Abstract
Recent studies have reported that adenosine is a significant mediator of regulatory T cell (Treg) function. Indeed, activation of the adenosine receptor subtypes expressed by a broad range of immune and inflammatory cells attenuates inflammation in several disease models. This anti-inflammatory response is associated with an increase in intracellular cAMP that inhibits cytokine responses of many immune/inflammatory cells, including T cells and APCs. Thus, adenosine produced by Tregs can provide a paracrine feedback that shapes the host response following an immunologic provocation. This review discusses the evidence that adenosine is an integral part of Treg biology and presents some of the mechanisms that may account for its contribution to the resolution of inflammation and the regulation of the immune/inflammatory cell phenotype.
Immune-inflammatory cells respond to local mediators, accessory molecules, and microbial products to assume a phenotype suitable for the tissue and physiologic stimulus. This plasticity allows the host to maintain the appropriate expression of genes so that immunity is optimized without excessive inflammation. CD4+ regulatory T cells (Tregs) produce mediators that contribute to this homeostasis by controlling gene expression in immune–inflammatory cells. One such anti-inflammatory mediator is adenosine, and the studies identifying its many anti-inflammatory effects are reviewed in detail elsewhere (1–3). However, like another mediator of Treg—that is, TGF-β1—adenosine has multiple effects. These mediators should not be considered as uniformly anti-inflammatory, but as part of a network of factors that, through their pleiotropic effects, shape the responses of target cells. Thus, the potential anti-inflammatory benefits of adenosine that can be exploited therapeutically have to be balanced against their possible detrimental effects.
Adenosine synthesis and the control of inflammation
Adenosine accumulates in response to inflammation and ischemia. There are several means by which adenosine is produced (4), beginning with the metabolism of intracellular ATP, ADP, and AMP via the action of cytoplasmic 5′-nucleotidases. Second, it can be generated by S-adenosylhomocysteine hydrolase. Finally, adenosine can be generated extracellularly from ATP and ADP via the combined action of ecto-nucleoside triphosphate diphosphohydrolase (ecto-NTPDase-1 [CD39])—or possibly other NTDPases—and ecto-5′-nucleotidase (CD73). Adenosine concentrations are also influenced by adenosine deaminase (ADA), a purine salvage pathway enzyme that degrades adenosine to inosine and adenosine kinase, which phosphorylates adenosine to AMP. The role of adenosine in immune regulation can be appreciated when its concentration is modulated by a deficiency of ADA, CD39, or CD73 expression. Loss of ADA elevates adenosine (5), whereas deletion of CD39 (6) or CD73 (7) results in less extracellular adenosine and impaired control of inflammation. For example, cd73–/– mice exhibit defects in kidney function (8), thromboregulation (9), physiologic responses to hypoxia (7), and enhanced responses to some infections (10).
Evidence for the generation of adenosine by Tregs
The first suggestion that adenosine played a role in Treg biology emerged from an in vivo study in mice in which the adoptive transfer of Tregs prevents the induction of colitis by effector Th cells (Teffs) (11). As elaborated below, Tregs failed to control colitis by Teffs that lacked the adenosine A2A receptor (A2AAR), suggesting that adenosine contributed to the anti-inflammatory role conferred by Tregs. Shortly thereafter, murine Tregs were shown to express CD73, which converts 5AMP into adenosine (12). However, CD73 is expressed by most activated Th cells, and its expression is not enriched on Tregs. In two subsequent studies, Tregs were shown to express CD73 and CD39, thus possessing the full synthetic pathway enabling Tregs to synthesize adenosine from ATP (6, 13). Importantly, these Tregs have been shown to regulate inflammation in an adenosine-dependent manner (6, 13), and translational studies report that CD39 is expressed selectively with CD73 on Tregs from different human tissues (10, 13–16).
Additional evidence associating adenosine production with Tregs comes from experiments in which the presence of Foxp3 is positively associated with CD39 expression (13, 17). The documented ability of Tregs to convert ATP to adenosine provides direct evidence of the functional capacity of these enzymes (6, 13) and supports the notion that adenosine is a mediator of Treg function (Fig. 1).
FIGURE 1.
CD39 and CD73 on Tregs generate extracellular adenosine, which can engage ARs on Teffs, APCs, and myeloid cells. This aspect of Treg function would be dependent on the presence of the substrates (local ATP, ADP, and/or AMP), which accumulate as a consequence of inflammation, hypoxia, metabolic stress, and/or cell death.
Tregs can be defined by the differential expression of surface markers, and subsets of Tregs appear to control different diseases (18). This diversity may be linked to the ability to synthesize adenosine, because Tregs that express CD39 preferentially control Th17 responses (19). More direct evidence in support of adenosine as a mediator of Tregs includes studies showing that Tregs from cd39–/– mice are less efficient at suppressing proliferation of Teffs from A2AAR-deficient mice (6). However, it is impossible to ascertain from this study whether the lack of CD39 on the Treg or the lack of A2AAR on the Teff targets was the most important factor. It is worth noting that the Teffs lacking the A2AAR, which could not be controlled in the colitis model in vivo, were inhibited in the proliferation inhibition assay (20). These studies suggest that Tregs use different mediators, including adenosine, that have varied effects on Teff function in distinct assays.
Adenosine as an anti-inflammatory mediator in disease models
The digestive tract is ideal for addressing the biologic relevance of adenosine-mediated immune regulation in vivo, because adaptive Tregs control responses to the shifting repertoire of luminal Ags in this organ. For example, Tregs regulate the magnitude of gastritis after infection with Helicobacter spp. (21–23). Consistent with a role for adenosine in the control of inflammation, gastritis was more severe when mice lacking CD73 or the A2AAR were infected with H. pylori or H. felis (10, 24). More direct evidence for adenosine production by Tregs emerged from the adoptive transfer of Tregs from cd73–/– mice, which failed to prevent gastritis as efficiently as Tregs from wild-type mice (10). Furthermore, the reduction in inflammation by adenosine in these models favored the persistence of these infections (10, 24). CD39+ and CD73+ Tregs are also found in human gastric mucosa (10), and these responses are associated with the lifelong infection by H. pylori (25).
Adenosine production by Tregs also contributes to immune regulation in other regions of the digestive tract. Tregs prevent the wasting and colitis that follows the adoptive transfer of wild-type pathogenic CD4+ CD45RBhi Th cells into scid mice (20). In this same model, A2AAR agonists alone prevented colitis induced by pathogenic Th cells in the absence of Tregs (11). As mentioned above, wild-type Tregs failed to prevent colitis induced by the adoptive transfer of pathogenic Teffs from A2AAR–/– mice, implicating an anti-inflammatory role for Treg-derived adenosine (11). A2AAR on recipient myeloid cells is also required for optimal control of intestinal inflammation, because the cotransfer of Tregs and pathogenic T cells from wild-type mice into rag/A2AAR–/– double knockout recipients still leads to colitis (C.C. Kurtz and P.B. Ernst, manuscript in preparation). The importance of adenosine in the control of intestinal inflammation is supported by the increased susceptibility of cd39–/– mice to colitis induced by dextran sodium sulfate and the association of mutations in the cd39 gene with Crohn's disease in humans (26).
Based on these studies in the gastrointestinal tract, we propose that adaptive Tregs are important to cope with the complex microbiota and that the production of adenosine by Tregs is significant in this context. However, details of the distribution and expression of the molecules responsible for the synthesis and response to adenosine in the gut remain to be determined.
Mechanisms for adenosine-mediated Treg function
Adenosine binds to four distinct subtypes of adenosine receptors (ARs): A1, A2A, A2B, and A3 (1, 4). Because of a lack of useful Abs for the assessment of cell surface AR expression, investigators must rely on multiple approaches to confirm the role of a specific AR subtype in each experimental model, including the use of cells or mice deficient in specific ARs and/or appropriate concentrations of highly selective agonists or antagonists. Using genetically engineered mice lacking the A2AAR, adenosine has been implicated in the control of a wide range of inflammatory responses (27). It is now widely appreciated that Th cells express the A2AAR predominantly (10, 24, 28–30), whereas APCs express the A2AAR and/or A2BAR (31–35). The A2AAR is induced ~8-fold in naive Th cells upon activation (28, 29), whereas induction in excess of 100-fold occurs in APCs in response to LPS (36). Because it has been known for many years that ARs can mediate anti-inflammatory effects, their induction upon immunologic provocation suggests that adenosine is a broadly relevant mediator providing a negative feedback to limit immune-mediated tissue damage. As such, ARs are an important target for Treg-derived adenosine.
Adenosine regulates T cell function indirectly by reducing the secretion of proinflammatory cytokines, including IL-12 and TNF-a, from stimulated APCs by as much as 80–90% (33, 34, 37). In the case of TNF-a, the impairment in expression is due to a decrease in mRNA stability (38). Adenosine also stimulates production of the anti-inflammatory cytokine IL-10 (31–34). In addition, the expression of CD86 and the immunogenicity of dendritic cells are impaired by adenosine acting through the A2BAR (13, 31, 37, 39), mimicking some mechanisms of immune regulation conferred by Tregs (13, 40, 41). Thus, the marked induction of ARs after activation provides a feedback mechanism to control the host response to an infection or immunization.
A2AAR signaling on activated Th cells directly inhibits the production of cytokines including IL-2, IL-4, IFN-γ, and TNF-α by as much as 90% (28, 42), again by decreasing mRNA stability (11). Adenosine acts directly as an anti-inflammatory mediator, and the administration of adenosine analogs increases the number of Tregs (42). A role for adenosine in the optimal development of Treg responses could explain why Tregs from A2AAR–/– mice failed to control disease caused by the adoptive transfer of wild-type pathogenic Th cells (11). The fact that Tregs produce and respond to adenosine implies that adenosine acts as an autocrine factor to optimize anti-inflammatory responses. This immunosuppressive action of adenosine may be mediated by increasing the concentration of intracellular cAMP. Indeed, Tregs can transfer intracellular cAMP to Teffs via gap junctions (43), and elevating cAMP in Tregs renders them more suppressive (44, 45).
The transfer of cAMP to a target Teff would occur only when Tregs have direct contact with the target cell. This requirement for intimate cell–cell contact for the optimal expression and function of Treg mediators is shared by membrane-associated TGF-β1 (46) and IL-35 (47). Furthermore, inhibition of host responses mediated by interactions of LAG3 (48) and CTLA-4 (41) on Tregs with their respective receptors on target cells requires intimate contact (40). Because the t1/2 of adenosine is measured in seconds, intimacy within the immunologic synapse shared by APCs, Tregs, and Teffs would be essential for any inhibitory role for adenosine (Fig. 2).
FIGURE 2.
Many molecules associated with Treg (CTLA-4, LAG3, IL-35, TGF-β1, adenosine, and cAMP) require cell–cell contact for their respective actions.
Coupling of ARs to the control of inflammatory responses
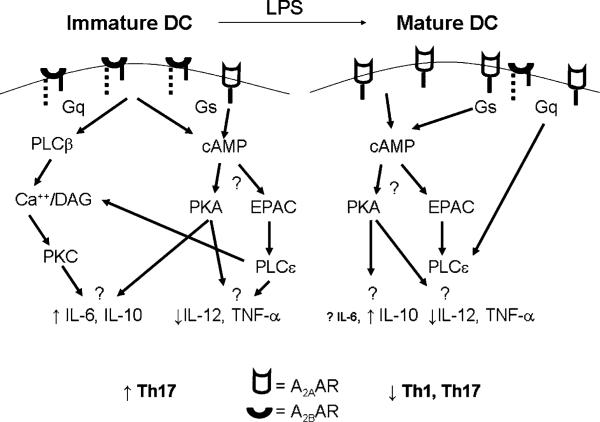
The A2AAR and A2BAR are distinguished by their ability to mediate increases in cAMP. The accumulation of cAMP is believed to responsible for the anti-inflammatory effects of adenosine delivered to T cells, APCs, or neutrophils (1, 4, 27). The A2AARs are coupled to members of the Gs α sub-family of G proteins, and their activation raises the level of cAMP (1, 49) (Fig. 3). Similarly, the activation of phosphodiesterases (PDEs) that degrade cAMP enhances responses of activated Th cells (50, 51). PDE activation is mediated in part through CD4. Mutation of class II MHC molecules on APCs so that they no longer bind CD4 prevents PDE activation in Th cells following stimulation and allows cAMP to accumulate (52). In association with this increase in cAMP, Th cells assume a phenotype resembling a Treg rather than an effector Th cell (53). Furthermore, the inflammatory potential of T cells is limited by blocking PDE activity pharmacologically (24, 54) or by providing exogenous cAMP analogs (55). These results all indicate the important role for cAMP in the inhibition of immune–inflammatory responses.
FIGURE 3.
The paracrine action of adenosine regulates the phenotype of adjacent cells, depending on the relative expression of AR subtypes or intracellular signaling molecules. Using APCs as an example, the resting cell expresses A2BAR predominantly, which accounts for the ability of adenosine to induce IL-6 and IL-10 (39) and inhibit IL-12 and TNF-α (37). Because the Gs coupling to the A2A and A2BAR accounts for the increase in cAMP, one might predict that responses mediated selectively through the A2BAR are attributable to Gq-activated pathways. A2AAR are the major receptor on dendritic cells after activation with LPS, (37) and adenosine (e.g., from Tregs) could provide a negative feedback through the A2AAR that impairs dendritic cell function and subsequently, a more general impairment in Th cell activation (35). Although many outcomes of adenosine on cytokine production have been reported, the linkages between specific signaling pathways and these eventual responses in the context of Treg biology remain to be defined more thoroughly.
The accumulation of cAMP leads to the activation of sensors, such as the cAMP-dependent protein kinase (PKA; Fig. 3). cAMP binds to the regulatory subunit of PKA, leading to the release of the active catalytic subunit and the phosphorylation of PKA substrates (56–58). Using PKA inhibitors such as H-89 suggested that activation of PKA is central to the ability of A2AAR to inhibit the oxidative burst in neutrophils (59).
The cAMP response element binding proteins are some of the substrates for PKA. Members of this leucine zipper family of transcription factors include CREB, the cAMP response element modulator and activating transcription factor 1 (60). Following activation of PKA, the free catalytic subunit enters the nucleus and phosphorylates the kinase inducible domain of CREB on Ser133, which increases its transcriptional activity. These transcription factors play multiple and sometimes paradoxical roles in Th cell responses. For example, the proteins are subject to alternative splicing and a particular variant of the cAMP response element modulator, termed inducible cAMP early repressor, attenuates cytokine induction after TCR stimulation (60). The expression of Foxp3, a major regulator of the development and function of Tregs is also regulated by CREB (61). Thus, the inhibition induced by adenosine from Tregs could be mediated, in part, through these pathways. However, raising the level of cAMP in T cells with forskolin followed by analysis with oligonucleotide arrays showed a significant induction of the genes for IL-2, IL-2Rα, and IFN-γ (62). This finding conflicts with evidence that phosphodiesterase inhibitors shift the T cell phenotype toward Th2 cells (63). The best explanation may be that the earliest stages of T cell activation include a role for cAMP while this reliance diminishes over time subsequent to the induction of PDE. This timing would match the induction of the A2AAR on Th cells during activation and allow adenosine to induce cAMP as a negative feedback mechanism subsequent to the initiation of Th cell responses. However, the full complement of PKA substrates responsible for the effects of adenosine in immune/inflammatory cells are not known and need to be defined in detail.
Originally, cAMP was thought to activate PKA exclusively, but it is now apparent that it regulates other cAMP sensors including the exchange protein directly activated by cAMP (Epac) (58, 64). Epac initiates a large number of cAMP-mediated events, including cell proliferation, cell survival, secretion, and Ca2+ metabolism (58, 64). There are two iso-forms of Epac—Epac1 and Epac2, with Epac1 being more widely expressed and present in T cells (58, 64). Epac1 is a guanine nucleotide exchange factor for the small GTP binding protein Rap (60), with major roles in integrin-mediated cell adhesion and the formation of cell–cell junctions (58, 64). Epac1 can signal dependently or independently from PKA to inhibit proinflammatory responses, in part through its effects on suppressor of cytokine signaling proteins (SOCS) (65). SOCS1 and SOCS3 both inhibit responses in Th cells and APCs by multiple mechanisms (66). Moreover, adenosine stimulates the accumulation of IL-10 (32, 34, 37, 67), which can act in an autocrine manner to induce SOCS3 (66).
A significant finding is that Epac1 and Rap activate a novel member of the phospholipase family—phospholipase (PLC) C-ε (64). PLC-ε is widely expressed, contains the requisite domains responsible for the phospholipase activity that hydrolyzes phosphatidylinositol bisphosphate to inositol trisphosphate (IP3) and diacylglycerol (DAG), and is a CDC25 binding domain with guanine nucleotide exchange factor activity for small GTP-binding proteins in the Ras family (64). Thus, in addition to raising Ca2+ levels in cells, PLC-ε, Epac, and Rap initiate signaling cross-talk and potential links between cell membrane receptors and signaling pathways regulated by small GTP-binding proteins (64). This pathway of activation resembles that mediated through the Gq α subunit associated with the A2BAR expressed by APCs (see below and Fig. 3). The roles of Epac1, Rap, and PLC-ε in the regulation of immune function by all of the immune/inflammatory cells are important subjects for future work.
The A2BAR couples to two different G protein α subunits, the Gs α subunit, and the Gq α subunit (68). In cells where the A2BAR is coupled to the Gs α subunit, activation of Gs increases the activity of adenylate cyclase, raises the level of cAMP, and can potentially activate PKA and Epac1 as well as their downstream targets. Although cAMP is associated with the inhibition of inflammatory responses, it should be remembered that cholera toxin, which stimulates large amounts of cAMP, is also one of the most potent adjuvants ever described (69). Thus, cAMP induction does not always equate with inhibition. Moreover, some proinflammatory cytokines, such as IL-6, are induced through the activation of the A2BAR in dendritic cells (39), possibly by cAMP-sensitive promoters that can be activated by adenosine (70, 71). It will be important to identify the targets for cAMP that confer inhibition of host responses.
In settings in which the A2BAR is coupled to the Gq α subunit, it can increase the activity of PLC-β, causing the breakdown of phosphatidylinositol bisphosphate to IP3 and DAG. This activity briefly elevates the IP3/Ca2+ and DAG signals in the activated cells, thereby activating Ca2+-calmodulin–dependent enzymes, especially the calmodulin-sensitive protein kinases, such as Ca2+-calmodulin kinase II (72) and the serine-threonine kinase, protein kinase C. Because protein kinase C has been implicated in the activation of T cells and APCs (73), it is possible that adenosine signaling through the A2BAR induces potentially proinflammatory effects.
Can there be too much of a good thing?
In some circumstances, adenosine can exacerbate immunopathology as illustrated in the murine model of multiple sclerosis—experimental autoimmune encephalomyelitis, (EAE). Disease in this model depends on the migration into the CNS of pathogenic T cells sensitized by prior immunization with a peptide derived from myelin oligodendrocyte protein. Contrary to expectations, cd73–/– mice were found to be highly resistant to EAE (74); this was not caused by a lack of T cell responsiveness to immunization with myelin oligodendrocyte protein, because T cells of cd73–/– mice were activated, secreted increased quantities of proinflammatory cytokines, and caused severe disease when adoptively transferred into tcrα–/– mice, which are cd73+/+. These findings strongly suggested that CD73-derived adenosine and perhaps AR signaling were needed for T cell entry into the CNS. This notion was confirmed by the observation that an A2AAR antagonist protected wild-type mice from EAE (74). Thus, this model demonstrates that the anti-inflammatory effects of adenosine can be offset by the effect of adenosine on the access of pathogenic T cells to the CNS.
Adenosine, like other Treg mediators, can provoke disparate responses. Forexample, TGF-β1 acting alone is anti-inflammatory and favors the emergence of adaptive Tregs (75); however, in combination with IL-4 or IL-6, it stimulates Th9 (76) and Th17 (77) cells, respectively. Similarly, the inhibition of IL-12 coupled with the concomitant induction of IL-6 in dendritic cells after exposure to adenosine analogs is sufficient to convert the anti-inflammatory potential of TGF-β1 into a proinflammatory milieu that induces Th17 cells (J.M. Wilson and P.B. Ernst, submitted for publication). We propose that adenosine complements TGF-β1 as a mediator of suppression and an inducer of Th17 cells.
Additional evidence suggests that adenosine exacerbates intestinal inflammation in response to bacteria or chemicals via its effects on the A2BAR (78). However, other investigators have reported the opposite (79). In the lung, adenosine is believed to contribute to inflammation, largely through the A2BAR (80). Other data indicate that adenosine contributes to fibrosis in the liver or lung through the A2AAR (81) or A2BAR (80). The stimulation of fibrosis may be an attempt to “wall off” inflammation, but the widespread fibrotic atrophy of functional tissue is not usually desirable.
Anti-inflammatory mediators from Tregs can also contribute to immunosuppression that limits the clearance of infections (10, 82) or tumors (83). For example, the absence of the A2AAR enhances immune reactivity, allowing the regression of tumors in most A2AAR-deficient mice tested, whereas no tumors were resolved in wild-type mice (84). Similarly, knockdown of CD73 expression in ovarian or breast tumors or treatment of mice with a specific CD73 inhibitor or anti-CD73 Ab slowed tumor growth (85–87). Adenosine has also been suggested to exacerbate sepsis (88), although its anti-inflammatory effects complement antibiotic treatment to improve survival in other models (89). Undoubtedly, there are situations in which adenosine production provides a premature anti-inflammatory feedback that competes with the host's ability to mount a sufficient response.
Conclusions
Adenosine is one of several mediators that accumulate in the inflammatory milieu, conferring pleiotropic effects in a paracrine or autocrine manner that may be either beneficial or undesirable. These diverse outcomes reflect patterns of AR expression associated with distinct cellular lineages and differentiation states, as well as linkage to divergent G protein-coupled signaling pathways. Although the outcome of AR signaling is sometimes paradoxical, this inconsistency points to the complexity of biology rather than arguing against a role for adenosine in Treg function. Similar to other mediators (e.g., TGF-β1, IL-10) adenosine is not produced solely by Tregs, nor is it always beneficial. Clearly, a deeper understanding of the effects of adenosine and its interaction with other anti-inflammatory mediators will be required before we are able to translate current findings into novel anti-inflammatory therapies for the treatment of human disease.
Acknowledgments
We thank Dr. Ken Tung and Jeff Wilson (University of Virginia) and Paul Kincade (Oklahoma Medical Research Foundation) for sharing constructive suggestions for the content of this review.
This work was supported by National Institutes of Health Grants AI 070491 and AI 079145 (to P.B.E.) and AI 18220 (to L.F.T.). L.F.T. holds the Putnam City Schools Distinguished Chair in Cancer Research.
Abbreviations used in this paper
- A2AAR
adenosine 2A adenosine receptor
- A2BAR
adenosine 2B adenosine receptor
- ADA
adenosine deaminase
- AR
adenosine receptor
- DAG
diacylglycerol
- ecto-NTPDase-1
ecto-nucleoside triphosphate diphosphohyrdolase
- Epac
exchange protein directly activated by cAMP
- IP3
phosphatidylinositol bisphosphate
- PDE
phosphodiesterase
- PKA
cAMP-dependent protein kinase
- PLC
phospholipase C
- SOCS
suppressor of cytokine signaling
- Teff
effector Th cell
- Treg
regulatory T cell
Footnotes
Disclosures
P.B.E. is the principal investigator on a grant that has a subcontract with PGXhealth, which makes adenosine analogues.
References
- 1.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linden J. New insights into the regulation of inflammation by adenosine. J. Clin. Invest. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukashev DE, Smith PT, Caldwell CC, Ohta A, Apasov SG, Sitkovsky MV. Analysis of A2a receptor-deficient mice reveals no significant compensatory increases in the expression of A2b, A1, and A3 adenosine receptors in lymphoid organs. Biochem. Pharmacol. 2003;65:2081–2090. doi: 10.1016/s0006-2952(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 4.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 5.Wakamiya M, Blackburn MR, Jurecic R, McArthur MJ, Geske RS, Cartwright J, Jr., Mitani K, Vaishnav S, Belmont JW, Kellems RE, et al. Disruption of the adenosine deaminase gene causes hepatocellular impairment and perinatal lethality in mice. Proc. Natl. Acad. Sci. USA. 1995;92:3673–3677. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J. Clin. Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koszalka P, Ozüyaman B, Huo Y, Zernecke A, Flögel U, Braun N, Buchheiser A, Decking UK, Smith ML, Sévigny J, et al. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ. Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 10.Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, Linden J, Crowe SE, Ernst PB. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J. Infect. Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J. Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 12.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J. Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 13.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell'Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J. Exp. Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, Sobande TO, Kissane S, Salmon M, Rustin MH, Akbar AN. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J. Clin. Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ring S, Oliver SJ, Cronstein BN, Enk AH, Mahnke K. CD4+ CD25+ regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J. Allergy Clin. Immunol. 2009;123:1287–1296. e2. doi: 10.1016/j.jaci.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 18.Alyanakian MA, You S, Damotte D, Gouarin C, Esling A, Garcia C, Havouis S, Chatenoud L, Bach JF. Diversity of regulatory CD4+ T cells controlling distinct organ-specific autoimmune diseases. Proc. Natl. Acad. Sci. USA. 2003;100:15806–15811. doi: 10.1073/pnas.2636971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 20.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 21.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(-/-) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 2003;170:3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 22.Rad R, Brenner L, Bauer S, Schwendy S, Layland L, da Costa CP, Reindl W, Dossumbekova A, Friedrich M, Saur D, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Peña A, Rollán A, Viviani P, Guiraldes E, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, Rieger JM, Figler RA, Linden J, Crowe SE, Ernst PB. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2:232–242. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. quiz 212–213. [DOI] [PubMed] [Google Scholar]
- 26.Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 28.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 29.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol. Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- 30.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 31.Ben Addi A, Lefort A, Hua X, Libert F, Communi D, Ledent C, Macours P, Tilley SL, Boeynaems JM, Robaye B. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur. J. Immunol. 2008;38:1610–1620. doi: 10.1002/eji.200737781. [DOI] [PubMed] [Google Scholar]
- 32.Németh ZH, Lutz CS, Csóka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Haskó G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J. Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haskó G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabó C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 34.Haskó G, Szabó C, Németh ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 35.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 36.Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem. J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson JM, Ross WG, Agbai ON, Frazier R, Figler RA, Rieger J, Linden J, Ernst PB. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J. Immunol. 2009;182:4616–4623. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fotheringham JA, Mayne MB, Grant JA, Geiger JD. Activation of adenosine receptors inhibits tumor necrosis factor-alpha release by decreasing TNF-alpha mRNA stability and p38 activity. Eur. J. Pharmacol. 2004;497:87–95. doi: 10.1016/j.ejphar.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 39.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 42.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bopp T, Dehzad N, Reuter S, Klein M, Ullrich N, Stassen M, Schild H, Buhl R, Schmitt E, Taube C. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J. Immunol. 2009;182:4017–4024. doi: 10.4049/jimmunol.0803310. [DOI] [PubMed] [Google Scholar]
- 45.Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, Zhang GM, Feng ZH. miR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009;10:180–185. doi: 10.1038/embor.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 47.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 48.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J. Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 49.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Seybold J, Newton R, Wright L, Finney PA, Suttorp N, Barnes PJ, Adcock IM, Giembycz MA. Induction of phosphodiesterases 3B, 4A4, 4D1, 4D2, and 4D3 in Jurkat T-cells and in human peripheral blood T-lymphocytes by 8-bromo-cAMP and Gs-coupled receptor agonists. Potential role in beta2-adrenoreceptor desensitization. J. Biol. Chem. 1998;273:20575–20588. doi: 10.1074/jbc.273.32.20575. [DOI] [PubMed] [Google Scholar]
- 51.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 52.Zhou W, König R. T cell receptor-independent CD4 signalling: CD4-MHC class II interactions regulate intracellular calcium and cyclic AMP. Cell. Signal. 2003;15:751–762. doi: 10.1016/s0898-6568(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 53.Denning TL, Qi H, König R, Scott KG, Naganuma M, Ernst PB. CD4+ Th cells resembling regulatory T cells that inhibit chronic colitis differentiate in the absence of interactions between CD4 and class II MHC. J. Immunol. 2003;171:2279–2286. doi: 10.4049/jimmunol.171.5.2279. [DOI] [PubMed] [Google Scholar]
- 54.Bjørgo E, Taskén K. Role of cAMP phosphodiesterase 4 in regulation of T-cell function. Crit. Rev. Immunol. 2006;26:443–451. doi: 10.1615/critrevimmunol.v26.i5.40. [DOI] [PubMed] [Google Scholar]
- 55.Staples KJ, Bergmann M, Tomita K, Houslay MD, McPhee I, Barnes PJ, Giembycz MA, Newton R. Adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent inhibition of IL-5 from human T lymphocytes is not mediated by the cAMP-dependent protein kinase A. J. Immunol. 2001;167:2074–2080. doi: 10.4049/jimmunol.167.4.2074. [DOI] [PubMed] [Google Scholar]
- 56.Taylor SS, Kim C, Cheng CY, Brown SH, Wu J, Kannan N. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim. Biophys. Acta. 2008;1784:16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009;61:394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim. Biophys. Sin. (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br. J. Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell. Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J. Exp. Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes-Fulford M, Sugano E, Schopper T, Li CF, Boonyaratanakornkit JB, Cogoli A. Early immune response and regulation of IL-2 receptor subunits. Cell. Signal. 2005;17:1111–1124. doi: 10.1016/j.cellsig.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 63.Claveau D, Chen SL, O'Keefe S, Zaller DM, Styhler A, Liu S, Huang Z, Nicholson DW, Mancini JA. Preferential inhibition of T helper 1, but not T helper 2, cytokines in vitro by L-826,141 [4-[2-(3,4-Bisdifluromethoxyphenyl)-2-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-phenyl]-ethyl]3-methylpyridine-1-oxide], a potent and selective phosphodiesterase 4 inhibitor. J. Pharmacol. Exp. Ther. 2004;310:752–760. doi: 10.1124/jpet.103.064691. [DOI] [PubMed] [Google Scholar]
- 64.Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377:345–357. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 65.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol. Cell. Biol. 2006;26:6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dimitriou ID, Clemenza L, Scotter AJ, Chen G, Guerra FM, Rottapel R. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol. Rev. 2008;224:265–283. doi: 10.1111/j.1600-065X.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 67.Csóka B, Németh ZH, Virág L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Haskó G. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell. Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Lycke N. The mechanism of cholera toxin adjuvanticity. Res. Immunol. 1997;148:504–520. doi: 10.1016/s0923-2494(98)80144-2. [DOI] [PubMed] [Google Scholar]
- 70.Németh ZH, Leibovich SJ, Deitch EA, Sperlágh B, Virág L, Vizi ES, Szabó C, Haskó G. Adenosine stimulates CREB activation in macrophages via a p38 MAPK-mediated mechanism. Biochem. Biophys. Res. Commun. 2003;312:883–888. doi: 10.1016/j.bbrc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Sun Y, Wu F, Sun F, Huang P. Adenosine promotes IL-6 release in airway epithelia. J. Immunol. 2008;180:4173–4181. doi: 10.4049/jimmunol.180.6.4173. [DOI] [PubMed] [Google Scholar]
- 72.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem. J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mills JH, Thompson LF, Mueller C, Waickman AT, Jalkanen S, Niemela J, Airas L, Bynoe MS. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 78.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J. Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Che J, Chan ES, Cronstein BN. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol. Pharmacol. 2007;72:1626–1636. doi: 10.1124/mol.107.038760. [DOI] [PubMed] [Google Scholar]
- 82.Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 2003;5:515–526. doi: 10.1016/s1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 83.Lukashev D, Sitkovsky M, Ohta A. From “Hellstrom Paradox” to anti-adenosinergic cancer immunotherapy. Purinergic Signal. 2007;3:129–134. doi: 10.1007/s11302-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou X, Zhi X, Zhou P, Chen S, Zhao F, Shao Z, Ou Z, Yin L. Effects of ecto-5′-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol. Rep. 2007;17:1341–1346. [PubMed] [Google Scholar]
- 87.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. USA. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Németh ZH, Csóka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Haskó G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J. Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J. Infect. Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]