.” [42739, 42740, 42741] (WAM).
Etymology
A western Australian species.
Description
Body in dorsal view rotundate-oval, convex, widest behind the middle. http://www.species-id.net/o/index.php?title=File:Antiporus_occidentalis_dorsal.jpg&oldid=109760.
Measurements
Total length of beetle = 4.6–4.9 mm (holotype 4.8 mm); total length without head = 4.4–4.7 mm (holotype 4.6 mm); maximum width = 2.3–2.5 mm (holotype 2.4 mm).
Colour
Upper side reddish brown; some portions with small and less extended dark brown or black patches. Head uniformly black, reddish brown on the anterior part. Antenna testaceous, distal joint apically darkened. Pronotum reddish brown with large patch on middle part which does not reach the anterior border. Elytra reddish brown with small and less extended dark brown or black patches (Fig. 5b). Venter black, including pronotum, epipleuron, metaventrite, metacoxal plate and prosternal process. Legs and abdominal sternites reddish brown.
Sculpture
Head finely microreticulated, regularly and densely punctured, coarser around the clypeal grooves. Interstices between punctures larger than the diameter of the punctures, particularly on the disc.
Pronotum semi-matt, very finely microreticulated. Sides of pronotum regularly and gently curved. Puncturation regular on the whole surface, except on a round area situated on both sides of the disc where the punctures are more sparse and on the lateral border where they are coarser and very close. Pronoto-elytral angles obtuse.
Puncturation on elytra regular and very dense, covering the whole surface. The interstices between punctures are narrower than the diameter of punctures, but less so on the apical half. Ground sculpture finely microreticulated, semi-matt on the basal half, shagreened on the apical half.
Ventral surface; prosternal process narrowly lanceolate, rounded tip, weakly carinate in cross section, slightly narrowed between procoxae. Metacoxal lines raised, moderately separated, subparallel in posterior half, diverging to about twice their narrowest width in anterior half. Metacoxae and sternites very strongly punctured.
Male
Pro- and mesotarsi moderately expanded, robust; single proclaw thickened, sharply curved and with a small tooth near base. Metafemora slightly incised into a triangular process near apex. Last abdominal sternite rounded in middle. Parameres broad and rounded. Median lobe of aedeagus in ventral view very broad, strongly bilobed towards tip (Fig. 5d), in lateral view rather thin and elongated. Minor differences between median lobi of A. femoralis and A. occidentalis sp.n. (Fig. 5) are attributed to individual variability.
Female
Pro- and mesotarsi narrower than in males, not expanded. Proclaws simple. Mesotibia narrow.
Affinities
The new species is the sister species of A. femoralis and cannot be separated using morphological characters such as size, colour and form of median lobe (Fig. 5). However, the species are allopatric: Antiporus occidentalis sp.n. occurs in south-western Australia, and A. femoralis in south-eastern Australia, south of Brisbane, along the east coast to Victoria, South Australia and Tasmania.
Distribution
South-western Australia. South of a line from Carnavon to the Stirling Ranges (Fig. 3).
Habitat
Antiporus occidentalis sp.n. was collected from shaded or at least half-shaded pools, peatland swamps and lakes, overgrown roadside ditches and rest pools of intermittent creeks (Fig. S2), from the coast (Preston Beach near sea level) up to 450 m in the Stirling Ranges. In contrast to the south-eastern Australian A. femoralis, it seems that the species prefers more peaty water with a dark bottom consisting of mud, peat and plant debris.
Antiporus femoralis (Boheman, 1858)
Hydroporus femoralis Boheman, 1858: 19.
Antiporus femoralis (Boheman, 1858): Watts 1978: 67; Brancucci 1984: 151; Watts 1997: 36.
Type locality
Australia: New South Wales, Sydney.
Material examined
New South Wales: 2 exs., C NSW, 25 km N Wollongong, Darkes Forest, Maddens Fall Lookout, 480 m, 29.X.2006, 34.13.335S 150.54.465E, L. & E. Hendrich leg. (NSW 86); 2 exs., C NSW, 17 km SE Nowra, Jerwis Bay NP, Coonemia Road, 54 m, 31.X.2006, 34.58.156S 150.43.045E, L. & E. Hendrich leg. (NSW 90); 2 exs., C NSW, 1 km N Nowra, Bomaderry, Bomaderry Creek, 71 m, 31.X.2006, 34.50.383S 150.35.509E, L. & E. Hendrich leg. (NSW 91); 3 exs., C NSW, 10 km S Nowra at Falls Creek, Parma Creek, 27 m, 1.XI.2006, 34.58.104S 150.35.415E, L. & E. Hendrich leg. (NSW 92); 2 exs., C NSW, 40 km SW Nowra, Braidwood Road, Tianjara Creek, 498 m, 1.XI.2006, 35.06.382S 150.20.037E, L. & E. Hendrich leg. (NSW 93); 2 exs., C NSW, Endrick River at Braidwood Road, 554 m, 1.XI.2006, 35.05.193S 150.07.182E, L. & E. Hendrich leg. (NSW 94); 8 exs., C NSW, 48 km NE Braidwood, Corang Creek, 589 m, 1.XI.2006, 35.10.488S 150.04.101E, L. & E. Hendrich leg. (NSW 95); 2 exs., C NSW, 10 km W Braidwood, Shoalhaven River at Bombay Bridge, 628 m, 2.XI.2006, 35.25.419S 149.42.582E, L. & E. Hendrich leg. (NSW 96); 10 exs., S NSW, 2 km NE Queanbeyan, Molonglo Gorge, 584 m, 12.XI.2006, 35.19.313S 149.15.029E, L. & E. Hendrich leg. (NSW 101); 2 exs., S NSW, 3 km N Jindabyne, Wollondibby Creek, 928 m, 13.XI.2006, 36.23.406S 148.35.533E, L. & E. Hendrich leg. (NSW 102); 2 exs., S NSW, Mt. Kosciusko NP, Diggers Creek (Alpine lake), 1517 m, 14.XI.2006, 36.21.357S 148.29.281E, L. & E. Hendrich leg. (NSW 106); 4 exs., S NSW, 12 km SW Delegate, Bog Road, 812 m, 15.XI.2006, 37.04.356S 148.53.260E, L. & E. Hendrich leg. (NSW 108); 1 ex., S NSW, Imlay Road, White Rock Picnic Area, 497 m, 15.XI.2006, 37.08.039S 149.21.324E, L. & E. Hendrich leg. (NSW 109); 1 ex., S NSW, Imlay Road, 8.5 km E from Monaro Hwy to Eden, 564 m, 15.XI.2006, 37.08.029S 149.25.028E, L. & E. Hendrich leg. (NSW 110); 2 exs., S NSW, 6.5 km SW Eden, Towamba Road 2 km N Nullica, 556 m, 16.XI.2006, 37.04.412S 149.51.200E, L. & E. Hendrich leg. (NSW 111); 1 ex., S NSW, Wallagaraugh River Picnic Area, 43 km SW Eden, 54 m, 17.XI.2006, 37.22.079S 149.43.073E, L. & E. Hendrich leg. (NSW 112). Victoria: 2 exs., E VIC, Tonghi River at Hwy 1 3–5 km SW Cann River, 126 m, 17.XI.2006, 37.33.503S 149.03.546E, L. & E. Hendrich leg. (VIC 115); 1 ex., S VIC, Simpsons Creek 12 km SW Orbost at Princess Hwy, 31 m, 18.XI.2006, 37.45.095S 149.20.436E, L. & E. Hendrich leg. (VIC 116); 2 exs., C VIC, Hughes Creek at Avenel, 161 m, 25.XI.2006, 36.54.221S 145.14.191E, L. & E. Hendrich leg. (VIC 120); 2 exs., C VIC, 5–7 km W Puckapunyal, street to Tooborac, 205 m, 25.XI.2006, 37.00.282S 144.58.415E, L. & E. Hendrich leg. (VIC 122); 3 exs., C VIC, Kyneton, Boggi Creek, Mineral Springs Picnic Area, 485 m, 26.XI.2006, 37.14.094S 144.25.259E, L. & E. Hendrich leg. (VIC 123); 3 exs., W VIC, Grampians, 7 km NW Dunkeld, street to Lavendish, 229 m, 27.XI.2006, 37.38.510S 142.17.507E, L. & E. Hendrich leg. (VIC 125); 3 exs., W VIC, Grampians, Wannon River, 5 km N Dunkeld, 236 m, 27.XI.2006, 37.37.494S 142.20.226E, L. & E. Hendrich leg. (VIC 126); 2 exs., W VIC, Grampians, Fyans Creek, 15 km S Halls Gap, 363 m, 27.XI.2006, 37.14.595S 142.32.240E, L. & E. Hendrich leg. (VIC 128). South Australia: 1 ex., SE SA, 10–12 km N Mt. Gambier, Mt. Gambier Forest Reserve, 77 m, 29.XI.2006, 37.42.308S 140.47.523E, L. & E. Hendrich leg. (SA 135); 3 exs., SA, Meadows Creek at Kuitpo Forest, 286 m, 3.XII.2006, 35.12.367S 138.42.004E, L. & E. Hendrich leg. (SA 137). Tasmania: 1 ex., NW TAS, Montagu River at Togari, 41 m, 12.XII.2006, 40.54.545S 144.52.399E, L. & E. Hendrich leg. (TAS 145); 3 exs., NW TAS, Welcome River at Hwy A 2, 44 m, 12.XII.2006, 40.57.004S 144.48.325E, L. & E. Hendrich leg. (TAS 146); 10 exs., C TAS, CPCA, 500 m E Lake Ada, pools, 1154 m, 14.XII.2006, 41.52.575S 146.28.432E, L. & E. Hendrich leg. (TAS 149).
Description
Morphology and size as in the above species. Minor differences between median lobi of A. femoralis and A. occidentalis sp.n. (Fig. 5) are attributed to individual variability.
Remarks. Specimens from Tasmania are larger and darker than specimens from the mainland.
Distribution
South-eastern Australia. From around Sydney along the east coast south to Victoria, Tasmania and South Australia, including Port Lincoln and Kangaroo Island (Watts 1978, 1997) (Fig. 3).
Habitat
The species inhabits a wide variety of freshwater habitats and can be found in slow-flowing creeks, rest pools of intermittent streams and rivers, ponds, old farm dams, ditches, and seasonal or permanent sedge swamps from near sea level up to an altitude of 1154 m. The ideal habitat should be rich in rotten leaves or plant debris and overgrown with sedges or reed (Fig. S2).
Discussion
Taxonomy
We strongly support the utilisation of Internet technology to enhance dissemination of taxonomic knowledge (e.g., Knapp [93] for an example from this journal), like SpeciesID (http://www.species-id.net/w/index.php?title=Antiporus_occidentalis&oldid=2012). WikiSpecies pages, in our opinion the best taxonomic information facility on the web (see also Page [94] on WikiPedia), are given in Text S1. The species pages have links to GenBank entries and additional material such as habitat photos. Where necessary, they will be updated to provide further data as they become available.
Phylogeny
We inferred a fully resolved phylogeny for 10 of the 16 species of the genus Antiporus, with four major lineages. Antiporus gottwaldi is the sister taxon to all other species. Next, A. hollingsworthi is the sister taxon to all remaining species except A. gottwaldi. The remaining species are divided into two clades. Species from the clade including A. femoralis are distributed in the southern parts of Australia (from southern New South Wales, Victoria, Tasmania, South Australia and the southern part of Western Australia), while members of the clade including A. bakewellii range from Northern Territory to northern Queensland (A. jenniferae) and down the east coast to Tasmania (A. blakeii). Although A. blakeii and A. femoralis belong to separate clades, their distribution is almost congruent. These broadly sympatric species belong to different clades. Occasionally, both species can be found in the same habitat (e.g., Tasmania, Victoria).
Ecological niche modelling of A. femoralis and A. occidentalis sp.n
Our results indicate that A. femoralis and A. occidentalis sp.n. differ in their realised ecological niches, represented here by their modelled climate envelopes. As suggested by the results shown in Fig. 6, the distributions of both species depend heavily on winter rain. However, the variables representing a high level of seasonal variation in climate (“precipitation warmest quarter” and “precipitation seasonality”) are more important in the A. occidentalis sp.n. model, while in the A. femoralis model they are of lower relative importance than “annual mean temperature” and “annual precipitation”.
Figure 6. Climate variables.
A projection of Antiporus femoralis (blue triangles) and Antiporus occidentalis sp.n. (red circles) localities on climate variables. Note that localities of both taxa are situated in areas with relatively high precipitation in the coldest quarter. In the warmest quarter, most localities of A. femoralis also receive high precipitation, while localities of A. occidentalis sp.n. are predominantly dry in this season. This effect is also visualised as precipitation seasonality, where A. femoralis inhabit areas with relatively low precipitation seasonality, and A occidentalis sp.n. inhabit areas with moderate precipitation seasonality.
As shown in Fig. 3, the distributions both of A. femoralis and A. occidentalis sp.n. cover areas highly suitable for both taxa according to the ENMs. However, A. femoralis is commonly found at localities that are less suitable for A. occidentalis sp.n. and vice versa.
Fig. 5 shows that areas of occupancy of both A. femoralis and A. occidentalis sp.n. correspond to relatively high precipitation in the Southern Hemisphere winter (coldest quarter). However, A. femoralis lives in areas where summer (warmest quarter) precipitation is at a level similar to that in winter, whereas A. occidentalis sp.n. inhabits areas with very dry summers. Apparently, the main difference between the climatic envelopes of these two Antiporus species is the summer drought in the area of A. occidentalis sp.n. The ecological validity of this difference is also confirmed by the decrease in regularized training gain if “precipitation warmest quarter” is omitted from the model of A. occidentalis sp.n. This pattern suggests a possible niche divergence between the two taxa, with A. occidentalis sp.n. showing a preference for areas with lower summer precipitation and A. femoralis preferring areas with relatively wet summers and low seasonal differences in precipitation. Given the nature of ENMs, especially the restricted set of abiotic variables and the complete exclusion of biotic variables from the analyses, such results must be treated with caution [33], [80]. The apparent divergence in climatic envelopes might be due to abiotic factors not included in the analysis, such as differences in microhabitat structures, soil or water chemistry. It might also be influenced by biotic factors. Thise might, for example, be predators or competing species present in the area of A. occidentalis sp.n. Both species occur in syntopy with several other species of dytiscid beetles with similar ecology, but none of these syntopic similar species are present in the ranges of both Antiporus species [53]. The presence of these other species might keep A. occidentalis sp.n. from occupying the niches of its Western sibling taxon, A. femoralis.
We used two approaches to model validation to address these possible problems. First, the hypothesis that A. femoralis and A. occidentalis sp.n. occupy different environmental niches was tested by comparing ENMs of the two species to a model based on both species together. As described in Raxworthy [24], species delimitation by ecological niche modelling is most reliable if models of each split clade alone are superior (according to better fit and more significant statistical model validation) to models of all clades lumped together. Models in which this is not the case might also be validated by including negative locality data (but see [95]), which in the present case has not been available. As all models have fits considered “very good”, this criterion does not contribute to the verification of species delimitation in the case of A. occidentalis sp.n.
Another method of model validation is the use of various statistical tests, as implemented in the ENMtools software [88], [90]. According to the results of the identity test, niche diversification between these two sibling species must be considered highly significant. The climate envelope of A. occidentalis sp.n. is very different from that of A. femoralis. The background test yields results in which the significance is much smaller in magnitude than that of the identity test results. Nevertheless, the background test results indicate that this divergence cannot be attributed to the ecological difference in the species' allopatric ranges alone. This suggests that in the area of occupancy of A. occidentalis sp.n., a different climate space is available than in the range of A. femoralis.
However, the results of the two test runs seem to contradict each other. The climate envelopes of both species are more divergent than expected based on localities of A. occidentalis sp.n. (and on random test samples drawn from the background of A. femoralis), but they are more similar than expected based on the reverse comparison (Fig. 4, explained in Fig. S3). Nakazato et al. [96] performed background tests for species distribution models of four sibling species pairs and obtained a variety of outcomes. Whereas identity tests yielded highly significant results, sibling species were either ecologically more divergent, less divergent or not significantly divergent according to the background tests. One case resembled that of Antiporus: species were either more or less divergent depending on the direction of the test. The authors explain this counterintuitive result by differences in the heterogeneity of the species' environmental backgrounds.
In our view, the identity tests clearly indicates that A. femoralis and A. occidentalis sp.n. are ecologically divergent. This divergence may result from their exposure to different environmental backgrounds alone, but it may also be result from evolutionary niche diversification. The results of the background test do not contradict the latter assumption. They simply state that this diversification is higher than expected if tested one way and lower than expected if tested the other way.
Speciation/species delimitation in A. femoralis and A. occidentalis sp.n
In our view, A. occidentalis sp.n. constitutes a valid species according to the unified species concept, as it represents a metapopulation lineage evolving separately from other metapopulation lineages, including that represented by its closest relative, A. femoralis. In this paper, we used two different approaches to validate this hypothesis. First, a taxonomic/phylogenetic approach using morphological and molecular genetic data was employed. The morphological analysis showed that A. occidentalis sp.n. is indistinguishable from A. femoralis. Genetic data, however, unambiguously supported presence of two clades, and the relatively high cox1 divergence (>6%) clearly suggested further investigation into the possible presence of a cryptic species [97]. The operational criterion applicable to this result is the genotypic cluster of Mallet [19]. This criterion defines species as identifiable clusters having no intermediates.
In our second approach, we used ecological data to test the ecological species concept, as proposed in Van Valen [17] and Andersson [18], as operational criterion. According to this concept, individuals occupying the same niche or adaptive zone constitute a species. The results of our modelling suggest that A. femoralis and A. occidentalis sp.n. do not occupy the same niche. The difference in their niches can be attributed largely, but not completely to the different environmental conditions prevailing in their distributional ranges. The distributional range of A. occidentalis sp.n. features drier summers and generally higher seasonal variation in precipitation than those experienced by A. femoralis. In our view, these two operational criteria support the assessment of A. occidentalis sp.n. as a separately evolving metapopulation lineage.
Precise estimation of the age of separation using a molecular clock approach is difficult due to the lack of reliable calibration points. Other pairs of dytiscid species (Hyderodes shuckardi Hope, 1838 and H. crassus Sharp, 1882, Spencerhydrus latecinctus Sharp, 1882 and S. pulchellus Sharp, 1882) are known to exhibit a distribution pattern similar to A. femoralis and A occidentalis sp.n., but no studies on molecular dating have yet been performed. The observed intraspecific distance suggests that A. femoralis and A. occidentalis sp.n. have remained in evolutionary separation for a long time. Applying the “molecular clock” evolution rates of about 3.54% divergence per million years (myr) of Papadopoulou et al. [98] to the minimum interspecific cytochrome c oxidase 1 distance suggests that the two lines have split around 1.0 to 1.9 myr ago. As shown by various studies, age estimations using standard mutation rates must be viewed with great caution [99]–[102]. Nevertheless, this result supports the view that speciation between A. femoralis and A. occidentalis sp.n. took place well after the Miocene transgression period, when the Nullarbor plain had already fallen dry. In this scenario, speciation probably followed a colonization event across the arid plain, possibly during a temporary phase of less arid conditions.
The scenario presented here attempts to connect present biodiversity with evidence from the geological record. It is based on several assumptions, for some of which evidence is scarce, but offers one possible explanation for the two morphologically indistinguishable, but genetically and ecologically divergent sibling species A. femoralis and A. occidentalis sp.n. It may be supported by future studies on similar speciation events, especially if more accurate age estimations are possible. We believe that the results of such studies may help elucidate the implications of geological history and past environmental changes for Australia's present biogeography.
Supporting Information
Sequences of primers used for PCR and sequencing. Forward (F) and reverse (R) primers are given. Mitochrondrial gene loci: coi = cytochrome C oxidase 1, cob = cytochrome B oxidase, 16S = 16S ribosomal RNA. Nuclear gene loci: H3 = histone 3, 18S = 18S ribosomal RNA, ArK = arginine kinase.
(DOC)
Coordinates of Antiporus femoralis and A. occidentalis used for modeling. Geographic latitude and longitude are given in decimal degrees.
(DOC)
A heuristic estimate of the contributions of the bioclimatic variables used for modelling. Results of the jackknife analysis of variable importance are given as ranks (1 to 5) for all variables. Isolation: rank of the variable's training gain when used in isolation. Omission: rank of the variable in decreasing the total regularised training gain when omitted.
(DOC)
Background selection in ecological niche modelling. This picture shows each two ecological niche models for Antiporus femoralis, A. occidentalis sp.n. and both species together. For each set of locality data, one model was created using a manually specified background, as indicated by the green frame, and another one using no specified background. Both models were tested for niche overlap. All resulting values of I and D are close to 1 and thus indicate high overlap between models, confirming the similarity apparent from visual comparison.
(TIF)
Habitat of Antiporus occidentalis sp.n. a) Pond near Preston Beach, Western Australia (Loc. 30) and b) seasonal swamp at “Nannup Wildflower Walk” near Nannup, Western Australia (Loc. 32,).
(TIF)
Apparent contradiction in the background test results. This picture (modified from Nakazato et al. [96]) shows the environmental spaces available to (red and blue lines) and occupied by (shaded areas) both allopatric Antiporus species. In the niche overlap test, true localities of both species are compared. In the background test, the true localities of each one species are compared to random samples points drawn from the background areas (i.e., available environmental spaces) of the other species. Here, background test (1) yields relatively more divergent results than the true calculated overlap because, although the same overlap exists, it includes much more non-overlapping environmental space. Background test (2) yields more similar results than the true calculated overlap because it includes far more overlap than non-overlap between niche spaces. See Fig. 3.
(TIF)
Web links. Antiporus femoralis and A. occidentalis sp.n. on Wikispecies.
(DOC)
Acknowledgments
We thank the Department of Environment and Conservation in Western Australia for giving permission to conduct scientific research in Western Australia (Permit numbers: SF 003017 and NE 002348), and the Parks and Wildlife Commission of the Northern Territory for giving permission to conduct scientific research in the Northern Territory (Permit Number: 23929 and RK- 400/RK- 660). We also thank the Department of Environment and Conservation in New South Wales (Scientific Licence No. S12040) for giving permission to conduct scientific research in National and State Parks. We are grateful to Dennis Rödder, Bonn, Germany, and two anonymous reviewers, whose comments contributed greatly to the quality of this article.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work, as well as ongoing research on the Australian predaceous water beetle fauna was supported by grants from the German Research Foundation (DFG) to Lars Hendrich (HE5729/1-1) and Michael Balke (BA2152/7-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward RD, Zemlak TS, Innes BS, Last PR, Hebert PDN. DNA barcoding Australia's fish species. Phil Trans R Soc Lond B Biol Sci. 2005;360:1847–1857. doi: 10.1098/rstb.2005.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MA, Woodley NE, Janzen DH, Hallwachs W, Hebert PDN. DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proc Natl Acad Sci U S A. 2006;103:3657–3662. doi: 10.1073/pnas.0511318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith MA, Wood DM, Janzen DH, Hallwachs W, Hebert PDN. DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (Diptera, Tachinidae) are not all generalists. Proc Nat Acad Sci USA. 2007;104:4967–4972. doi: 10.1073/pnas.0700050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajibabaei M, Singer GAC, Hebert PDN, Hickey DA. DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 2007;23 doi: 10.1016/j.tig.2007.02.001. doi: 10.1016/j.tig.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Miller SE. DNA barcoding and the renaissance of taxonomy. Proc Natl Acad Sci U S A. 2007;104:4775–4776. doi: 10.1073/pnas.0700466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janzen DA, Hallwachs W, Blandin P, Burns JM, Cadiou JM, et al. Integration of DNA barcoding into an ongoing inventory of complex tropical biodiversity. Mol Ecol Res. 2009;9:1–26. doi: 10.1111/j.1755-0998.2009.02628.x. [DOI] [PubMed] [Google Scholar]
- 8.Monaghan MT, Wild R, Elliot M, Fujisawa T, Balke M, et al. Accelerated Species Inventory on Madagascar Using Coalescent-Based Models of Species Delineation. Syst Biol. 2009;58:298–311. doi: 10.1093/sysbio/syp027. [DOI] [PubMed] [Google Scholar]
- 9.Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci U S A. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfenninger M, Schwenk K. Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evol Biol. 2007;7 doi: 10.1186/1471-2148-7-121. doi: 10.1186/1471-2148-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns JM, Janzen DH, Hajibabaei M, Hallwachs W, Hebert PDN. DNA barcodes and cryptic species of skipper butterflies in the genus Perichares in Area de Conservación Guanacaste, Costa Rica. Proc Natl Acad Sci U S A. 2008;105:6350–6355. doi: 10.1073/pnas.0712181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dépraz A, Hausser J, Pfenninger M. A species delimitation approach in the Trochulus sericeus/hispidus complex reveals two cryptic species within a sharp contact zone. BMC Evol Biol. 2009;9 doi: 10.1186/1471-2148-9-171. doi: 10.1186/1471-2148-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuart BL, Inger RF, Voris HK. High level of cryptic species diversity revealed by sympatric lineages of Southeast Asian forest frogs. Biol Letters. 2006;2:470–474. doi: 10.1098/rsbl.2006.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns JM, Janzen DH, Hajibabaei M, Hallwachs W, Hebert PDN. DNA barcodes of closely related (but morphologically and ecologically distinct) species of skipper butterflies (Hesperiidae) can differ by only one to three nucleotides. J Lepid Soc. 2008;61:138–153. [Google Scholar]
- 15.De Queiroz K. Species Concepts and Species Delimitation. Syst Biol. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- 16.Mayr E. New York: Columbia University Press; 1942. Systematics and the origin of species. [Google Scholar]
- 17.Van Valen L. Ecological species, multispecies, and oaks. Taxon. 1976;25:233–239. [Google Scholar]
- 18.Andersson L. The driving force: Species concepts and ecology. Taxon. 1990;39:375–382. [Google Scholar]
- 19.Mallet J. A species definition for the modern synthesis. Trends Ecol Evol. 1995;10:294–299. doi: 10.1016/0169-5347(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 20.Guisan A, Zimmermann N. Predictive habitat distribution models in ecology. Ecol Model. 2000;135:147–189. [Google Scholar]
- 21.Elith J, Leathwick J. Species distribution models: Ecological explanation and prediction across space and time. Annu RevEcol Evol S. 2009;40:677–697. [Google Scholar]
- 22.Johnson NK, Cicero C. The role of ecologic diversification in sibling speciation of Empidonax flycatchers (Tyrannidae): Multigene evidence from mtDNA. Mol Ecol. 2002;11:2065–2081. doi: 10.1046/j.1365-294x.2002.01588.x. [DOI] [PubMed] [Google Scholar]
- 23.Graham CH, Ron SR, Santos JC, Schneider CJ, Moritz C. Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution. 2004;58:1781–1793. doi: 10.1111/j.0014-3820.2004.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 24.Raxworthy CJ, Ingram CM, Rabibisoa N, Pearson RG. Applications of ecological niche modelling for species delimitation: a review and empirical evaluation using day geckos (Phelsuma) from Madagascar. Syst Biol. 2007;56:907–923. doi: 10.1080/10635150701775111. [DOI] [PubMed] [Google Scholar]
- 25.Rissler LJ, Apodaca JJ. Adding More Ecology into Species Delimitation: Ecological Niche Models and Phylogeography Help Define Cryptic Species in the Black Salamander (Aneides flavipunctatus). Syst Biol. 2007;56:924–942. doi: 10.1080/10635150701703063. [DOI] [PubMed] [Google Scholar]
- 26.Sattler T, Bontadina F, Hirzel AH, Arlettaz R. Ecological niche modelling of two cryptic bat species calls for a reassessment of their conservation status. J ApplEcol. 2007;44:1188–1199. [Google Scholar]
- 27.Murienne J, Guilbert E, Grandcolas P. Species' diversity in the New Caledonian endemic genera Cephalidiosus and Nobarnus (Insecta: Heteroptera: Tingidae), and approach using phylogeny and species' distribution modelling. Biol J Linn Soc. 2009;97:177–184. [Google Scholar]
- 28.Chefaoui RM, Hortal J, Lobo JM. Potential distribution modelling, niche characterization and conservation status assessment using GIS tools: a case study of Iberian Copris species. Biol Conserv. 2005;122:327–338. [Google Scholar]
- 29.Lobo JM, Verdú JR, Numa C. Environmental and geographical factors affecting the Iberian distribution of flightless Jekelius species (Coleoptera: Geotrupidae). Divers Distrib. 2006;12:179–188. [Google Scholar]
- 30.Porch N. Climate space, bioclimatic envelopes and coexistence methods for the reconstruction of past climates: a method using Australian beetles and significance for Quaternary reconstruction. Quaternary Sci Rev. 2010;29:633–647. [Google Scholar]
- 31.Rödder D, Lötters S. Potential distribution of the alien invasive Brown Tree Snake, Boiga irregularis (Reptilia: Colubridae). Pac Sci. 2010;64:11–22. [Google Scholar]
- 32.Lord J, Westoby M, Leishman M. Seed size and phylogeny in six temperate floras: constraints, niche conservatism, and adaptation. Am Nat. 1995;146:349–364. [Google Scholar]
- 33.Peterson AT, Soberon J, Sanchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285:1265–1267. doi: 10.1126/science.285.5431.1265. [DOI] [PubMed] [Google Scholar]
- 34.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- 35.Kozak KH, Wiens JJ. Does niche conservatism promote speciation? A case study in North American salamanders. Evolution. 2006;60:2604–2621. [PubMed] [Google Scholar]
- 36.Rödder D, Lötters S. Niche shift versus niche conservatism? Climatic characteristics within the native and invasive ranges of the Mediterranean housegecko (Hemidactylus turcicus). Global Ecol Biogeogr. 2009;18:674–687. [Google Scholar]
- 37.Rödder D, Schmidtlein S, Veith M, Lötters S. Alien invasive slider turtle in unpredicted habitat: a matter of niche shift or of predictors studied? PLoS One. 2009;4 doi: 10.1371/journal.pone.0007843. doi: 10.1371/journal.pone.0007843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Losos JB, Leal M, Glor RE, deQueiroz K, Hertz PE. Niche lability in the evolution of a Caribbean lizard community. Nature. 2003;424:542–550. doi: 10.1038/nature01814. [DOI] [PubMed] [Google Scholar]
- 39.Rice NHE, Martinez-Meyer E, Peterson AT. Ecological niche differentiation in the Aphelocoma jays: a phylogenetic perspective. Biol J Linn Soc. 2003;80:369–383. [Google Scholar]
- 40.Jakob S, Heibl C, Rödder D, Blattner FR. Population demography influences climatic niche evolution: evidence from diploid American Hordeum species (Poaceae). Mol Ecol. 2010;19:1423–1438. doi: 10.1111/j.1365-294X.2010.04582.x. [DOI] [PubMed] [Google Scholar]
- 41.Hendrich L, Pons J, Ribera I, Balke M. mtDNA sequences reliably estimate species richness for regional and phylogenetic subsamples of a near-complete species level sequenced Australian fauna. PLoS ONE: submitted 2010 [Google Scholar]
- 42.Cooper SJB, Hinze S, Leys R, Watts CHS, Humphreys WF. Islands under the desert: molecular systematics and evolutionary origins of stygobitic water beetles (Coleoptera: Dytiscidae) from central Western Australia. Invertebr Syst. 2002;16:589–590. [Google Scholar]
- 43.Leys R, Watts CHS, Cooper SJB, Humphreys WF. Evolution of subterranean Diving Beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution. 2003;57:2819–2834. doi: 10.1111/j.0014-3820.2003.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 44.Hendrich L, Watts CHS. Taxonomic revision of the Australian genus Sternopriscus Sharp, 1882 (Coleoptera: Dytiscidae: Hydroporinae). Koleopterologische Rundschau. 2004;74:75–142. [Google Scholar]
- 45.Watts CHS, Humphreys WF. Twenty-six New Dytiscidae (Coleoptera) of the Genera Limbodessus Guignot and Nirripirti Watts & Humphreys, from Underground Waters in Australia. T Roy Soc South Aus. 2004;130:123–185. [Google Scholar]
- 46.Balke M, Pons J, Ribera I, Sagata K, Vogler AP. Infrequent and unidirectional colonization of hyperdiverse Papuadytes diving beetles in New Caledonia and New Guinea. Mol Phylogenet Evol. 2007;42:505–516. doi: 10.1016/j.ympev.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Hendrich L, Hawlitschek O, Balke M. The epigean Australasian species of Neobidessodes gen.n. diving beetles – a revision integrating morphology, cybertaxonomy, DNA taxonomy and phylogeny (Coleoptera: Dytiscidae, Bidessini). Zootaxa. 2009;2288:1–41. [Google Scholar]
- 48.Williams WD, Campbell IC. The inland aquatic environment and fauna. In: Dyne GR, Walton DW, editors. Fauna of Australia. General Articles. Canberra: Australian Government Publishing Service Vol. 1 A; 1987. pp. 156–183. [Google Scholar]
- 49.Kemp EM. Tertiary palaeogeography and the evolution of Australian climate. In: Keast A, editor. Ecological Biogeography of Australia. The Hague: Junk vol. 1; 1981. pp. 31–49. [Google Scholar]
- 50.Bowler JM. Age, origin and landform expression of aridity in Australia. In: Barker WR, Greenslade PJM, editors. Evolution of the Flora and Fauna of Arid Australia. Frewville: Peacock Publications; 1982. pp. 35–46. [Google Scholar]
- 51.Frakes LA, McGowran B, Bowler JM. Evolution of Australian Environments. In: Dyne GR, Walton, DW, editors. Fauna of Australia. General Articles. Canberra: Australian Government Publishing Service Vol. 1 A; 1987. pp. 1–18. [Google Scholar]
- 52.Byrne M, Yeats DK, Joseph L, Kearney M, Bowler J, et al. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Mol Ecol. 2008;17:4398–4417. doi: 10.1111/j.1365-294X.2008.03899.x. [DOI] [PubMed] [Google Scholar]
- 53.Watts CHS. A revision of the Australian Dytiscidae (Coleoptera). Aust J Zool, Supplementary Series. 1978;57:1–166. [Google Scholar]
- 54.Watts CHS. Four new species of Antiporus Sharp (Coleoptera; Dytiscidae) from Australia, with notes on A. femoralis (Boheman) and A. interrogationis (Clark). Rec South Austr Mus. 1997;30:35–42. [Google Scholar]
- 55.Watts CHS, Pinder A. Two new species of Antiporus from Western Australia (Coleoptera: Dytiscidae). Rec South Austr Mus. 2000;33:17–19. [Google Scholar]
- 56.Hendrich L. A new species of Antiporus Sharp, 1882 from peatland swamps of south-western Australia (Coleoptera: Dytiscidae). Linzer Biologische Beiträge. 2001;33:299–308. [Google Scholar]
- 57.Hendrich L, Watts CHS. An endemic predaceous water beetle from the Murchison River in Western Australia–Antiporus kalbarriensis sp.n. (Coleoptera: Dytiscidae, Hydroporinae, Hydroporini). Zootaxa. 2010;2338:35–42. [Google Scholar]
- 58.Brancucci M. Notes on some species of the genus Antiporus (Coleoptera: Dytiscidae). Aquat Insect. 1984;6:149–152. [Google Scholar]
- 59.Balke M, Ribera I, Miller M, Hendrich L, Sagata K, et al. New Guinea highland origin of a widespread arthropod supertramp. Proc R Soc Lon B. 2009;276:2359–2367. doi: 10.1098/rspb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hendrich L, Balke M. Kakadudessus tomweiri, a new genus and species of diving beetle from tropical northern Australia, based on molecular phylogenetic and morphological data (Coleoptera, Dytiscidae, Bidessini). Zootaxa. 2009;2134:49–59. [Google Scholar]
- 61.Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, et al. 2010. The CIPRES Portals. CIPRES. URL: http://www.phylo.org/sub_sections/portal. Accessed 2010-07-28.
- 62.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Posada D. jModelTest: Phylogenetic Model Averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 64.Zwickl DJ. PhD dissertation, The University of Texas at Austin; 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. [Google Scholar]
- 65.Huelsenbeck JP, Ronquist F. MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 66.Goloboff P, Farris S, Nixon K. Argentina: 1.1. Published by the authors, Tucumán; 2000. TNT (Tree analysis using New Technology) (BETA) ver. [Google Scholar]
- 67.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 68.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modelling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 69.Jaynes ET. Information theory and statistical mechanics. Phys Rev. 1957;106:620–630. [Google Scholar]
- 70.Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, et al. Novel methods improve prediction of species' distributions form occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 71.Wisz MS, Hijmans RJ, Peterson AT, Graham CH, Guisan A, et al. Effects of sample size on the performance of species distribution models. Divers Distrib. 2008;14:763–773. [Google Scholar]
- 72.Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT. Predicting species distributions from small numbers of occurrence records: A testcase using cryptic geckos in Madagascar. J Biogeogr. 2007;34:102–117. [Google Scholar]
- 73.Peterson AT, Vieglais DA. Predicting species invasions using ecological niche modelling: new approaches from bioinformatics attack a pressing problem. BioScience. 2001;51:363–371. [Google Scholar]
- 74.Jeschke JM, Strayer DL. Usefulness of bioclimatic models for studying climate change and invasive species. Ann N Y Acad Sci. 2008;1134:1–24. doi: 10.1196/annals.1439.002. [DOI] [PubMed] [Google Scholar]
- 75.Rödder D, Schmidtlein S, Schick S, Lötters S. Climate Envelope Models in systematics and evolutionary research: theory and practice. In: Hodkinson T, Jones M, Pamell J, Waldren S, editors. Systematics and climate change. Cambridge, UK (in press): Cambridge University Press; 2010. [Google Scholar]
- 76.Habel JC, Néve G, Rödder D, Schmitt T. Global warming will affect the genetic diversity and uniqueness of Lycaena helle populations. Global Change Biology: in press 2010 [Google Scholar]
- 77.Habel JC, Schmitt T, Meyer M, Finger A, Rödder D, et al. Biogeography meets conservation: the genetic structure of the endangered lycaenid butterfly Lycaena helle (Denis & Schiffermüller, 1775). Biol J Linn Soc: in press. 2010.
- 78.Cordellier M, Pfenninger M. Inferring the past to predict the future: climate modelling predictions and phylogeography for the freshwater gastropod Radix balthica (Pulmonata, Basommatophora). Mol Ecol. 2009;18:534–544. doi: 10.1111/j.1365-294X.2008.04042.x. [DOI] [PubMed] [Google Scholar]
- 79.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 80.Waltari E, Hijmans RJ, Peterson AT, Nyári AS, Perskins SL, et al. Locating Pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000563. doi: 10.1371/journal.pone.0000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beaumont LJ, Hughes L, Poulsen M. Predicting species distributions: use of climatic parameters in BIOCLIM and its impact on predictions of species' current and future distributions. Ecol Model. 2005;186:251–270. [Google Scholar]
- 82.Heikkinen RK, Luoto M, Araújo MB, Virkkala R, Thuiller W, et al. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog Phys Geog. 2006;30:751–777. [Google Scholar]
- 83.Rödder D, Lötters S. Naturwissenschaften; 2010. Explanative power of variables used in species distribution modelling: an issue of general model transferability or niche shift in the invasive Greenhouse frog (Eleutherodactylus planirostris). doi: 10.1007/s00114-010-0694-7. [DOI] [PubMed] [Google Scholar]
- 84.Phillips SJ, Dudík M. Modelling of species distributions with Maxent: new extensions and comprehensive evaluation. Ecography. 2008;31:161–175. [Google Scholar]
- 85.Hanley J, McNeil B. The meaning of the use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 86.Swets K. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 87.Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, et al. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- 88.Warren DL, Glor RE, Turelli M. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography: in press. 2010. doi: 10.1111/j.1600-0587.2009.06142.x.
- 89.Schoener TW. Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology. 1968;49:704–726. [Google Scholar]
- 90.Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution. 2008;62:2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 91.Buffington M, Gates M. Advanced imaging techniques II: Using a compound microscope for photographing point-mount specimens. Am Entomologist. 2008;54:222–224. [Google Scholar]
- 92.Miller KB, Nilsson AN. Homology and terminology: Communicating information about rotated structures in water beetles. Latissimus. 2003;17:1–4. [Google Scholar]
- 93.Knapp S. Four New Vining Species of Solanum (Dulcamaroid Clade) from Montane Habitats in Tropical America. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010502. doi: 10.1371/journal.pone.0010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Page RDM. Wikipedia as an encyclopaedia of life. Nature Proceedings. 2010 hdl:10101/npre.2010.4242.1: Posted 26 Feb 2010. [Google Scholar]
- 95.Anderson RP. Real vs. artefactual absences in species distributions: Tests for Oryzomys albigularis (Rodentia: Muridae) in Venezuela. J Biogeogr. 2003;30:591–605. [Google Scholar]
- 96.Nakazato T, Warren DL, Moyle LC. Ecological and geographic modes of species divergence in wild tomatoes. Am J Bot. 2010;97:680–693. doi: 10.3732/ajb.0900216. [DOI] [PubMed] [Google Scholar]
- 97.Hebert PDN, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B (Suppl.) 2003;03BL0066:1–4. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papadopoulou A, Anastasiou I, Vogler AP. Revisiting the insect mitochondrial molecular clock: Mid-Aegean trench calibration. Mol Biol Evol. 2010;27:1659–1672. doi: 10.1093/molbev/msq051. [DOI] [PubMed] [Google Scholar]
- 99.Britten RJ. Rates of DNA sequence evolution differ between taxonomic groups. Science. 1986;231:1393–1398. doi: 10.1126/science.3082006. [DOI] [PubMed] [Google Scholar]
- 100.Hasegawa M, Kishino H. Heterogeneity of tempo and mode of mitochondrial DNA evolution among mammalian orders. Jpn J Genet. 1989;64:243–258. doi: 10.1266/jjg.64.243. [DOI] [PubMed] [Google Scholar]
- 101.Ayala FJ. Vagaries of the molecular clock. Proc Nat Acad Sci U S A. 1997;94:7776–7783. doi: 10.1073/pnas.94.15.7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biology. 2006;4 doi: 10.1371/journal.pbio.0040088. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences of primers used for PCR and sequencing. Forward (F) and reverse (R) primers are given. Mitochrondrial gene loci: coi = cytochrome C oxidase 1, cob = cytochrome B oxidase, 16S = 16S ribosomal RNA. Nuclear gene loci: H3 = histone 3, 18S = 18S ribosomal RNA, ArK = arginine kinase.
(DOC)
Coordinates of Antiporus femoralis and A. occidentalis used for modeling. Geographic latitude and longitude are given in decimal degrees.
(DOC)
A heuristic estimate of the contributions of the bioclimatic variables used for modelling. Results of the jackknife analysis of variable importance are given as ranks (1 to 5) for all variables. Isolation: rank of the variable's training gain when used in isolation. Omission: rank of the variable in decreasing the total regularised training gain when omitted.
(DOC)
Background selection in ecological niche modelling. This picture shows each two ecological niche models for Antiporus femoralis, A. occidentalis sp.n. and both species together. For each set of locality data, one model was created using a manually specified background, as indicated by the green frame, and another one using no specified background. Both models were tested for niche overlap. All resulting values of I and D are close to 1 and thus indicate high overlap between models, confirming the similarity apparent from visual comparison.
(TIF)
Habitat of Antiporus occidentalis sp.n. a) Pond near Preston Beach, Western Australia (Loc. 30) and b) seasonal swamp at “Nannup Wildflower Walk” near Nannup, Western Australia (Loc. 32,).
(TIF)
Apparent contradiction in the background test results. This picture (modified from Nakazato et al. [96]) shows the environmental spaces available to (red and blue lines) and occupied by (shaded areas) both allopatric Antiporus species. In the niche overlap test, true localities of both species are compared. In the background test, the true localities of each one species are compared to random samples points drawn from the background areas (i.e., available environmental spaces) of the other species. Here, background test (1) yields relatively more divergent results than the true calculated overlap because, although the same overlap exists, it includes much more non-overlapping environmental space. Background test (2) yields more similar results than the true calculated overlap because it includes far more overlap than non-overlap between niche spaces. See Fig. 3.
(TIF)
Web links. Antiporus femoralis and A. occidentalis sp.n. on Wikispecies.
(DOC)