Abstract
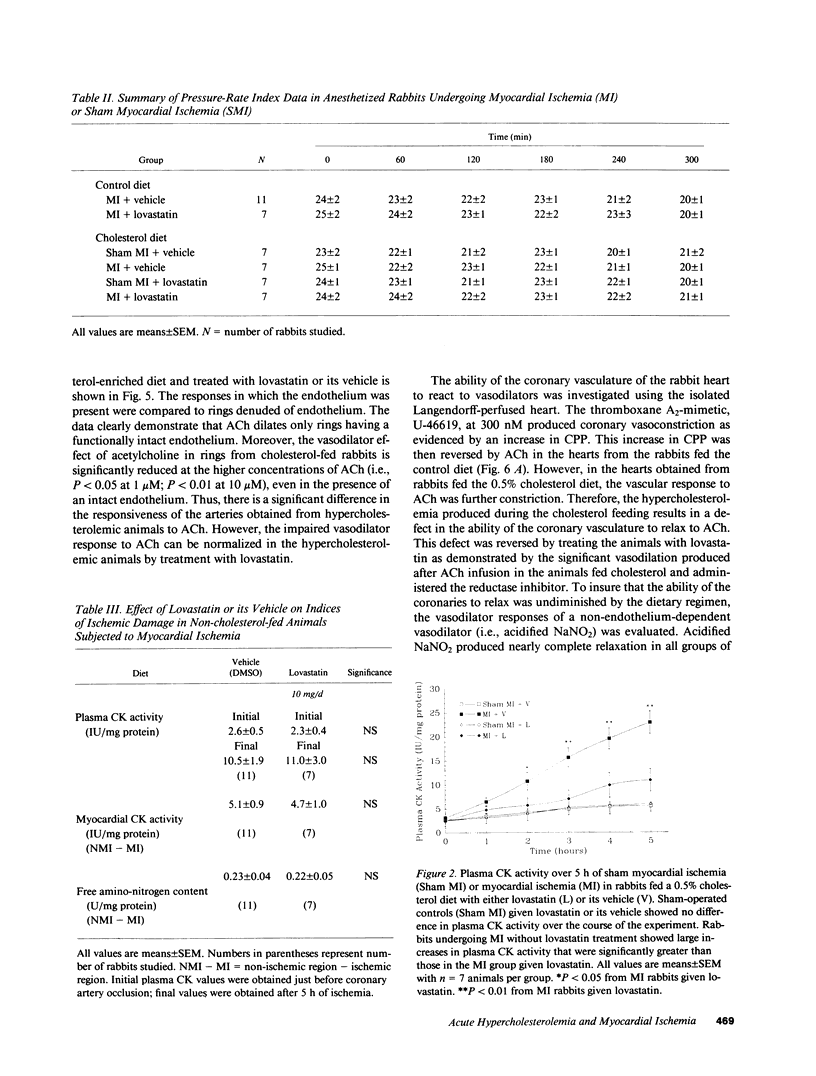
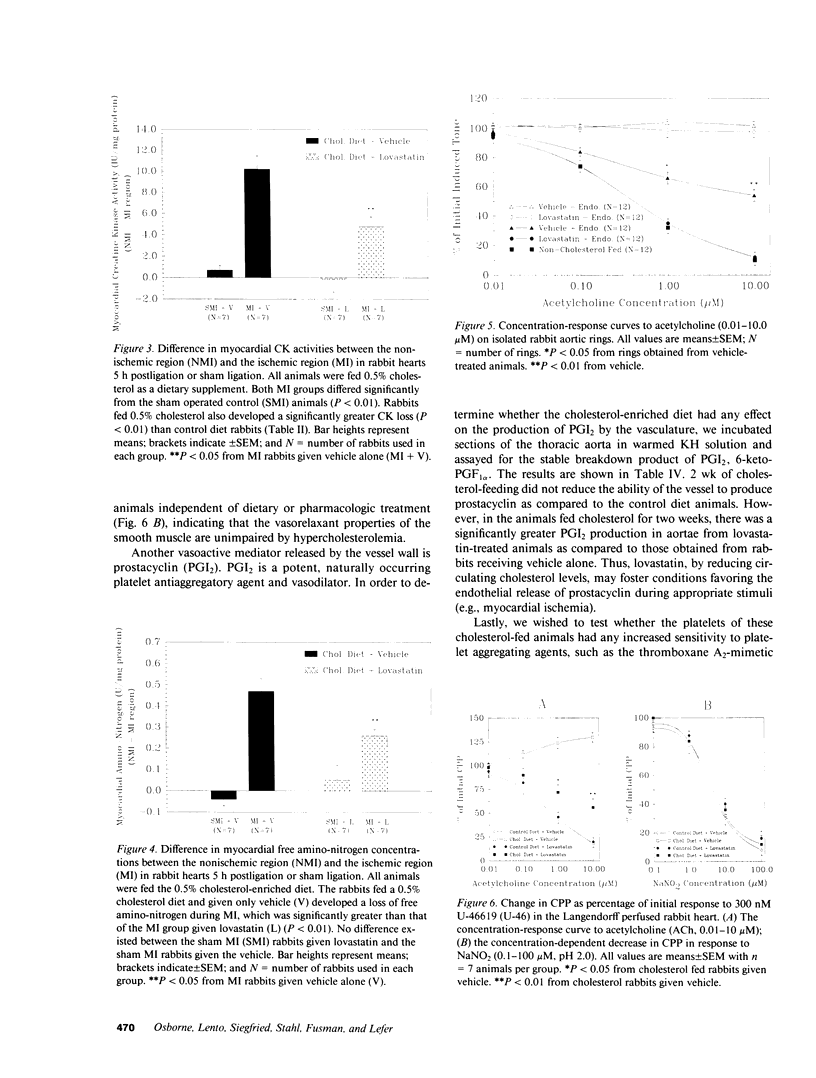
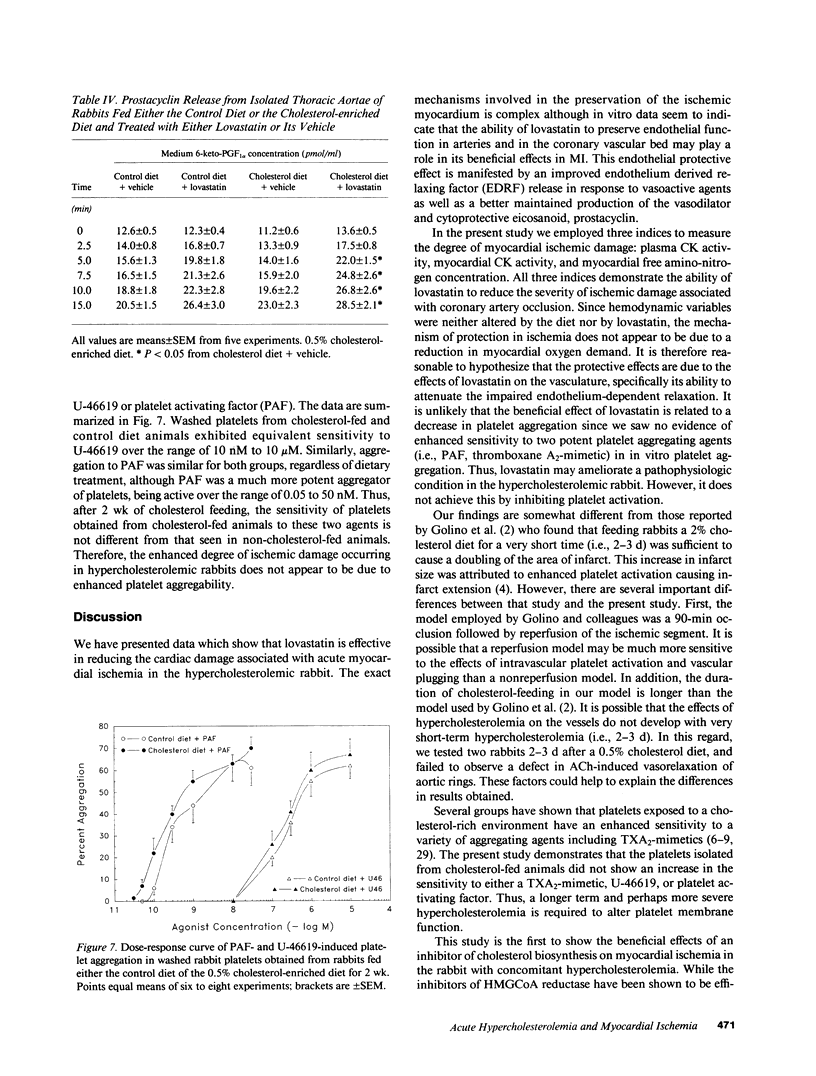
Hypercholesterolemia was induced in New Zealand white rabbits by feeding them a 0.5% cholesterol-enriched rabbit chow for 2 wk. Half of the cholesterol-fed rabbits were given lovastatin, a potent inhibitor of hydroxymethylglutaryl-coenzyme A reductase (HMG-CoA reductase), the rate limiting enzyme in cholesterol biosynthesis, and the other half were given its vehicle (i.e., DMSO). At the end of 2 wk, the rabbits underwent experimental myocardial ischemia or a sham ischemia procedure. Ischemic animals fed the cholesterol-enriched diet for 2 wk experienced much greater cardiac damage than ischemic rabbits fed the control diet, despite the absence of any atherosclerosis. Lovastatin was shown to protect the ischemic rabbit myocardium by three different indices of ischemic damage: (a) maintenance of creatine kinase (CK) activity in the ischemic myocardium; (b) reduced loss of free amino-nitrogen containing compounds from the ischemic myocardium; and (c) blunting the rise of plasma CK activity. These effects were not due to differences in myocardial oxygen demand between the groups. Arteries isolated from animals fed the cholesterol-enriched diet developed defects in endothelium-dependent relaxation in both large vessels as well as coronary resistance vessels. Acute hypercholesterolemia increases the severity of myocardial ischemia while at the same time impairing endothelium-dependent relaxation. These deleterious changes can be significantly attenuated by treatment with lovastatin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Andrews H. E., Bruckdorfer K. R., Dunn R. C., Jacobs M. Low-density lipoproteins inhibit endothelium-dependent relaxation in rabbit aorta. Nature. 1987 May 21;327(6119):237–239. doi: 10.1038/327237a0. [DOI] [PubMed] [Google Scholar]
- Araki H., Lefer A. M. Role of prostacyclin in the preservation of ischemic myocardial tissue in the perfused cat heart. Circ Res. 1980 Nov;47(5):757–763. doi: 10.1161/01.res.47.5.757. [DOI] [PubMed] [Google Scholar]
- Carvalho A. C., Colman R. W., Lees R. S. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974 Feb 21;290(8):434–438. doi: 10.1056/NEJM197402212900805. [DOI] [PubMed] [Google Scholar]
- Freiman P. C., Mitchell G. G., Heistad D. D., Armstrong M. L., Harrison D. G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res. 1986 Jun;58(6):783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Golino P., Maroko P. R., Carew T. E. Efficacy of platelet depletion in counteracting the detrimental effect of acute hypercholesterolemia on infarct size and the no-reflow phenomenon in rabbits undergoing coronary artery occlusion-reperfusion. Circulation. 1987 Jul;76(1):173–180. doi: 10.1161/01.cir.76.1.173. [DOI] [PubMed] [Google Scholar]
- Golino P., Maroko P. R., Carew T. E. The effect of acute hypercholesterolemia on myocardial infarct size and the no-reflow phenomenon during coronary occlusion-reperfusion. Circulation. 1987 Jan;75(1):292–298. doi: 10.1161/01.cir.75.1.292. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Armstrong M. L., Marcus M. L., Piegors D. J., Mark A. L. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984 Jun;54(6):711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- Ingerman-Wojenski C., Silver M. J., Smith J. B., Macarak E. Bovine endothelial cells in culture produce thromboxane as well as prostacyclin. J Clin Invest. 1981 May;67(5):1292–1296. doi: 10.1172/JCI110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjekshus J. K., Sobel B. E. Depressed myocardial creatine phosphokinase activity following experimental myocardial infarction in rabbit. Circ Res. 1970 Sep;27(3):403–414. doi: 10.1161/01.res.27.3.403. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S. A., Klurfeld D. M. Influence of mevinolin on experimental atherosclerosis in rabbits. Pharmacol Res Commun. 1981 Nov;13(10):921–926. doi: 10.1016/s0031-6989(81)80063-x. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Osborne J. A., Yanagisawa A., Sun J. Z. Influence of atherosclerosis on vascular responsiveness in isolated rabbit vascular smooth muscle. Cardiovasc Drugs Ther. 1987 Dec;1(4):385–391. doi: 10.1007/BF02209080. [DOI] [PubMed] [Google Scholar]
- Mickelson J. K., Carlson C. J., Margaretten W., Rapaport E. Streptokinase alters myocardial creatine kinase depletion after ischaemia and reperfusion in rabbits. Clin Exp Pharmacol Physiol. 1986 Sep;13(9):637–646. doi: 10.1111/j.1440-1681.1986.tb02392.x. [DOI] [PubMed] [Google Scholar]
- Moscat J., Perez P., Gavilanes F. G., Acin F., Schuller A., Municio A. M. Membrane fluidity and thromboxane synthesis in platelets from patients with severe atherosclerosis. Thromb Res. 1986 Oct 15;44(2):197–205. doi: 10.1016/0049-3848(86)90135-0. [DOI] [PubMed] [Google Scholar]
- Ogletree M. L., Lefer A. M. Prostaglandin-induced preservation of the ischemic myocardium. Circ Res. 1978 Feb;42(2):218–224. doi: 10.1161/01.res.42.2.218. [DOI] [PubMed] [Google Scholar]
- Ogletree M. L., Lefer A. M., Smith J. B., Nicolaou K. C. Studies on the protective effect of prostacyclin in acute myocardial ischemia. Eur J Pharmacol. 1979 Jun;56(1-2):95–103. doi: 10.1016/0014-2999(79)90438-2. [DOI] [PubMed] [Google Scholar]
- Osborne J. A., Mentley R. K., Lefer A. M. Increased severity of acute myocardial ischemia in experimental atherosclerosis. Heart Vessels. 1987;3(2):73–79. doi: 10.1007/BF02058522. [DOI] [PubMed] [Google Scholar]
- Renaud J. F., Schmid A., Romey G., Nano J. L., Lazdunski M. Mevinolin, an inhibitor of cholesterol biosynthesis, drastically depresses Ca2+ channel activity and uncouples excitation from contraction in cardiac cells in culture. Proc Natl Acad Sci U S A. 1986 Oct;83(20):8007–8011. doi: 10.1073/pnas.83.20.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Shattil S. J., Anaya-Galindo R., Bennett J., Colman R. W., Cooper R. A. Platelet hypersensitivity induced by cholesterol incorporation. J Clin Invest. 1975 Mar;55(3):636–643. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H., Tomoike H., Nabeyama S., Yamamoto H., Araki H., Nakamura M., Ishii Y., Tanaka K. Coronary artery spasm induced in atherosclerotic miniature swine. Science. 1983 Aug 5;221(4610):560–562. doi: 10.1126/science.6408736. [DOI] [PubMed] [Google Scholar]
- Tobert J. A., Bell G. D., Birtwell J., James I., Kukovetz W. R., Pryor J. S., Buntinx A., Holmes I. B., Chao Y. S., Bolognese J. A. Cholesterol-lowering effect of mevinolin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a reductase, in healthy volunteers. J Clin Invest. 1982 Apr;69(4):913–919. doi: 10.1172/JCI110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka T., Kamishiro T., Fumino H., Masaki T., Hosoda S. Rabbit hearts for the critical evaluation of drugs to reduce the size of experimentally produced acute myocardial infarction. Jpn Heart J. 1984 Jul;25(4):623–632. doi: 10.1536/ihj.25.623. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Akita H., Mizutani T., Fukuzaki H., Watanabe Y. Hyperreactivity of coronary arterial smooth muscles in response to ergonovine from rabbits with hereditary hyperlipidemia. Circ Res. 1983 Jul;53(1):63–71. doi: 10.1161/01.res.53.1.63. [DOI] [PubMed] [Google Scholar]
- Zmuda A., Dembinska-Kiec A., Chytkowski A., Gryglewski R. J. Experimental atherosclerosis in rabbits: platelet aggregation, thromboxane A2 generation and anti-aggregatory potency of prostacyclin. Prostaglandins. 1977;14(6):1035–1042. doi: 10.1016/0090-6980(77)90283-0. [DOI] [PubMed] [Google Scholar]




