Abstract
Chronic myeloid leukemia (CML) results from expression of the BCR/ABL oncogene in a primitive hematopoietic cell. However BCR/ABL-activated signaling mechanisms are dependent on the cellular context in which it is expressed, and mechanisms underlying primitive human hematopoietic cell transformation by BCR-ABL are not well understood. Our previous studies have shown that BCR/ABL-Y177 plays an essential role in Ras activation and human hematopoietic progenitor transformation in CML. The adapter protein growth factor receptor binding protein-2 (Grb2) can bind phosphorylated BCR/ABL-Y177, induce Grb2-SoS complex formation, and activate Ras signaling. We investigated the role of Grb2 in CML progenitor transformation by co-transducing human CD34+ cells with lentivirus vectors expressing shRNA to Grb2 and retrovirus vectors expressing BCR/ABL. We show that Grb2 knockdown significantly inhibits proliferation and survival of BCR-ABL-expressing CD34+ cells, but not control CD34+ cells. Grb2 knockdown reduced MAPK activity in BCR-Abl-expressing hematopoietic cells. We conclude that inhibition of Grb2 expression demonstrates an important role in BCR-ABL mediated MAPK activation and transformation of primary human hematopoietic cells. These results support further investigation of downstream effectors of Grb2-mediated signals and targeting of Grb2 interactions in the treatment of CML.
Keywords: Chronic myelogenous leukemia, leukemia stem cells, Signal transduction, Gene transfer
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell malignancy characterized by a balanced translocation between chromosomes 9 and 22, also known as the Philadelphia chromosome 1,2. The resulting BCR-ABL fusion oncogene encodes a cytoplasmic protein tyrosine kinase with elevated and dysregulated enzymatic activity that plays an essential role in the pathogenesis of CML 3,4. Expression of the BCR-ABL gene results in abnormal expansion of myeloid progenitors and more differentiated myeloid cells related to increased hematopoietic progenitor proliferation, reduced apoptosis and disturbed cell adhesion and migration in the malignant clone5. Imatinib mesylate (IM), a small molecule inhibitor of the BCR-ABL kinase, is very effective in the treatment of CML 6,7. However imatinib treatment does not eliminate leukemia stem cells (LSC) in CML patients 8–10. Residual LSC persist in patients who achieve cytogenetic and molecular response and continued drug treatment is required to maintain remission 11. Development of an improved understanding of critical molecular mechanisms underlying human hematopoietic progenitor transformation in CML is essential to development of alternative approaches to target leukemogenic cells in CML.
Although downstream signaling underlying BCR-ABL transformation have been intensively studied in cell lines and in murine models, the mechanisms responsible for transformation of primitive human hematopoietic cells in CML are less well understood. Since mechanisms of BCR-ABL mediated transformation can differ depending to the cellular context in which the oncogene is expressed it is important to determine the pathogenic role of specific signaling mechanisms in the context of the primitive human hematopoietic cells in which the disease arises in patients. We have developed a model of BCR-ABL transformation of human hematopoietic progenitor cells based on retrovirus-mediated BCR-ABL expression in human cord blood CD34+ cells 12. This model recapitulates several phenotypic characteristics of malignant progenitors from CML patients including increased proliferation, reduced apoptosis, and altered adhesion and migration, and facilitates investigation of molecular mechanisms of hematopoietic transformation in CML. We have used this model to show that abnormal tyrosine kinase activity plays an essential role in increased proliferation of BCR-ABL transformed human progenitors, but that both kinase-dependent and independent mechanisms contribute to altered adhesion and migration. We have also shown that tyrosine 177 (BCR/ABL-Y177) in BCR/ABL plays an essential role in Ras and Akt activation and human progenitor transformation in CML 13. BCR/ABL-Y177 represents a phosphorylation site that can bind the adapter protein growth factor receptor binding protein-2 (Grb2), induce Grb2-SoS complex formation, and activate Ras signaling 14. Grb2 binding to Y177 may also lead to association with the scaffold adapter protein Grb2-associated binder (Gab2), Gab2 phosphorylation and association with PI-3K and Shp2, PI-3K and Ras activation, and induction of CML-like disease in mice15. Interactions with Grb2 are also involved in the pathogenesis of Tel-ABL (ETV6-ABL) induced leukemia 16. However the requirement for Grb2 expression for BCR-ABL-mediated transformation has not been directly studied.
The role of individual genes in human hematopoietic progenitor cells can be accurately analyzed using RNA interference (RNAi) by transducing cells with shRNA expressing HIV- based lentivirus vectors. We studied the function of Grb2 in BCR-ABL transformed and normal human progenitors using shRNA-mediated knockdown of Grb2 expression. This approach required robust methods to reliably coexpress the BCR-ABL gene and shRNA constructs in human CD34+ cells using separate vectors with different reporter genes. Although cytomegalovirus (CMV), spleen focus-forming virus (SF), human phosphoglycerate kinase 1 (PGK)17, 18 have been used for transgene expression in CD34+ cells using enhanced Green Fluorescent Protein (eGFP) as a reporter, their efficiency in expressing red fluorescent protein (RFP) in human CD34+ cells has not been studied. We found that the strong SF promoter was required to adequately express RFP in CD34+ cells and identify cells co-expressing RFP and eGFP by flow cytometry. We used dual transduction with (1) a retroviral vector expressing eGFP and BCR-ABL, and (2) a lentivirus vector expressing RFP from a SF promoter and a shRNA construct from a U6 promoter, to investigate the role of Grb2 in BCR-ABL-transformed progenitor cells.
Patients, material and methods
Samples
CB samples were provided by StemCyte (Arcadia, CA). All donors signed an informed consent form. Sample acquisition was approved by the Institutional Review Board at the COHNMC and met all requirements of the Declaration of Helsinki. Cord blood mononuclear cells were isolated by Ficoll-Hypaque (Sigma Diagnostics, St Louis, MO) density gradient centrifugation. CD34+ cells were selected from cord blood cells using immunomagnetic column separation (Miltenyi Biotech Inc., Auburn, CA).
Synthesis of shRNA expression vectors
The lentivirus pHIV7, pHIV7/C-GFP and pHIV7/SF-GFP were previously described 17. To generate pHIV7-SF-RFP, a fragment containing RFP was amplified from pCMV-DsRed-Express by PCR, KpnI and BamHI sites added at the 5′ and 3′ ends, and ligated to a BamHI-KpnI fragment containing the SFFV LTR 19. The SFFV LTR-RFP fragment was inserted into a unique BamHI site in pHIV7 to generate pHIV7-SF-RFP. To generate pHIV7-CMV-RFP, a CMV IE-RFP fragment obtained by PCR of pCMV-DsRed-Express was inserted into pHIV7. Vectors expressing GFP and RFP from the PGK promoter (pHIV7-PGK-GFP and pHIV7-PGK-RFP) were generated by amplifying the PGK promoter the PGK-KS plasmid (a kind gift from Dr. Donald Kohn, UCLA), generating PGK-GFP and PGK-RFP cassettes, and insertion into pHIV7 (Supplementary Figure S1). We designed shRNAs targeting human Grb2 (NM_203506.2). The targeted Grb2 sequences corresponded to positions 363–381 (sense 5′ AAGCCATCGCCAAATATGA 3′, antisense 5′ TCATATTTGGCGATGGCTT 3′), 782–804 (sense 5′ CCAUGUCAUGGAUAACUCATT 3′, antisense 5′ UGAGUUAUCCAUGACAUGGTT 3′), 892–910 (sense 5′ GUCAAGAAGCAAUUAUUUATT 3′, antisense 5′ UAAAUAAUUGCUUCUUGACTT 3′). Short hairpin expression cassettes were constructed by PCR amplification of pTZ U6+1, using a forward primer complementary to the sequence upstream of the U6 promoter and reverse primer covering the antisense loop, sense of the siRNAs and 3′-end of the U6 promoter (Supplementary Figure S1B). A BamHI site was included at the 5′-end of the forward primer and an EcoRI site at 5′-end of the reverse primer. The PCR products were digested with BamHI and EcoRI and ligated into pHIV7-SF-RFP to generate pHIV7-SF-RFP-siGrb2-2, −4 and −5. A lentiviral vector with a shRNA targeting HIV-1 tat and rev designated as pHIV7-U6-TR-SF-RFP was used as a control for off-target shRNA effects.
Vectors and virus production
The MIG R1 and MIG 210 retroviral vectors (kind gifts from Dr Warren Pear, University of Pennsylvania) have been described previously 20. Replication incompetent retroviruses were obtained by transient transfection of 293 cells with MIG R1, MIG 210 retroviral plasmids and the pCL-ampho plasmid 20. To produce infectious HIV vectors, 293T cells were co-transfected with pCMV gp-2, 4 pCMV-rev, pCMV-VSV-G and vector plasmid. Culture supernatants were collected 24 hours and 36 hours after transfection, pooled, filtered, concentrated by ultracentrifugation, and stored at −80°C until use. Vector titers were determined by transduction of HT1080 cells and analysis of RFP expression using flow cytometry. Stocks were tested for replication-competent lentivirus.
Transduction of CD34+ cells
CD34+ cells were cultured on Retronectin (Pan Vera, Madison, WI) coated plates in serum-free medium (SFM) (Stem Cell Technologies, Vancouver, BC, Canada) containing growth factors (GFs) [Interleukin-3 (IL-3) (25 ng/ml); interleukin-6 (IL-6) (10 ng/ml); Flt-3 ligand (100 ng/ml); Stem Cell Factor (SCF) (50 ng/ml) and Thrombopoietin (100 ng/ml)] at 37°C in 5% CO2. After 48 hours cells were co-transduced with retroviral vectors expressing BCR-ABL and GFP (MIG 210) or control vectors expressing the GFP alone (MIG R1) at multiplicity of infection (MOI) of 10, together with lentivirus vectors expressing Grb2 shRNA or the non specific shRNA at MOI of 5. Infection was repeated after 24 hours. After additional culture for 48 hours, CD34+ cells were labelled with anti-CD34-APC antibodies (Becton Dickinson, San Jose, CA) and CD34+GFP+RFP+ cells selected using flow cytometry (Dako-Cytomation Inc., Fort Collins, CO).
Western blot and Immunoprecipitation
Cells were lysed in 50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.5% NP40, and 0.5% sodium deoxycholate, with protease and phosphatase inhibitors. Proteins were resolved on SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked with 10% non fat dry milk with 0.1% Tween and labelled with primary antibody anti-Abl (Abl-3, CalBiochem, OP 20); anti-Actin (AC-15; Sigma-Aldrich Co., A 5441); anti-phosphotyrosine (4G10, Millipore, 05-777); anti-phosphorylated p42/44 MAPK (sc-7383), (Santa Cruz Biotechnology, Santa Cruz, CA); anti-phosphorylated Stat5 (pY694, 611964) (BD Biosciences); and anti-phosphorylated Akt (Ser473, 9271) (Cell Signaling Technology); followed by mouse or rabbit horseradish peroxidase-conjugated secondary antibody (1:6000; Jackson ImmunoResearch Laboratories). Proteins bands were visualized using the Superfemto kit (Pierce Biotechnology, Rockford, IL). Relative quantitation of protein levels was performed using densitometric analysis. For immunoprecipitation, protein extracts were cleared with Protein A beads (Pierce Chemical Company) at 4°C for 1 hour. Primary antibody (2μg) was added to 1.5 mg total protein and incubated overnight at 4°C, followed by incubation with True Blot beads (eBioscience) for 2 hours. Beads were isolated by centrifugation, washed with PBS plus 1% NP-40, boiled with 2x sample loading buffer and resolved by SDS-PAGE followed by Western blotting with anti-Abl and anti-Grb2 antibodies.
Progenitor Culture
CD34+GFP+RFP+ cells were incubated in SFM with low concentrations of GF similar to those found in stroma-conditioned medium (granulocyte-macrophage colony-stimulation factor [GM-CSF], 200 pg/mL; granulocyte colony-stimulating factor [G-CSF], 1 ng/mL; SCF, 200 pg/mL; leukemia inhibitory factor [LIF], 50 pg/mL; macrophage inflammatory protein α [MIP-1 α], 200 pg/mL; and IL-6 1 ng/ml) 21–23 at 37°C with 5% CO2. The number of viable cells was enumerated after 7 and 14 days of culture. Expression of myeloid and erythroid differentiation antigens was evaluated by labeling with antibodies to CD33, CD11b and Glycophorin A (Gly A) and flow cytometry (LSRII; Becton Dickinson). MTS assay were performed by culturing cells in triplicate in 96-well plates (104 cells/well) and quantifying viable cells using an MTS assay kit (Promega, Madison, WI). Cells were also cultured in IMDM with 30% FBS and high GF conditions [erythropoietin (3u/ml); SCF (5ng/ml); granulocyte- macrophage colony stimulating factor (GM-CSF) (20ng/ml); granulocyte colony stimulating factor (G-CSF) (20ng/ml) and IL-3 (5ng/ml)]. For assessment of apoptosis cells were labeled with Annexin V-Cy-5 and DAPI (BD PharMingen, San Diego, CA), and analyzed by flow cytometry 20.
Statistics
Results of data obtained from multiple experiments were reported as the mean ± one SEM. Significance levels were determined by Student’s paired t-test analysis or where indicated by one-way or two-way ANOVA.
Results
BCR-ABL-Y177F mediates BCR-ABL and Grb2 association in human hematopoietic progenitor cells
We have previously shown that the Y177 motif in the BCR portion of the protein plays a critical role in human hematopoietic progenitor transformation by BCR-ABL and in BCR-ABL induced activation of Ras, Akt and STAT5 signaling. In cell lines phosphorylated Y177 has been shown to directly bind the SH2 domain of Grb2 24–26. Immunoprecipitation of BCR-ABL using anti-ABL antibodies followed by Western blotting with anti-Grb2 antibodies confirmed Grb2 association with BCR-ABL in BCR-ABL-expressing hematopoietic cells. Grb2 association with BCR-ABL was markedly diminished in BCR-ABL-Y177F expressing cells (Supplementary Figure S2). Similar results were obtained on immunoprecipitation with anti-Grb2 antibodies and Western blotting with anti-ABL antibodies. These results indicate that Y177 mediates BCR-ABL and Grb2 association in human hematopoietic cells and support further investigation of the role of Grb2 in BCR-ABL-mediated transformation of primary human CD34+ cells.
Dual BCR-ABL and shRNA gene expression in human CD34+ cells
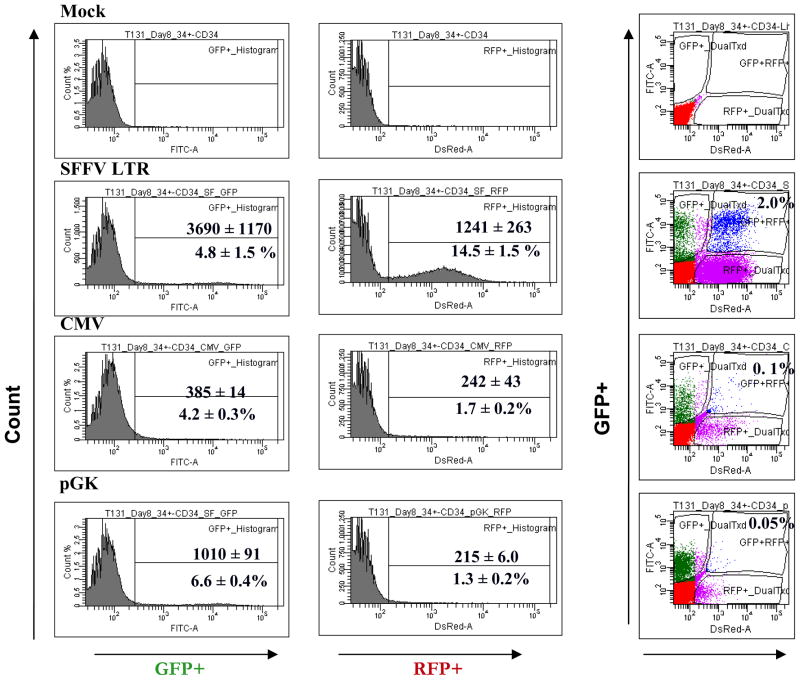
Investigation of the effect of Grb2 knockdown in BCR-ABL-mediated transformation requires coexpression of the BCR-ABL gene and Grb2 shRNA constructs in human CD34+ cells. Coexpression of multiple transgene necessitates use of different reporters to identify cells transduced with different vectors. In preliminary studies we observed that GFP and RFP expression from the CMV promoter in human CD34+ cells was low, resulting in poor delineation of dual transduced cells and making this approach impractical. We systematically compared the expression of GFP and RFP reporters in human CD34+ cells from CMV, SFFV (SF) and PGK promoters (Supplementary Figure S1). CD34+ cells were prestimulated with GF for 48 hours followed by exposure to infectious pHIV7 virus particles expressing GFP and RFP from different promoters (CMV-GFP, CMV-RFP, SF-GFP; SF-RFP, PGK-GFP, PGK-RFP) at MOI of 5 for two consecutive days. Cells were analyzed by flow cytometry 48 hours after the second virus exposure (Figure 1). The percentage of GFP+ cells were not significantly different when GFP was expressed from the different promoters, but the median fluorescent intensity (MFI) of GFP expressed from the SF promoter was 4-fold higher compared to the CMV promoter, and 6.5-fold higher compared to the PGK promoter. In contrast the percentage of RFP+ cells significantly higher when expressed from the SF promoter compared to CMV and PGK promoters (14.5 ± 1.5%, 1.7 ± 0.2%, 1.3 ± 0.2% respectively) and MFI of RFP expressed from the SF promoter was 5-fold higher compared to the CMV and PGK promoters (MFI 1241 ± 263, 242 ± 43, 215 ± 6.0 respectively). The low percentage of RFP+ cells expressed from CMV or PGK promoters resulted in a minimal percentage of cells expressing both GFP and RFP (0.6 ± 0.2%, 0.1 ± 0.01%, CMV and PGK respectively), compared to the SF promoter (3.0 ± 1.0%). These results indicate that the expression of RFP requires a stronger promoter such as SF.
Figure 1. Transfer and expression of GFP and RFP gene in human CD34+ cells.
CD34+ cells were transduced with the indicated vectors at an MOI of 5 and analyzed by flow cytometry for GFP+, RFP+ and GFP+/RFP+ expression. A) Histograms of GFP fluorescent intensity and RFP fluorescent intensity. The upper number represents the median fluorescent intensity while the lower number represents the percentage of positive cells. B) Representative flow cytometry plot of GFP+ versus RFP+ cells.
Grb2 knockdown is associated with reduced proliferation and survival of BCR-ABL transformed cells
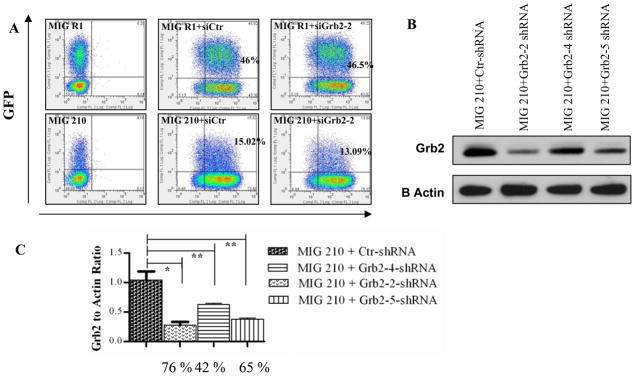
We used retrovirus vectors to express BCR-ABL and GFP (MIG 210) or GFP alone (MIG R1) in human CD34+ cells together with lentivirus vectors to express Grb2 shRNAs or control shRNAs. Efficient dual transduction with GFP-expressing retrovirus and RFP-expressing lentivirus could be achieved. CD34+ cells coexpressing GFP and RFP were selected by flow cytometry sorting (Figure 2A). Western blotting analysis showed that anti-Grb2 shRNA-transduced cells demonstrated significantly reduced Grb2 protein levels compared to control shRNA transduced cells (Figure 2B, C). These results confirmed the efficacy of the anti-Grb2 shRNAs to inhibit Grb2 expression and the utility of this approach to investigate the role of Grb2 in normal as well as BCR-ABL expressing CD34+ cells.
Figure 2. Efficient knock down of Grb2 in BCR-ABL-transformed human CD34+ cells.
(A) Representative data for GFP and RFP expression in CD34+ cells transduced with BCR-ABL and control GFP vectors. (B) Representative results of Western blotting for Grb2 protein expression. Sorted GFP+RFP+ cells -transduced with MIG210 and shRNA vectors were cultured for seven days followed by preparation of protein extracts. Western blotting was performed using anti-Grb2 antibodies and anti-Actin antibodies. (C) Grb2 to actin ratios were obtained by densitometric analysis. Results shown are normalized to Grb2 expression in control shRNA expressing cells. The percentage inhibition of Grb2 expression by the different shRNA with MIG210+Grb2-2 shRNA was 76.11±1.31; MIG210+Grb2-4 shRNA, 42.43±3.1; and MIG210+Grb2-5 shRNA, 64.98±2.29 compared to MIG210+ctr shRNA. Significance levels for differences in Grb2 expression between siCtr and siGrb2-2 * P < 0.001, n = 7; siCtr and siGrb2-4, siGrb2-5 ** P < 0.03, n = 2.
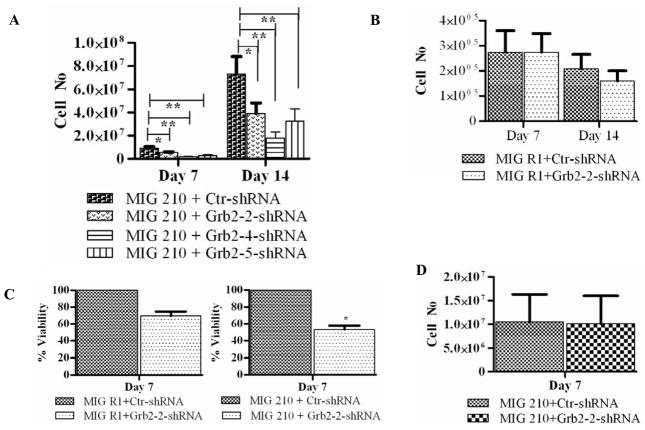
Selected CD34+GFP+RFP+ cells were cultured with low GF and viable cell numbers were enumerated on day 7 and day 14. Grb2 shRNA-expressing BCR-ABL transformed CD34+ progenitor cells generated significantly fewer cells after 7 and 14 days of culture compared to the control shRNA transduced cells (Figure 3A). Expression of Grb2 shRNA did not significantly reduce expansion of control CD34+ cells expressing GFP alone (Figure 3B). We also confirmed significantly reduced growth of Grb2 knockdown BCR-ABL expressing cells using the MTS assay (Figure 3C). In contrast, Grb2 shRNA-expressing BCR-ABL-transformed CD34+ cells did not show reduced growth compared to control shRNA-expressing cells in the presence of high concentrations of GF (Figure 3D). These results suggest that GF signaling can overcome the effects of Grb2 knockdown on BCR-ABL transformed CD34+ cells, and suggest an essential role for Grb2 in BCR-ABL-dependent but not in GF-dependent proliferation.
Figure 3. Effect of Grb2 shRNAs on normal and BCR-ABL-transformed CD34+ progenitor cell growth.
(A) BCR-ABL-transformed or (B) control CB CD34+ cells expressing the indicated shRNAs were cultured with low concentrations of GF and the number of viable cells counted on day 7 and 14. Viable cells per 100,000 input CD34+GFP+RFP+ cells are shown. Significance levels: * p < 0.03, n=8; ** p < 0.002, n=2, compared to controls. (C) BCR-ABL-transformed or control CD34+ cells expressing the indicated shRNAs were cultured with low GF concentrations for five days and cell proliferation determined using an MTS assay. Results are from three independent experiments each done in triplicate. Significance levels: *, P < 0.03, MIG210+ctr shRNA versus MIG210+Grb2-2 shRNA. (D) BCR-ABL-transformed cells expressing the indicated shRNAs were cultured in high GF conditions for seven days, n = 3.
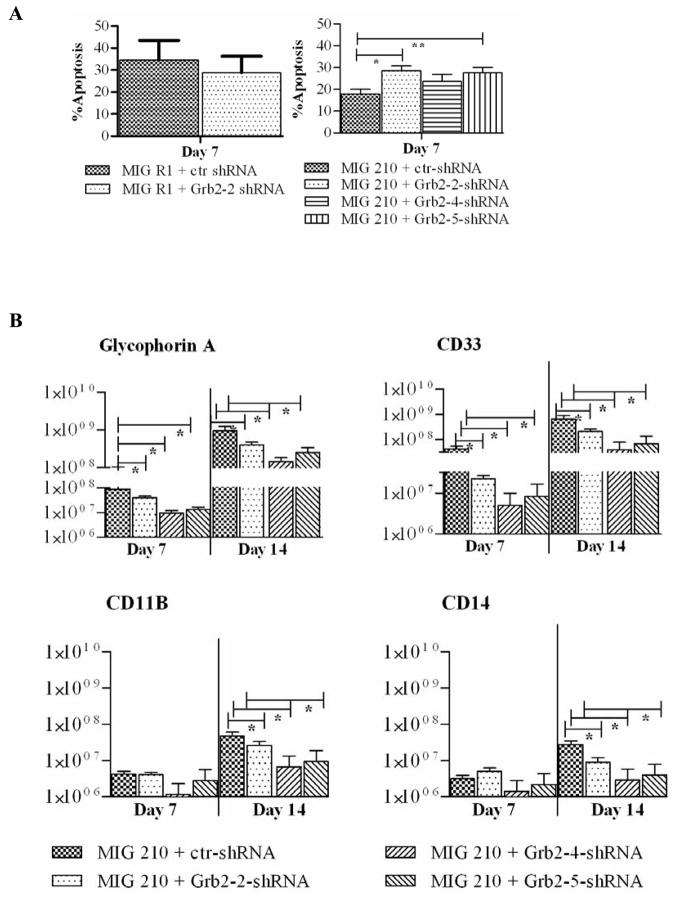
Grb2-2 and Grb2-5 shRNA expressing BCR-ABL transformed cells cultured in low GF demonstrated a significant increase in apoptosis, analyzed by Annexin V and DAPI labeling, compared to control shRNA expressing cells (Figure 4A). This data suggests that Grb2 contributes to reduced apoptosis in BCR-ABL-transformed hematopoietic cells. In contrast Grb2 knockdown did not increase apoptosis in normal CD34+ cells.
Figure 4. Effect of Grb2 knock down on CD34+ cell apoptosis and differentiation.
Transduced CD34+ cells were cultured with low GF for indicated periods and analyzed by flow cytometry for apoptosis and erythroid and myeloid markers. (A) Cells were labeled with Annexin-V Cy-5 and DAPI and apoptosis assessed by flow cytometry. Significance values: * P < 0.015, MIG 210+ctr shRNA versus Grb2-2 shRNA; ** P < 0.006, MIG 210+ctr shRNA versus Grb2-5 shRNA. (B) Cells were analyzed for glycophorin A (GlyA), CD 11b, CD 14 and CD 33 expression on day 7 and 14. The figure shows absolute number (log10) of cells per 100,000 input cells. Significance values for differences between MIG 210 + ctr shRNA and various Grb2 shRNAs are indicated * P < 0.05.
Immunophenotypic analyses indicated that BCR-ABL expressing cells generated higher number of both myeloid (CD11b and CD33 expressing) and erythroid (Glycophorin A expressing) cells compared to control cells (data not shown) 20. Knockdown of Grb2 resulted in significantly reduced expansion of both erythroid and myeloid cells from BCR-ABL-transformed CD34+ progenitor cells (Figure 4B). These results indicate a crucial role for Grb2 in both abnormal myeloid and erythroid cell expansion in CML.
Grb2 knockdown results in reduced MAPK activity in BCR-ABL-transformed human hematopoietic cells
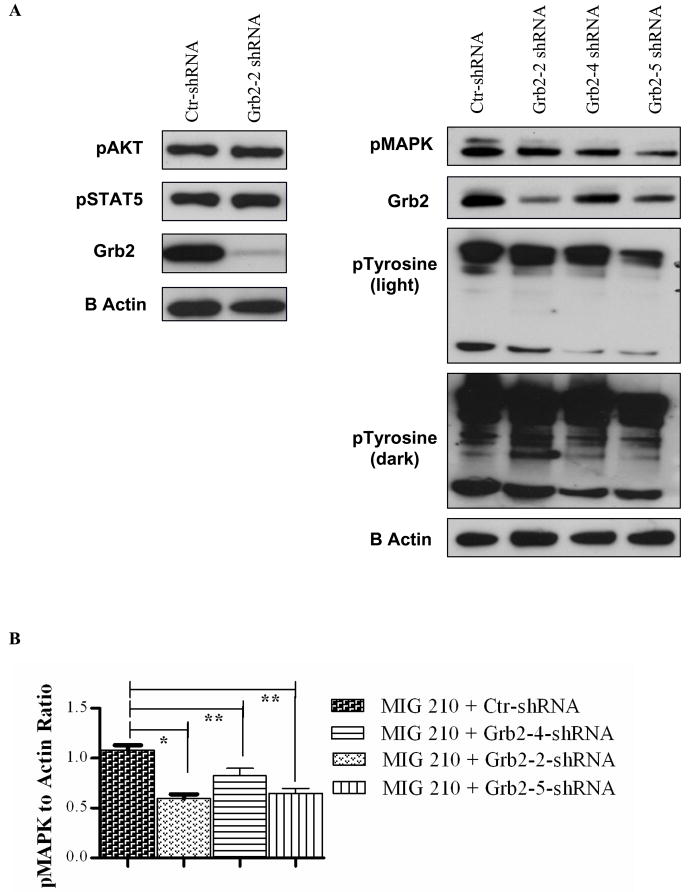
Grb2 through one of its SH3 domains (mainly the C-terminal domain) may mediate BCR-ABL association with the guanine nucleotide exchange factor SOS, which can activate Ras by stimulating exchange of GDP for GTP 27. Abnormal Ras activation may play a central role in mitogenic signaling in BCR-ABL expressing cells 28, leading to activation of downstream signaling through Raf/MEK/MAPK. Western blotting with phospho-MAPK antibodies [anti-Phospho-p44/42M AP Kinase (Thr202/Tyr204)] showed that Grb2 shRNA expression resulted in reduced p-MAPK levels in BCR-ABL-expressing cells (Figure 5) (n=3). These results suggest an important role for Grb2 in enhanced Raf/MEK/MAPK activity in BCR-ABL-transformed hematopoietic cells. Grb2 may also mediate activation of the phosphoinositide-3-kinase (PI3K) through interactions with Gab2 and the regulatory 85-kDa sub-unit of PI3K 15. PI3K signaling may contribute to enhanced proliferation and survival of BCR-ABL-expressing cells. However we did not observe a consistent reduction in phosphorylation of AKT (Ser473), a key downstream effector of PI3K signaling, in Grb2-knockdown BCR-ABL-expressing cells. STAT5 activity is increased in BCR-ABL-transformed cells. However no change in STAT5 phosphorylation was seen on Grb2 knockdown. These results indicate that mechanisms other than Grb2 may contribute to Akt and STAT5 activation in BCR-ABL expressing cells. Western blotting for tyrosine phosphorylated proteins indicated that Grb2 knockdown was associated with reduced tyrosine phosphorylation of BCR-ABL.
Figure 5. Effect of Grb2 knock down on BCR-ABL signaling in human hematopoietic cells.
BCR-ABL expressing CD34+ cells were grown in low GF concentrations for seven days followed by preparation of protein extracts for Western blotting. (A) Representative Western blots for phosphorylated AKT (n=4), MAPK (n=4) and pTyr (n=2) in control shRNA, Grb2-2 shRNA (n=4), and Grb2-4, 5 (n=2) expressing cells. (B) Cumulative results from densitometric analysis of indicate that p value (< 0.05) is significant for pMAPK and pTyr.
Discussion
In this study we show that Grb2 knockdown using lentivirus mediated shRNA expression results in reduced proliferation and survival of BCR-ABL-transformed primary human CD34+ progenitor cells. Grb2 inhibition did not significantly inhibit proliferation and survival of control CD34+ cells that did not express BCR-ABL. Grb2 knockdown inhibits MAPK activation in BCR-ABL-transformed hematopoietic cells. These results are important because this study identify Grb2 as an important mediator of BCR-ABL mediated human hematopoietic progenitor transformation in CML.
BCR-Tyrosine 177 plays a key role in signal transduction initiated by BCR-ABL leading to myeloid leukemogenesis in mice likely through binding to the SH2 domain of the Grb2 adaptor protein 14. We have shown that mutation of Y177 significantly reversed BCR-ABL mediated transformation of human hematopoietic cells 12. The Y177 motif represents a Grb2 SH2 domain binding motif. Here we confirmed that mutation of BCR-Y177 abrogates interaction with Grb2 in BCR-ABL expressing CD34+ cells. The role of Grb2 on BCR-ABL induced transformation in primary hematopoietic cells has been indirectly evaluated through mutation of Y177 or Grb2-SH3 domain peptides that inhibit association with SoS, but has not been directly studied by inhibition of gene expression 31. Grb2 expression is critical for normal development, and mouse embryos with homozygous deletions of grb2 die early in embryonic development, impeding investigation of the role of Grb2 later in development 29. The use of shRNA-mediated Grb2 knockdown in this study allowed us to address the role of Grb2 in normal and leukemic CD34+ progenitors.
Grb2 knockdown in BCR-ABL expressing CD34+ cells required dual transduction of CD34+ cells with separate vectors expressing the BCR-ABL gene and a Grb2 shRNA construct while coexpressing GFP and RFP respectively. Whereas various internal promoters express GFP reporter gene well in cell lines and primary progenitor cells 17, 18, their efficiency for expression of RFP has not been studied. We found that use of CMV or PGK promoters resulted in a very low percentage of cells double positive for GFP and RFP. In contrast the SFFV LTR promoter resulted in significantly higher levels of RFP expression in CD34+ cells compared to CMV or PGK promoters. Therefore expression of RFP and GFP in hematopoietic stem cells using the strong promoter SFFV LTR allowed us to successfully coexpress Grb2 shRNA with the BCR-ABL gene in CD34+ cells.
We observed that shRNA mediated inhibition of Grb2 expression significantly inhibited BCR-ABL-stimulated proliferation and survival of human CD34+ hematopoietic cells. These findings suggest that BCR-ABL expressing CD34+ cells are dependent on Grb2 signaling at least in part for the fully transformed phenotype. Interestingly similar levels of Grb2 knockdown had a significantly less prominent effect on the growth of control CD34+ cells, although prolonged Grb2 inhibition may result in modest inhibition of proliferation of normal hematopoietic cells. These results are important since Grb2 signaling is reported to play an important role in normal GF receptor signaling and suggest redundancies leading to lack of dependence on Grb2 in GF signaling in normal CD34+ cells.
The canonical model of Grb2 function is based on the constitutive association of one of the Grb2 SH3 domains with proline-rich sequences in SoS, a guanine nucleotide exchange factor that promotes GDP-GTP exchange on Ras, leading to activation of Ras and the MAPK cascade. Consistent with this we observed that Grb2 knockdown in BCR-ABL transformed human hematopoietic cells was associated with inhibition of MAPK phosphorylation. Grb2 can also bind members of the mammalian Gab family, which can enhance signaling through GF and cytokine receptors29. However we did not observe significant inhibition of Akt or STAT5 phosphorylation, suggesting that signaling through Gab2 may be less affected by Grb2 knockdown in this cell type.
There has been considerable interest in developing strategies to target Grb2 using novel protein interaction blocker drugs that abrogate Grb2 binding to downstream effectors such as SoS, blocking the activation of Ras and MAPK. High affinity Grb2 SH3 (N) domain blocker peptides (HAGBP) have been developed by modification of the naturally occurring proline-rich motif in the SoS protein 32. Our results support the potential utility of targeting Grb2 as an alternative or an adjuvant treatment for CML in addition to tyrosine kinase blockade. Of note, CML primitive progenitors/stem cells in particular have been found to be resistant to elimination by tyrosine kinase inhibitor treatment. However, the functional role of Grb2 in the primitive CML CD34+CD38- subpopulation could not be determined in the present study because of technical limitations related to conditions required for efficient transduction of hematopoietic cells. In addition although cord blood CD34+ cells were used in these studies because of their ease of transduction, it is possible that Grb2 signaling in adult CD34+ cells could differ from that observed in cord blood cells. Further technical improvements in gene expression in primitive adult human hematopoietic stem and progenitor cells will be required to address these issues in future studies.
In conclusion our studies confirm an important role for Grb2 mediated activation of MAPK in BCR-ABL mediated transformation of human hematopoietic cells, and demonstrate selectivity of effects of Grb2 inhibition for BCR-ABL-transformed as compared to normal hematopoietic cells. These results support further investigation of downstream effectors of Grb2-mediated signals and targeting of Grb2 interactions in the treatment of CML.
Supplementary Material
Acknowledgments
Supported by NIH grant R01 HL77847 and R01CA095684 to Ravi Bhatia. We acknowledge the support of the COHNMC Analytical Cytometry and Sequencing cores. We are grateful to StemCyte for their generous gift of cord blood samples.
Footnotes
Conflict of interests: The authors have no conflicts of interest to report.
References
- 1.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 2.de Klein A, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–7. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 3.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–82. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–40. [PubMed] [Google Scholar]
- 5.Deininger MW, Druker BJ. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol Rev. 2003;55:401–23. doi: 10.1124/pr.55.3.4. [DOI] [PubMed] [Google Scholar]
- 6.Druker BJ, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 7.Talpaz M, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928–37. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 8.Holtz MS, et al. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia R, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–7. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 10.Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19:1034–41. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 11.Rousselot P, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 12.Ramaraj P, et al. Effect of mutational inactivation of tyrosine kinase activity on BCR/ABL-induced abnormalities in cell growth and adhesion in human hematopoietic progenitors. Cancer Res. 2004;64:5322–31. doi: 10.1158/0008-5472.CAN-03-3656. [DOI] [PubMed] [Google Scholar]
- 13.Chu S, Li L, Singh H, Bhatia R. BCR-tyrosine 177 plays an essential role in Ras and Akt activation and in human hematopoietic progenitor transformation in chronic myelogenous leukemia. Cancer Res. 2007;67:7045–53. doi: 10.1158/0008-5472.CAN-06-4312. [DOI] [PubMed] [Google Scholar]
- 14.Puil L, et al. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. Embo J. 1994;13:764–73. doi: 10.1002/j.1460-2075.1994.tb06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattler M, et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1:479–92. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 16.Million RP, Harakawa N, Roumiantsev S, Varticovski L, Van Etten RA. A direct binding site for Grb2 contributes to transformation and leukemogenesis by the Tel-Abl (ETV6-Abl) tyrosine kinase. Mol Cell Biol. 2004;24:4685–95. doi: 10.1128/MCB.24.11.4685-4695.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yam PY, et al. Design of HIV vectors for efficient gene delivery into human hematopoietic cells. Mol Ther. 2002;5:479–84. doi: 10.1006/mthe.2002.0558. [DOI] [PubMed] [Google Scholar]
- 18.Ramezani A, Hawley TS, Hawley RG. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther. 2000;2:458–69. doi: 10.1006/mthe.2000.0190. [DOI] [PubMed] [Google Scholar]
- 19.Baum C, Hegewisch-Becker S, Eckert HG, Stocking C, Ostertag W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J Virol. 1995;69:7541–7. doi: 10.1128/jvi.69.12.7541-7547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modi H, et al. Role of BCR/ABL gene-expression levels in determining the phenotype and imatinib sensitivity of transformed human hematopoietic cells. Blood. 2007;109:5411–21. doi: 10.1182/blood-2006-06-032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Zhao RC, Verfaillie CM. Abnormal integrin-mediated regulation of chronic myelogenous leukemia CD34+ cell proliferation: BCR/ABL up-regulates the cyclin-dependent kinase inhibitor, p27Kip, which is relocated to the cell cytoplasm and incapable of regulating cdk2 activity. Proc Natl Acad Sci U S A. 2000;97:10538–43. doi: 10.1073/pnas.190104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta P, McCarthy JB, Verfaillie CM. Stromal fibroblast heparan sulfate is required for cytokine-mediated ex vivo maintenance of human long-term culture-initiating cells. Blood. 1996;87:3229–36. [PubMed] [Google Scholar]
- 23.Bhatia R, McGlave PB, Dewald GW, Blazar BR, Verfaillie CM. Abnormal function of the bone marrow microenvironment in chronic myelogenous leukemia: role of malignant stromal macrophages. Blood. 1995;85:3636–45. [PubMed] [Google Scholar]
- 24.Pendergast AM, et al. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993;75:175–85. [PubMed] [Google Scholar]
- 25.Zhang X, Subrahmanyam R, Wong R, Gross AW, Ren R. The NH(2)-terminal coiled-coil domain and tyrosine 177 play important roles in induction of a myeloproliferative disease in mice by Bcr-Abl. Mol Cell Biol. 2001;21:840–53. doi: 10.1128/MCB.21.3.840-853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma G, Lu D, Wu Y, Liu J, Arlinghaus RB. Bcr phosphorylated on tyrosine 177 binds Grb2. Oncogene. 1997;14:2367–72. doi: 10.1038/sj.onc.1201053. [DOI] [PubMed] [Google Scholar]
- 27.Gishizky ML, Cortez D, Pendergast AM. Mutant forms of growth factor-binding protein-2 reverse BCR-ABL-induced transformation. Proc Natl Acad Sci U S A. 1995;92:10889–93. doi: 10.1073/pnas.92.24.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–37. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 29.Cheng AM, et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 30.Giubellino A, Burke TR, Jr, Bottaro DP. Grb2 signaling in cell motility and cancer. Expert Opin Ther Targets. 2008;12:1021–33. doi: 10.1517/14728222.12.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kardinal C, et al. Chronic myelogenous leukemia blast cell proliferation is inhibited by peptides that disrupt Grb2-SoS complexes. Blood. 2001;98:1773–81. doi: 10.1182/blood.v98.6.1773. [DOI] [PubMed] [Google Scholar]
- 32.Feller SM, Tuchscherer G, Voss J. High affinity molecules disrupting GRB2 protein complexes as a therapeutic strategy for chronic myelogenous leukaemia. Leuk Lymphoma. 2003;44:411–27. doi: 10.1080/1042819021000037930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.