Abstract
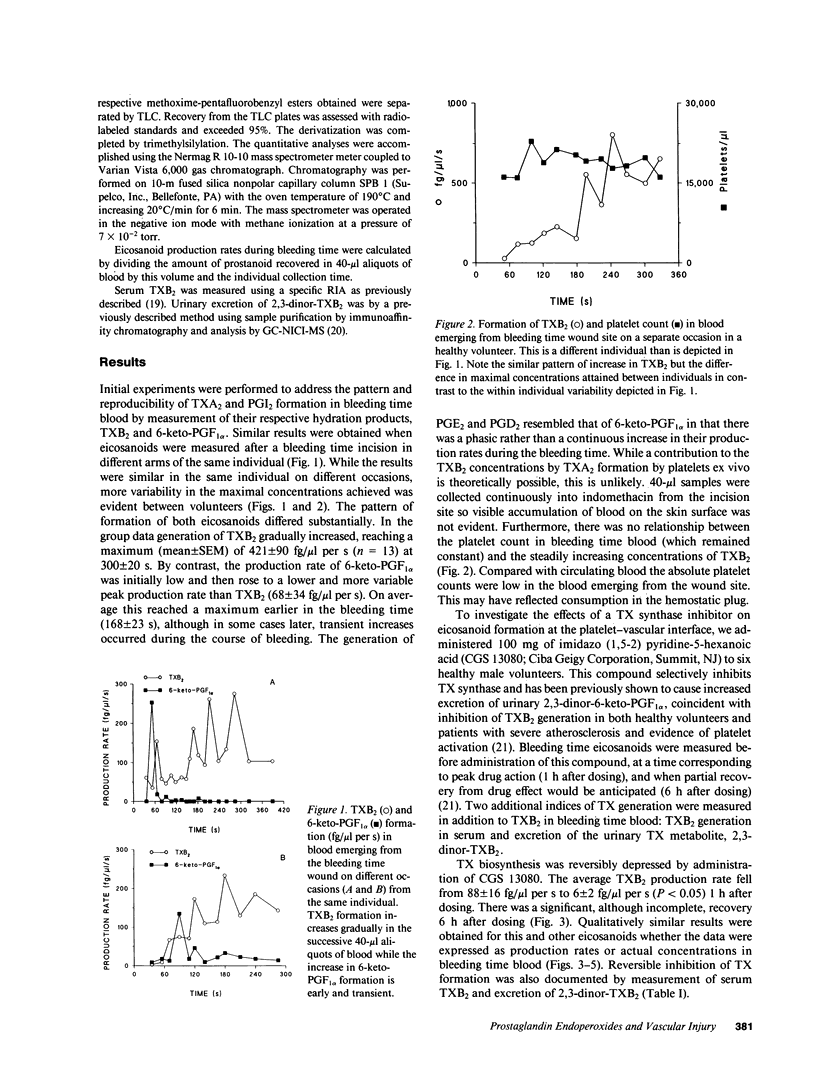
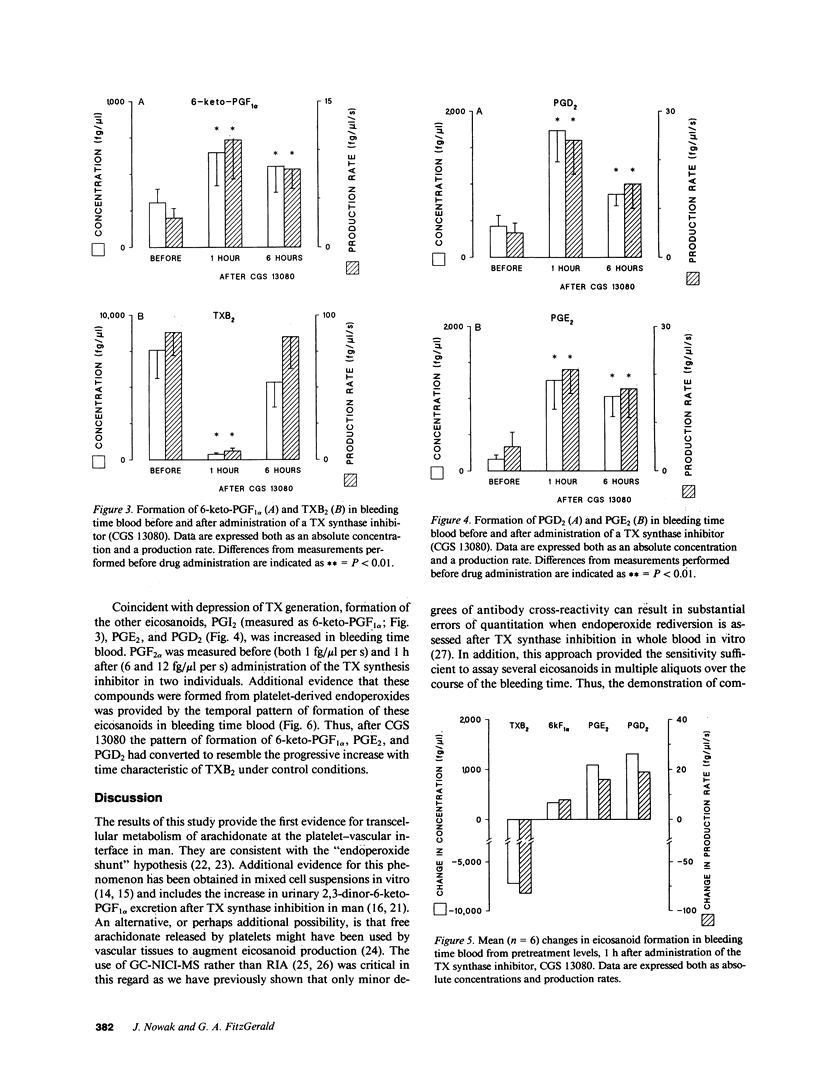
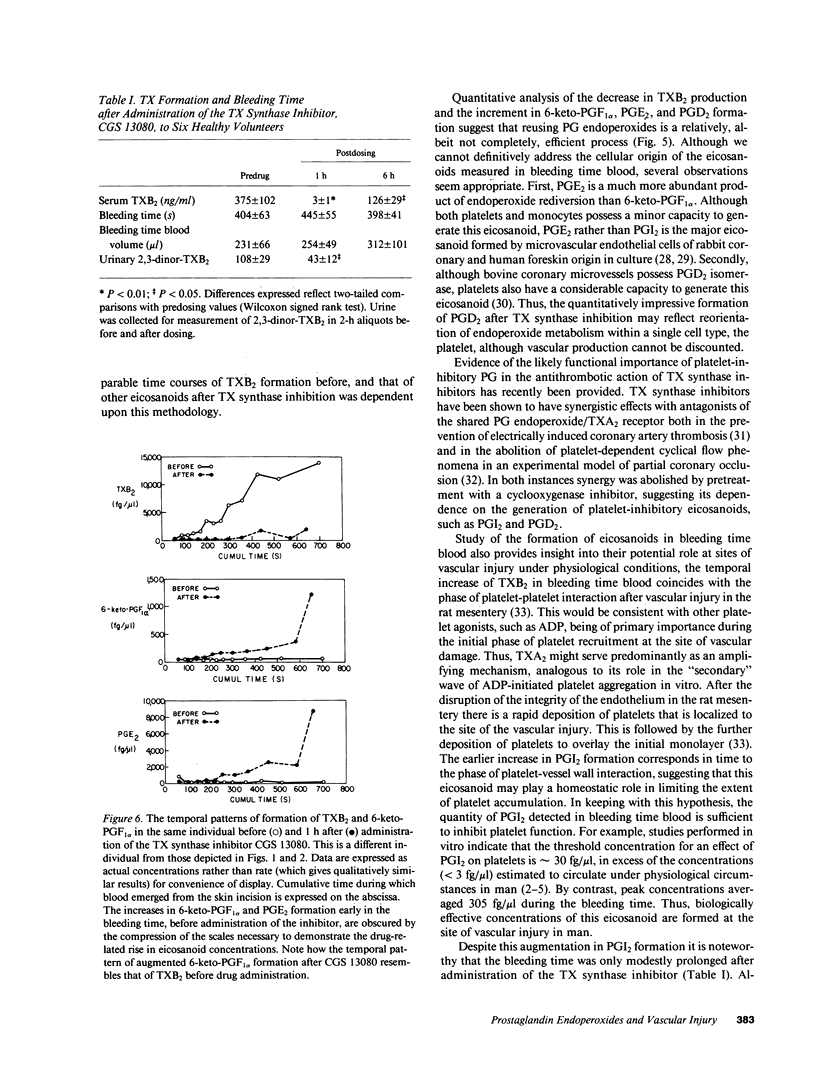
Prostacyclin (PGI2) is an inhibitor of platelet function in vitro. We tested the hypothesis that PGI2 is formed in biologically active concentrations at the platelet-vascular interface in man and can be pharmacologically modulated to enhance its inhibitory properties. This became feasible when we developed a microquantitative technique that permits the measurement of eicosanoids in successive 40-microliters aliquots of whole blood emerging from a bleeding time wound. In 13 healthy volunteers the rate of production of thromboxane B2 (TXB2) gradually increased, reaching a maximum of 421 +/- 90 (mean +/- SEM) fg/microliters per s at 300 +/- 20 s. The hydration product of PGI2, 6-keto-PGF1 alpha, rose earlier and to a lesser degree, reaching a peak (68 +/- 34 fg/microliters per s) at 168 +/- 23 s. The generation of prostaglandins PGE2 and D2 resembled that of PGI2. Whereas the threshold concentration of PGI2 for an effect on platelets in vitro is approximately 30 fg/microliters, only less than 3 fg/microliters circulates under physiological conditions. By contrast, peak concentrations of 6-keto-PGF1 alpha obtained locally after vascular damage averaged 305 fg/microliters. Pharmacological regulation of PG endoperoxide metabolism at the platelet-vascular interface was demonstrated by administration of a TX synthase inhibitor. The rate of production of PGI2, PGE2, and PGD2 increased coincident with inhibition of TXA, as reflected by three indices; the concentration of TXB2 in bleeding time blood and serum, and excretion of the urinary metabolite, 2,3-dinor-TXB2. These studies indicate that PGI2 is formed locally in biologically effective concentrations at the site of vessel injury and provide direct evidence in support of transcellular metabolism of PG endoperoxides in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertele V., De Gaetano G. Potentiation by dazoxiben, a thromboxane synthetase inhibitor, of platelet aggregation inhibitory activity of a thromboxane receptor antagonist and of prostacyclin. Eur J Pharmacol. 1982 Dec 3;85(3-4):331–333. doi: 10.1016/0014-2999(82)90220-5. [DOI] [PubMed] [Google Scholar]
- Blair I. A., Barrow S. E., Waddell K. A., Lewis P. J., Dollery C. T. Prostacyclin is not a circulating hormone in man. Prostaglandins. 1982 Apr;23(4):579–589. doi: 10.1016/0090-6980(82)90118-6. [DOI] [PubMed] [Google Scholar]
- Born G. V., Richardson P. D. Activation time of blood platelets. J Membr Biol. 1980 Dec 15;57(2):87–90. doi: 10.1007/BF01868994. [DOI] [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Shak S., Karasek M. A., Davison P. M., Goldstein I. M. Prostaglandin I2 is not a major metabolite of arachidonic acid in cultured endothelial cells from human foreskin microvessels. J Clin Invest. 1984 Sep;74(3):914–919. doi: 10.1172/JCI111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ-Hazelhof E., Nugteren D. H. Prostacyclin is not a circulating hormone. Prostaglandins. 1981 Nov;22(5):739–746. doi: 10.1016/0090-6980(81)90213-6. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Zetter B. R., Antoniades H. N., Levine L. Platelet-dependent stimulation of prostacyclin synthesis by platelet-derived growth factor. Nature. 1980 Dec 11;288(5791):600–602. doi: 10.1038/288600a0. [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A., Brash A. R., Falardeau P., Oates J. A. Estimated rate of prostacyclin secretion into the circulation of normal man. J Clin Invest. 1981 Nov;68(5):1272–1276. doi: 10.1172/JCI110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Brash A. R., Oates J. A., Pedersen A. K. Endogenous prostacyclin biosynthesis and platelet function during selective inhibition of thromboxane synthase in man. J Clin Invest. 1983 Oct;72(4):1336–1343. doi: 10.1172/JCI111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Oates J. A., Hawiger J., Maas R. L., Roberts L. J., 2nd, Lawson J. A., Brash A. R. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983 Mar;71(3):676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Reilly I. A., Pedersen A. K. The biochemical pharmacology of thromboxane synthase inhibition in man. Circulation. 1985 Dec;72(6):1194–1201. doi: 10.1161/01.cir.72.6.1194. [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A., Smith B., Pedersen A. K., Brash A. R. Increased prostacyclin biosynthesis in patients with severe atherosclerosis and platelet activation. N Engl J Med. 1984 Apr 26;310(17):1065–1068. doi: 10.1056/NEJM198404263101701. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. J., Fragetta J., FitzGerald G. A. Prostaglandin endoperoxides modulate the response to thromboxane synthase inhibition during coronary thrombosis. J Clin Invest. 1988 Nov;82(5):1708–1713. doi: 10.1172/JCI113784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. J., Roy L., Catella F., FitzGerald G. A. Platelet activation in unstable coronary disease. N Engl J Med. 1986 Oct 16;315(16):983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Gorman R. R. A comparison of imidazole and 9,11-azoprosta-5,13-dienoic acid. Two selective thromboxane synthetase inhibitors. Biochim Biophys Acta. 1978 Mar 1;539(2):162–172. doi: 10.1016/0304-4165(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Bundy G. L., Peterson D. C., Sun F. F., Miller O. V., Fitzpatrick F. A. Inhibition of human platelet thromboxane synthetase by 9,11-azoprosta-5,13-dienoic acid. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4007–4011. doi: 10.1073/pnas.74.9.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresele P., Arnout J., Deckmyn H., Huybrechts E., Pieters G., Vermylen J. Role of proaggregatory and antiaggregatory prostaglandins in hemostasis. Studies with combined thromboxane synthase inhibition and thromboxane receptor antagonism. J Clin Invest. 1987 Nov;80(5):1435–1445. doi: 10.1172/JCI113223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrle P. A., Eichler H. G., Jäger U., Lechner K. Inhibition of prostacyclin and thromboxane A2 generation by low-dose aspirin at the site of plug formation in man in vivo. Circulation. 1987 May;75(5):1025–1029. doi: 10.1161/01.cir.75.5.1025. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A., Broekman M. J. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980 Nov;66(5):979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Unstable metabolites of arachidonic acid and their role in haemostasis and thrombosis. Br Med Bull. 1978 May;34(2):129–135. doi: 10.1093/oxfordjournals.bmb.a071482. [DOI] [PubMed] [Google Scholar]
- Needleman P. Prostacyclin in blood vessel-platelet interactions: perspectives and questions. Nature. 1979 May 3;279(5708):14–15. doi: 10.1038/279014a0. [DOI] [PubMed] [Google Scholar]
- Nowak J., Murray J. J., Oates J. A., FitzGerald G. A. Biochemical evidence of a chronic abnormality in platelet and vascular function in healthy individuals who smoke cigarettes. Circulation. 1987 Jul;76(1):6–14. doi: 10.1161/01.cir.76.1.6. [DOI] [PubMed] [Google Scholar]
- Oelz O., Oelz R., Knapp H. R., Sweetman B. J., Oates J. A. Biosynthesis of prostaglandin D2. 1. Formation of prostaglandin D2 by human platelets. Prostaglandins. 1977 Feb;13(2):225–234. doi: 10.1016/0090-6980(77)90004-1. [DOI] [PubMed] [Google Scholar]
- Pedersen A. K., Watson M. L., FitzGerald G. A. Inhibition of thromboxane biosynthesis in serum: limitations of the measurement of immunoreactive 6-keto-PGF1 alpha. Thromb Res. 1984 Jan 1;33(1):99–103. doi: 10.1016/0049-3848(84)90159-2. [DOI] [PubMed] [Google Scholar]
- Reilly I. A., Doran J. B., Smith B., FitzGerald G. A. Increased thromboxane biosynthesis in a human preparation of platelet activation: biochemical and functional consequences of selective inhibition of thromboxane synthase. Circulation. 1986 Jun;73(6):1300–1309. doi: 10.1161/01.cir.73.6.1300. [DOI] [PubMed] [Google Scholar]
- Schafer A. I., Crawford D. D., Gimbrone M. A., Jr Unidirectional transfer of prostaglandin endoperoxides between platelets and endothelial cells. J Clin Invest. 1984 Apr;73(4):1105–1112. doi: 10.1172/JCI111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W., Dray F. Very low levels of 6-keto-prostaglandin F1 alpha in human plasma. J Lab Clin Med. 1982 Mar;99(3):388–398. [PubMed] [Google Scholar]
- Vermylen J., Defreyn G., Carreras L. O., Machin S. J., Van Schaeren J., Verstraete M. Thromboxane synthetase inhibition as antithrombotic strategy. Lancet. 1981 May 16;1(8229):1073–1075. doi: 10.1016/s0140-6736(81)92241-8. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynalda M. A., Fitzpatrick F. A. Albumins stabilize prostaglandin I2. Prostaglandins. 1980 Nov;20(5):853–861. doi: 10.1016/0090-6980(80)90138-0. [DOI] [PubMed] [Google Scholar]


