Abstract
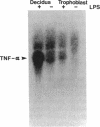
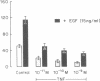
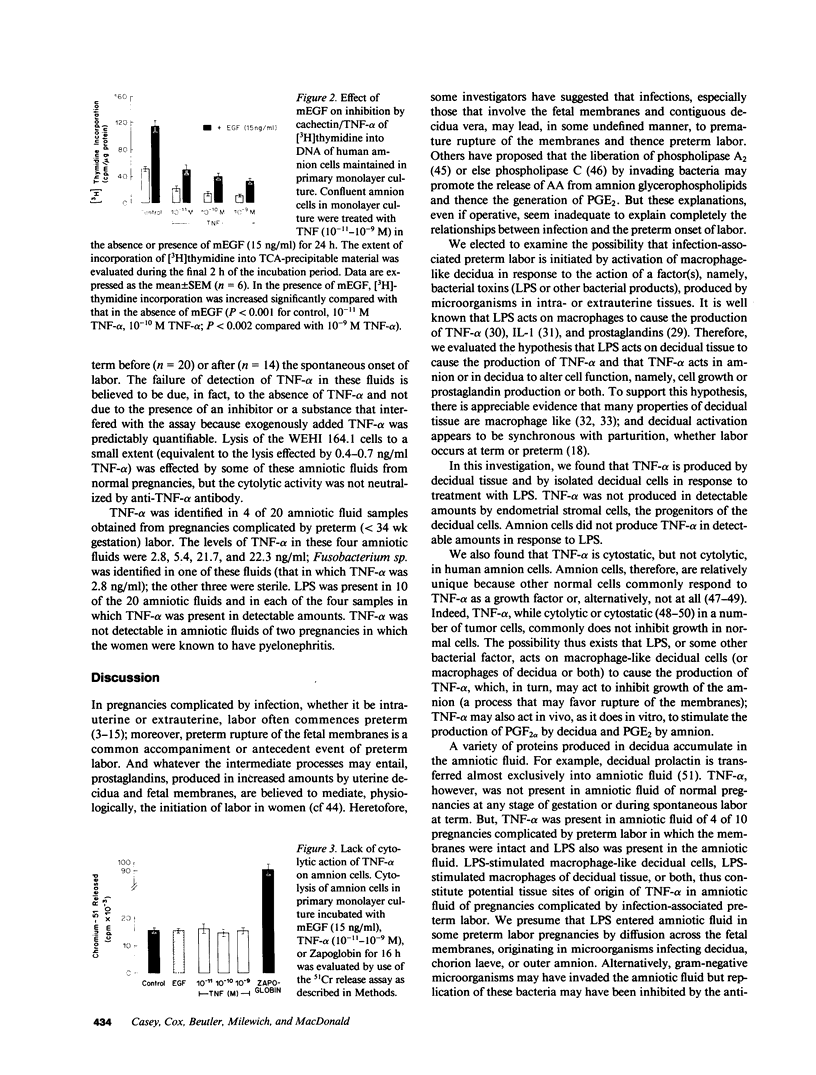
This study was conducted as part of an investigation to evaluate the hypothesis that bacterial toxins (LPS or lipoteichoic acid), acting on macrophage-like uterine decidua to cause increased formation of cytokines, may be involved in the pathogenesis of infection-associated preterm labor. We found that cachectin/tumor necrosis factor-alpha (TNF-alpha) was synthesized and secreted into the culture medium by human decidual cells and explants in response to treatment with LPS. LPS treatment also caused an increase in PGF2 alpha production by decidual cells and explants. In amnion cells in monolayer culture, TNF-alpha stimulated PGE2 formation, and TNF-alpha was cytostatic (inhibited [3H]thymidine incorporation into DNA) but not cytolytic in amnion cells. TNF-alpha was not detectable (less than 0.34 ng/ml) in the amniotic fluid of normal pregnancies at midtrimester or at term before or after the onset of labor (n = 44); but TNF-alpha was present at concentrations between 2.8 and 22.3 ng/ml in amniotic fluids of 4 of 20 pregnancies with intact membranes complicated by preterm labor (less than 34 wk gestational age). LPS was present in 10 of the 20 amniotic fluids of preterm labor pregnancies, including all four in which TNF-alpha was present. Bacteria were identified in only one of the four LPS-positive, TNF-alpha-positive fluids. Cytokine formation in macrophage-like decidua may serve a fundamental role in the pathogenesis of preterm labor, including increased prostaglandin formation and premature rupture of the membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bejar R., Curbelo V., Davis C., Gluck L. Premature labor. II. Bacterial sources of phospholipase. Obstet Gynecol. 1981 Apr;57(4):479–482. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bobitt J. R., Hayslip C. C., Damato J. D. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981 Aug 15;140(8):947–952. doi: 10.1016/0002-9378(81)90090-9. [DOI] [PubMed] [Google Scholar]
- Bobitt J. R., Ledger W. J. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977 Jul;19(1):8–12. [PubMed] [Google Scholar]
- Braverman M. B., Bagni A., de Ziegler D., Den T., Gurpide E. Isolation of prolactin-producing cells from first and second trimester decidua. J Clin Endocrinol Metab. 1984 Mar;58(3):521–525. doi: 10.1210/jcem-58-3-521. [DOI] [PubMed] [Google Scholar]
- Brennecke S. P., Castle B. M., Demers L. M., Turnbull A. C. Maternal plasma prostaglandin E2 metabolite levels during human pregnancy and parturition. Br J Obstet Gynaecol. 1985 Apr;92(4):345–349. doi: 10.1111/j.1471-0528.1985.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Sunderland C. A. Bone-marrow origin of endometrial granulocytes in the early human placental bed. J Reprod Immunol. 1983 Nov;5(6):383–387. doi: 10.1016/0165-0378(83)90247-4. [DOI] [PubMed] [Google Scholar]
- Casey M. L., MacDonald P. C. Biomolecular processes in the initiation of parturition: decidual activation. Clin Obstet Gynecol. 1988 Sep;31(3):533–552. doi: 10.1097/00003081-198809000-00005. [DOI] [PubMed] [Google Scholar]
- Casey M. L., MacDonald P. C. The initiation of labor in women: regulation of phospholipid and arachidonic acid metabolism and of prostaglandin production. Semin Perinatol. 1986 Oct;10(4):270–275. [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chamberlain G. Epidemiology and aetiology of the preterm baby. Clin Obstet Gynaecol. 1984 Aug;11(2):297–314. [PubMed] [Google Scholar]
- Cox S. M., MacDonald P. C., Casey M. L. Assay of bacterial endotoxin (lipopolysaccharide) in human amniotic fluid: potential usefulness in diagnosis and management of preterm labor. Am J Obstet Gynecol. 1988 Jul;159(1):99–106. doi: 10.1016/0002-9378(88)90501-7. [DOI] [PubMed] [Google Scholar]
- Dray F., Frydman R. Primary prostaglandins in amniotic fluid in pregnancy and spontaneous labor. Am J Obstet Gynecol. 1976 Sep 1;126(1):13–19. doi: 10.1016/0002-9378(76)90457-9. [DOI] [PubMed] [Google Scholar]
- Duff P. Pyelonephritis in pregnancy. Clin Obstet Gynecol. 1984 Mar;27(1):17–31. doi: 10.1097/00003081-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Fransen L., Van der Heyden J., Ruysschaert R., Fiers W. Recombinant tumor necrosis factor: its effect and its synergism with interferon-gamma on a variety of normal and transformed human cell lines. Eur J Cancer Clin Oncol. 1986 Apr;22(4):419–426. doi: 10.1016/0277-5379(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodgaonkar R. B., Dubin N. H., Blake D. A., King T. M. 13, 14-dihydro-15-keto-prostaglandin F2alpha concentrations in human plasma and amniotic fluid. Am J Obstet Gynecol. 1979 Jun 1;134(3):265–269. doi: 10.1016/s0002-9378(16)33031-9. [DOI] [PubMed] [Google Scholar]
- Gonik B., Creasy R. K. Preterm labor: its diagnosis and management. Am J Obstet Gynecol. 1986 Jan;154(1):3–8. doi: 10.1016/0002-9378(86)90383-2. [DOI] [PubMed] [Google Scholar]
- Gravett M. G. Causes of preterm delivery. Semin Perinatol. 1984 Oct;8(4):246–257. [PubMed] [Google Scholar]
- Hameed C., Tejani N., Verma U. L., Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol. 1984 Aug 1;149(7):726–730. doi: 10.1016/0002-9378(84)90111-x. [DOI] [PubMed] [Google Scholar]
- KASS E. H. Effect of corticosteroids and of hormones of pregnancy on the lethal action of bacterial endotoxin. Ann N Y Acad Sci. 1960 Jun 21;88:107–115. doi: 10.1111/j.1749-6632.1960.tb20012.x. [DOI] [PubMed] [Google Scholar]
- Kass E. H., McCormack W. M., Lin J. S., Rosner B., Munoz A. Genital mycoplasmas as a cause of excess premature delivery. Trans Assoc Am Physicians. 1981;94:261–266. [PubMed] [Google Scholar]
- Kass E. H. Pregnancy, pyelonephritis and prematurity. Clin Obstet Gynecol. 1970 Jun;13(2):239–254. doi: 10.1097/00003081-197006000-00003. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986 Apr;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Korte K., MacDonald P. C., Johnston J. M., Okita J. R., Casey M. L. Metabolism of arachidonic acid and prostanoids in human endometrial stromal cells in monolayer culture. Biochim Biophys Acta. 1983 Aug 1;752(3):423–433. doi: 10.1016/0005-2760(83)90272-2. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W. The role of arachidonic acid metabolites in mononuclear phagocytic cell interactions. Int J Dermatol. 1986 Mar;25(2):83–89. doi: 10.1111/j.1365-4362.1986.tb04543.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacDonald P., Alexander D., Catz C., Edelman R. Summary of a workshop on maternal genitourinary infections and the outcome of pregnancy. J Infect Dis. 1983 Mar;147(3):596–605. doi: 10.1093/infdis/147.3.596. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Ebenhack K., Kraemer D. L., Cox K., Cutrer S., Strickland D. M. A sensitive radioimmunoassay for 11-deoxy-13, 14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2: application as an index of prostaglandin E2 biosynthesis during human pregnancy and parturition. Prostaglandins Leukot Med. 1982 Nov;9(5):549–557. doi: 10.1016/0262-1746(82)90036-1. [DOI] [PubMed] [Google Scholar]
- Nakano K., Abe S., Sohmura Y. Recombinant human tumor necrosis factor--I. Cytotoxic activity in vitro. Int J Immunopharmacol. 1986;8(3):347–355. doi: 10.1016/0192-0561(86)90117-7. [DOI] [PubMed] [Google Scholar]
- Nehemiah J. L., Schnitzer J. A., Schulman H., Novikoff A. B. Human chorionic trophoblasts, decidual cells, and macrophages: a histochemical and electron microscopic study. Am J Obstet Gynecol. 1981 Jun 1;140(3):261–268. doi: 10.1016/0002-9378(81)90271-4. [DOI] [PubMed] [Google Scholar]
- Novy M. J., Liggins G. C. Role of prostaglandins, prostacyclin, and thromboxanes in the physiologic control of the uterus and in parturition. Semin Perinatol. 1980 Jan;4(1):45–66. [PubMed] [Google Scholar]
- Okazaki T., Casey M. L., Okita J. R., MacDonald P. C., Johnston J. M. Initiation of human parturition. XII. Biosynthesis and metabolism of prostaglandins in human fetal membranes and uterine decidua. Am J Obstet Gynecol. 1981 Feb 15;139(4):373–381. [PubMed] [Google Scholar]
- Okita J. R., Sagawa N., Casey M. L., Snyder J. M. A comparison of human amnion tissue and amnion cells in primary culture by morphological and biochemical criteria. In Vitro. 1983 Feb;19(2):117–126. doi: 10.1007/BF02621895. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason L. H., Mathieson B. J., Liang S. M., Flick D. A., Herberman R. B. Mediation of mouse natural cytotoxic activity by tumour necrosis factor. Nature. 1986 Jun 12;321(6071):700–702. doi: 10.1038/321700a0. [DOI] [PubMed] [Google Scholar]
- Ruggiero V., Latham K., Baglioni C. Cytostatic and cytotoxic activity of tumor necrosis factor on human cancer cells. J Immunol. 1987 Apr 15;138(8):2711–2717. [PubMed] [Google Scholar]
- Rush R. W., Keirse M. J., Howat P., Baum J. D., Anderson A. B., Turnbull A. C. Contribution of preterm delivery to perinatal mortality. Br Med J. 1976 Oct 23;2(6042):965–968. doi: 10.1136/bmj.2.6042.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Yasumizu T., Fukuoka H., Kinoshita K., Kaneko Y., Tsuchiya M., Sakamoto S. Prostaglandin F2 alpha metabolite levels in plasma, amniotic fluid, and urine during pregnancy and labor. Am J Obstet Gynecol. 1979 Apr 15;133(8):886–890. doi: 10.1016/0002-9378(79)90306-5. [DOI] [PubMed] [Google Scholar]
- Satyaswaroop P. G., Bressler R. S., de la Pena M. M., Gurpide E. Isolation and culture of human endometrial glands. J Clin Endocrinol Metab. 1979 Apr;48(4):639–641. doi: 10.1210/jcem-48-4-639. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh C. J., Hill G. B., Eden R. D., Gall S. A. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol. 1984 Mar 15;148(6):739–743. doi: 10.1016/0002-9378(84)90558-1. [DOI] [PubMed] [Google Scholar]
- Weingold A. B. Appendicitis in pregnancy. Clin Obstet Gynecol. 1983 Dec;26(4):801–809. doi: 10.1097/00003081-198312000-00005. [DOI] [PubMed] [Google Scholar]