Abstract
Thalassemic red cells show irregular morphology and maldistribution of glycoproteins and sialic acids. These changes are compatible with damage to the red cell membrane skeleton. To test this possibility, we systematically studied the interconnections of skeletal proteins in patients with a form of alpha thalassemia (HbH disease), in patients with beta thalassemia intermedia, and in normal individuals. Alpha- and beta-thalassemic spectrin functions normally in spectrin self-association, binding to normal inside-out vesicles (IOVs), and binding to actin in the presence and absence of normal protein 4.1. Binding of normal spectrin to beta: thalassemic IOVs is normal but alpha-thalassemic IOVs are defective and bind only half the normal amount of spectrin (66 +/- 5 vs. 120 +/- 16 micrograms spectrin dimer/mg IOV protein, respectively). A different defect is detected in beta thalassemia, in which protein 4.1 shows markedly reduced ability (48 +/- 7% of normal) to enhance the binding of normal spectrin to actin and a decreased ability to bind normal spectrin in a binary interaction, compared with normal protein 4.1 (24 +/- 1 and 43 +/- 1 micrograms protein 4.1/mg spectrin, respectively). As no quantitative deficiency of beta-thalassemic protein 4.1 is detected, we assume an acquired lesion is present, which affects about half of the protein 4.1 molecules. These findings indicate that specific, localized, yet different defects exist in the skeletal proteins of alpha- and beta-thalassemic red cells. The different molecular lesions imply that the mechanism of hemolysis and probably the interaction of unpaired globin chains with the membrane differs in the two diseases.
Full text
PDF

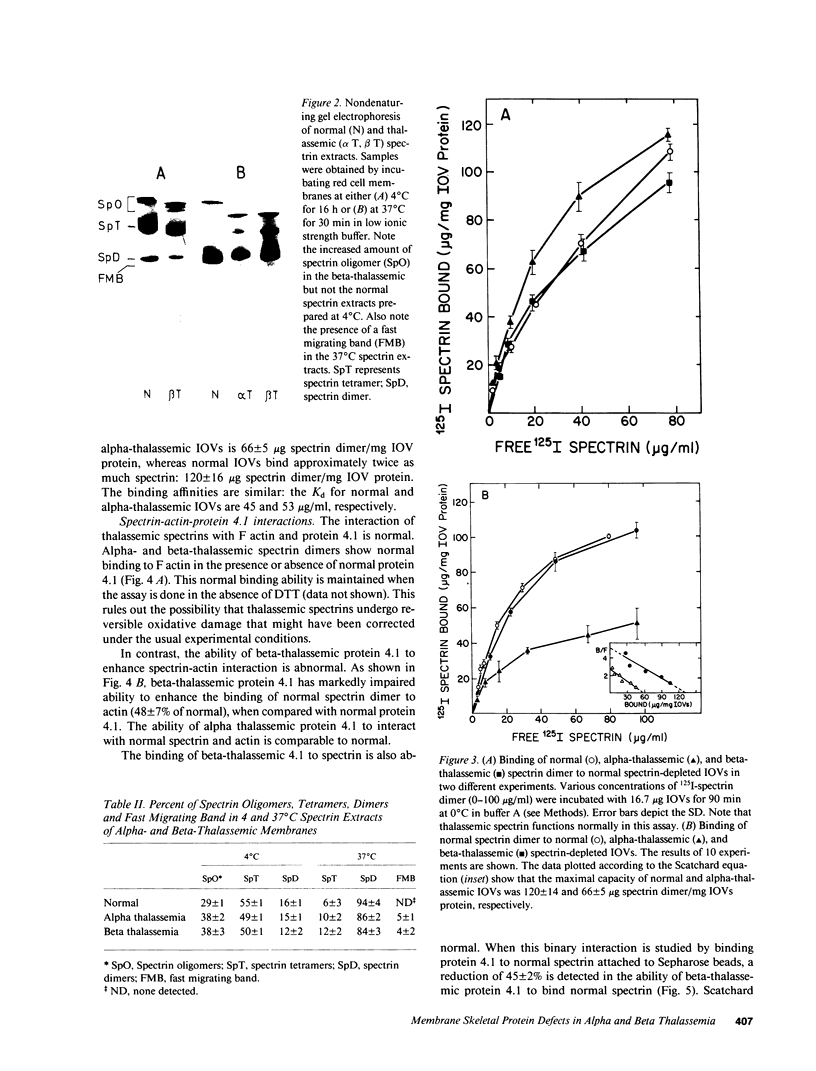
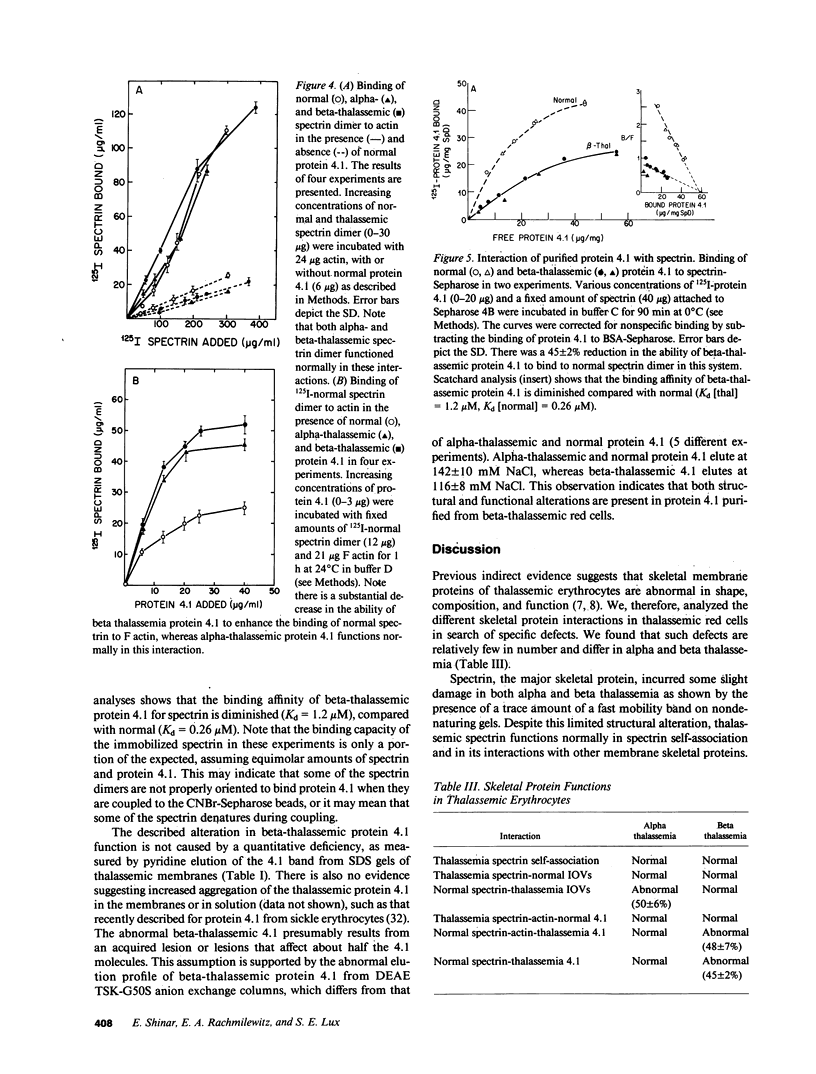


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Orringer E. P., Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982 May 13;306(19):1155–1161. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- Allen D. W., Cadman S., McCann S. R., Finkel B. Increased membrane binding of erythrocyte catalase in hereditary spherocytosis and in metabolically stressed normal cells. Blood. 1977 Jan;49(1):113–123. [PubMed] [Google Scholar]
- Anderson R. A., Lovrien R. E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984 Feb 16;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Marchesi V. T. Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature. 1985 Nov 21;318(6043):295–298. doi: 10.1038/318295a0. [DOI] [PubMed] [Google Scholar]
- Aragoncillo C., Rodriguez-Loperena M. A., Carbonero P., Garcia-Olmeda F. Nigrosine staining of wheat endosperm proteolipid patterns on starch gels. Anal Biochem. 1975 Feb;63(2):603–606. doi: 10.1016/0003-2697(75)90387-5. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Cohen C. M., Lux S. E. The effect of mild diamide oxidation on the structure and function of human erythrocyte spectrin. J Biol Chem. 1986 Apr 5;261(10):4620–4628. [PubMed] [Google Scholar]
- Becker P. S., Spiegel J. E., Wolfe L. C., Lux S. E. High yield purification of protein 4.1 from human erythrocyte membranes. Anal Biochem. 1983 Jul 1;132(1):195–201. doi: 10.1016/0003-2697(83)90447-5. [DOI] [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cividalli G., Kerem H., Rachmilewitz E. A. Globin synthesis in severe and intermediate homozygous beta thalassemia in Israel. Ann N Y Acad Sci. 1980;344:132–140. doi: 10.1111/j.1749-6632.1980.tb33656.x. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Liu S. C., Derick L. H., Palek J. Ultrastructural studies of the interaction of spectrin with phosphatidylserine liposomes. Blood. 1986 Oct;68(4):920–926. [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F. Biochemical characterization of complex formation by human erythrocyte spectrin, protein 4.1, and actin. Biochemistry. 1984 Dec 4;23(25):6091–6098. doi: 10.1021/bi00320a029. [DOI] [PubMed] [Google Scholar]
- Correas I., Leto T. L., Speicher D. W., Marchesi V. T. Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J Biol Chem. 1986 Mar 5;261(7):3310–3315. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galili U., Korkesh A., Kahane I., Rachmilewitz E. A. Demonstration of a natural antigalactosyl IgG antibody on thalassemic red blood cells. Blood. 1983 Jun;61(6):1258–1264. [PubMed] [Google Scholar]
- Goodman S. R., Weidner S. A. Binding of spectrin alpha 2-beta 2 tetramers to human erythrocyte membranes. J Biol Chem. 1980 Sep 10;255(17):8082–8086. [PubMed] [Google Scholar]
- Harris H. W., Jr, Lux S. E. Structural characterization of the phosphorylation sites of human erythrocyte spectrin. J Biol Chem. 1980 Dec 10;255(23):11512–11520. [PubMed] [Google Scholar]
- Kahane I., Rachmilewitz E. A. Alterations in the red blood cell membrane and the effect of vitamin E on osmotic fragility in beta-thalassemia major. Isr J Med Sci. 1976 Jan;12(1):11–15. [PubMed] [Google Scholar]
- Kahane I., Shifter A., Rachmilewitz E. A. Cross-linking of red blood cell membrane proteins induced by oxidative stress in beta thalassemia. FEBS Lett. 1978 Jan 15;85(2):267–270. doi: 10.1016/0014-5793(78)80470-0. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A., Shohet S. B. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983 Jul;20(3):225–242. [PubMed] [Google Scholar]
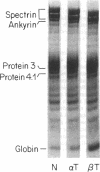
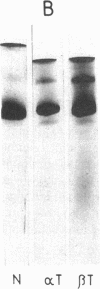
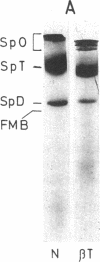
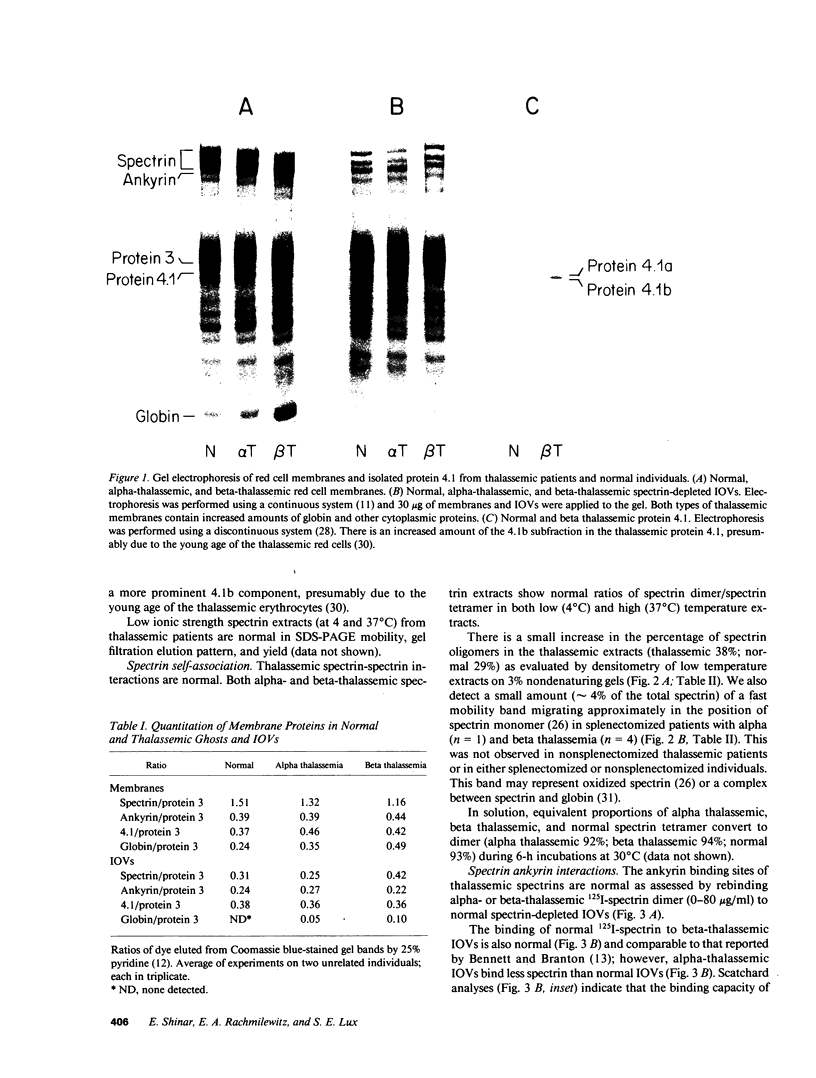
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian V., Wolfe L. C., John K. M., Pinder J. C., Lux S. E., Gratzer W. B. Analysis of the ternary interaction of the red cell membrane skeletal proteins spectrin, actin, and 4.1. Biochemistry. 1984 Sep 11;23(19):4416–4420. doi: 10.1021/bi00314a027. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):45–87. [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Platt O. S., Falcone J. F., Lux S. E. Molecular defect in the sickle erythrocyte skeleton. Abnormal spectrin binding to sickle inside-our vesicles. J Clin Invest. 1985 Jan;75(1):266–271. doi: 10.1172/JCI111684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt O. S., Falcone J. F. Membrane protein lesions in erythrocytes with Heinz bodies. J Clin Invest. 1988 Sep;82(3):1051–1058. doi: 10.1172/JCI113661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polliack A., Rachmilewitz E. A. Ultrastructural studies in -thalassaemia major. Br J Haematol. 1973 Mar;24(3):319–326. doi: 10.1111/j.1365-2141.1973.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Bradley T. B., Blumberg W. E. Role of haemichromes in the formation of inclusion bodies in haemoglobin H disease. Nature. 1969 Apr 19;222(5190):248–250. doi: 10.1038/222248a0. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., Shinar E., Shalev O., Galili U., Schrier S. L. Erythrocyte membrane alterations in beta-thalassaemia. Clin Haematol. 1985 Feb;14(1):163–182. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Shohet S. B., Lubin B. H. Lipid membrane peroxidation in beta-thalassemia major. Blood. 1976 Mar;47(3):495–505. [PubMed] [Google Scholar]
- Sato S. B., Ohnishi S. Interaction of a peripheral protein of the erythrocyte membrane, band 4.1, with phosphatidylserine-containing liposomes and erythrocyte inside-out vesicles. Eur J Biochem. 1983 Jan 17;130(1):19–25. doi: 10.1111/j.1432-1033.1983.tb07111.x. [DOI] [PubMed] [Google Scholar]
- Sauberman N., Fortier N. L., Fairbanks G., O'Connor R. J., Snyder L. M. Red cell membrane in hemolytic disease. Studies on variables affecting electrophoretic analysis. Biochim Biophys Acta. 1979 Sep 21;556(2):292–313. doi: 10.1016/0005-2736(79)90049-x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Rybicki A. C., Heath R. H., Lubin B. H. Protein 4.1 in sickle erythrocytes. Evidence for oxidative damage. J Biol Chem. 1987 Nov 15;262(32):15666–15672. [PubMed] [Google Scholar]
- Shinar E., Shalev O., Rachmilewitz E. A., Schrier S. L. Erythrocyte membrane skeleton abnormalities in severe beta-thalassemia. Blood. 1987 Jul;70(1):158–164. [PubMed] [Google Scholar]
- Snyder L. M., Leb L., Piotrowski J., Sauberman N., Liu S. C., Fortier N. L. Irreversible spectrin-haemoglobin crosslinking in vivo: a marker for red cell senescence. Br J Haematol. 1983 Mar;53(3):379–384. doi: 10.1111/j.1365-2141.1983.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Takakuwa Y., Tchernia G., Rossi M., Benabadji M., Mohandas N. Restoration of normal membrane stability to unstable protein 4.1-deficient erythrocyte membranes by incorporation of purified protein 4.1. J Clin Invest. 1986 Jul;78(1):80–85. doi: 10.1172/JCI112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Waugh S. M., Low P. S. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry. 1985 Jan 1;24(1):34–39. doi: 10.1021/bi00322a006. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B. Thalassemia revisited. Cell. 1982 May;29(1):7–9. doi: 10.1016/0092-8674(82)90084-8. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N., Hughes M., Hollán S. R., Horányi M., Szelényi J. Electron microscope and high resolution autoradiographic studies of the erythroblasts in haemoglobin H disease. Br J Haematol. 1980 Jul;45(3):401–404. doi: 10.1111/j.1365-2141.1980.tb07160.x. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]