Abstract
Previous studies from this laboratory have shown that maternal-derived cholesterol can be effluxed from trophoblasts to fetal HDL and plasma. We had the opportunity to study for the first time the ability of HDL and plasma from a fetus with the Smith–Lemli–Opitz syndrome (SLOS) to efflux cholesterol from trophoblasts. It was unclear whether cholesterol could be effluxed to fetuses with SLOS since lipoprotein levels are often very low. To answer this question, cord blood was collected from the placentas of an SLOS fetus and unaffected fetuses just after delivery. Plasma cholesterol concentrations were very low in the affected fetus; cholesterol, 7-dehydrocholesterol, and 8-dehydocholesterol concentrations were 14.1, 4.5, and 5.2 mg/dl, respectively. The HDL from the fetal SLOS effluxed ≈50% more cholesterol from a trophoblast cell line, were smaller in size, and had a lower cholesterol to phospholipid ratio as compared to HDL from unaffected fetuses or adults. Plasma from the SLOS fetus effluxed cholesterol to a similar percentage as unaffected fetal plasma or adult plasma, possibly due to fewer HDL particles as demonstrated in previous SLOS patients. These novel data demonstrate that the cholesterol-deficient SLOS fetus is able to obtain cholesterol from trophoblasts at a time when cholesterol is playing a critical role in development, and has implications for design of treatments for cholesterol deficiency syndromes as well as understanding of prenatal cholesterol transport in humans.
Keywords: Fetus, Trophoblast, BeWo cells, Pregnancy, Cholesterol transport
Smith–Lemli–Opitz syndrome (SLOS) is a metabolic disorder that presents with a spectrum of congenital abnormalities. Individuals with a severe phenotype have many congenital abnormalities and mental retardation whereas those with a mild phenotype may have only subtle learning disorders and minor dysmorphic features [1–3]. The biochemical cause of this syndrome is a reduction in the activity of the enzyme 7-dehydrocholesterol-Δ7-reductase (DHCR7) [4,5], which converts 7-dehydrocholesterol (7-DHC) to cholesterol, due to mutations in DHCR7 [6–8]. As might be expected, SLOS is characterized by a reduction in cholesterol and an increase in cholesterol precursors, including 7- and 8-DHC. Recent studies demonstrate that the incidence of this syndrome may be much greater than previously believed at a predicted incidence of 1:1590–1:13,500 [9,10], though observed incidence is lower (perhaps 1 in 20,000 births). The discrepancy in observed versus predicted incidence is likely due in part to pregnancy loss as well as undiagnosed mild cases. Nevertheless, SLOS is probably the second most prevalent, potentially lethal, recessive genetic condition behind cystic fibrosis with an incidence of 1:2500 [11]. The congenital malformations associated with this disease can begin to appear as early as 3 weeks post-conception when Sonic Hedgehog (SHH) is needed for patterning of the forebrain [12,13], since sterols activate SHH [14–16] and sterol deficiency is thought to play a role in abnormal SHH signaling in SLOS subjects [16,17]. Abnormal signaling may also occur downstream of SHH, perhaps at the level of smoothened [18]. Congenital malformations continue to develop and progress throughout gestation since cholesterol is a major component of all cell membranes and is critical to membrane function. It would seem the ability to increase cholesterol delivery to the SLOS fetus could ameliorate the phenotype.
Though the dogma has been that the fetus lacked a source of cholesterol besides that synthesized de novo, recent studies have suggested that the fetus does indeed have an exogenous source of cholesterol. In humans, SLOS fetuses and newborns with no DHCR7 activity due to null mutations in DHCR7 and who therefore must synthesize little or no cholesterol, often survive to birth or later with measurable cholesterol in tissues and plasma in the newborn [19,20]. Additionally, the severity of the SLOS phenotype is affected by the apoE isoform expressed in the mothers, but not the fathers [21], suggesting that maternal lipoprotein uptake or levels play a role in fetal development [22–24]. Though the reported rates of transport varied, some of the early human studies demonstrated significant transfer of maternal cholesterol to the fetal circulation [25–27]. Finally, a change in maternal cholesterol can actually affect the formation of fatty streaks in fetuses [28,29].
Since the fetus does not come in direct contact with the maternal circulation, cholesterol would need to be taken up by tissues that surround the fetus or lend nutritional support, such as the yolk sac and trophoblasts early in the first trimester and the placenta in the second and third trimesters [30]. Sources of early histotropic nutrition could be the uterine glands since these tissues secrete nutrients early in gestation, including lipids, into intervillous space and could be blood which has leaked from plugged spiral arteries [31]. The trophoblasts can take up the nutrients and secrete them into the coelomic cavity of the fetal unit where they are absorbed by the yolk sac and passed to the developing embryo or fetus [32]. Since the embryo/fetus begins to accrue significant mass by the middle to end of the first trimester, a more directed supply of nutrients forms, that being the placenta. The placenta, which has the capability to transport a wide variety of maternal nutrients to the fetus, becomes efficient at transport late in the first trimester [32–34]. A single layer of multinucleated syncytiotrophoblasts and the fetal capillary endothelium separate the maternal and fetal circulations. Thus, maternal cholesterol, likely in the form of lipoproteins, would need to be taken up on the apical side of trophoblasts, be transported across the cells to the basolateral side, and exit the cells towards the fetal circulation. One mechanism by which cholesterol can exit cells is through efflux of cholesterol to various acceptors in the circulation or interstitial fluid. A common acceptor for effluxed cellular cholesterol is HDL [35]. The composition of the acceptor is important in that efflux can be enhanced or impaired merely by changing HDL composition or size [36]. Other acceptors include lipid-poor apoA-I, apoA-IV, or apoE, or sterol-poor phospholipid vesicles [36–39]. As in other cell types, cholesterol originating from the apical (maternal) side can be effluxed from the basolateral (fetal) side of trophoblasts to HDL as well as to phospholipid vesicles [40].
Since the SLOS fetus is cholesterol deficient as compared to unaffected fetuses [19,20,41–43], we questioned whether there would there be sufficient fetal HDL to efflux cholesterol from trophoblasts. We had the opportunity to study for the first time the ability of HDL and plasma from an SLOS fetus to efflux cholesterol from trophoblasts. The SLOS HDL in fact showed enhanced efflux of cholesterol from a trophoblastic cell line as compared to HDL from unaffected fetuses or adults. Interestingly, HDL from the SLOS fetus was smaller as compared to HDL from control Fetuses and adults and sterol poor. Efflux to plasma from all sources was similar, however. Thus, the cholesterol-deficient SLOS fetus is able to obtain exogenous cholesterol from trophoblasts at a time when cholesterol is needed to ensure normal development.
Materials and methods
Collection of human samples
The mother of a previous child with SLOS became pregnant and chose to chose to carry the fetus to term even if the child was affected. The fetus was suspected of being affected with SLOS prenatally based on low maternal serum oestriol concentrations and abnormal ultrasound findings [44,45]. A diagnosis of SLOS in the male infant was subsequently confirmed by plasma sterol analysis and DNA mutation analysis. At the time of the vaginal delivery, umbilical cord blood was collected. Plasma derived from cord blood was shipped overnight on wet ice to the University of Cincinnati. Protocols for collection of samples were approved by the Oregon Health & Science University Institutional Review Board, and consent was obtained from the mother. Additional samples of cord blood from unaffected fetuses were obtained from the University Hospital at the University of Cincinnati (n = 5) and samples of blood were collected from unaffected adults (n = 3). Protocols for collection of samples were approved by the University of Cincinnati Institutional Review Board, and consent was obtained from the mothers and the adults.
Plasma and HDL compositions
HDL (1.063 g/ml < d < 1.21 g/ml) was isolated by sequential ultracentrifugation from samples that had been collected at least 24 h previously and stored at 4 °C to match the fetal SLOS sample handling. Cholesterol and 7- and 8-DHC concentrations in plasma were determined by gas liquid chromatography [46]. In HDL, protein concentrations were determined by the Lowry assay [47], cholesterol concentrations were determined by gas liquid chromatography [46], and phospholipid concentrations were determined chemically [48].
HDL sizing
HDL particles isolated from one randomly chosen control fetus, one randomly chosen adult, and the SLOS fetus were separated under native conditions using a PhastSystem (Pharmacia Biotech Inc.) on a precast 8–25% polyacrylamide gradient gel using native buffer strips (GE Healthcare) [36]. Standards of known sizes (Amersham) were run simultaneously. Protein was visualized with Coomassie Blue stain, and densities of bands determined by Image J software.
HDL apolipoprotein composition
HDL apolipoproteins were separated on 18% polyacrylamide gels (Biorad) under denaturing conditions. Protein was visualized with Coomassie Blue stain.
Cholesterol efflux studies
Studies were performed on confluent, polarized BeWo cells (subclone b30; a gift from Dr. Ken Audus); BeWo cells are a human-derived choriocarcinoma cell line that has sterol metabolism similar to the placenta in vivo and displays directional transport of lipids [40,49,50]. Cells were plated on transwells at 75,000 cells/ml as described [40]. Two days after plating, cells were treated with [3H]cholesterol. After 24 h, the media was removed from the upper chamber (apical side of cells) as well as the lower chamber (basolateral side) and cells were washed extensively. Serum-free media (SFM) was added to the apical side. SFM containing no acceptors or a variety of acceptors was added to the basolateral side. As a positive control, phospholipid vesicles were prepared by sonication [51] and used as acceptors (50 µg/ml). To study the ability of HDL to take up trophoblast-derived cholesterol, equal amounts of HDL-phospholipid were added to each well (11.5 µg phospholipid/well). To study the ability of plasma to take up trophoblast-derived cholesterol, 10% plasma was added to the media in the lower chamber. Twenty-four hours after the addition of the acceptors, media was collected, passed through a 0.45 µm filter, and [3H] measured by liquid scintillation counting. Cells were lysed with 1 N NaOH, and [3H] present in lysates were measured. Data are presented as the amount of [3H]cholesterol in the basolateral media as a percentage of total cellular [3H]cholesterol taken up.
Statistics
All data are presented as means ± 1 SD. For HDL composition (cholesterol to phospholipid ratio) and efflux to HDL, the SD is determined for the means of the different samples (fetal control—5; adult—3). Comparisons between groups were deemed significant by Student’s t-test (P < 0.05). For efflux to plasma, the SD is determined for one sample of the fetal control, one fetal SLOS, and one adult that was assayed in triplicate.
Results
Recent studies suggest that the fetus can obtain exogenous cholesterol by effluxing cholesterol from the basolateral side of trophoblasts to HDL or phospholipid vesicles [40]. In the present studies, we had the unique opportunity to test the ability of HDL from a fetus known to have SLOS to efflux cholesterol from trophoblasts.
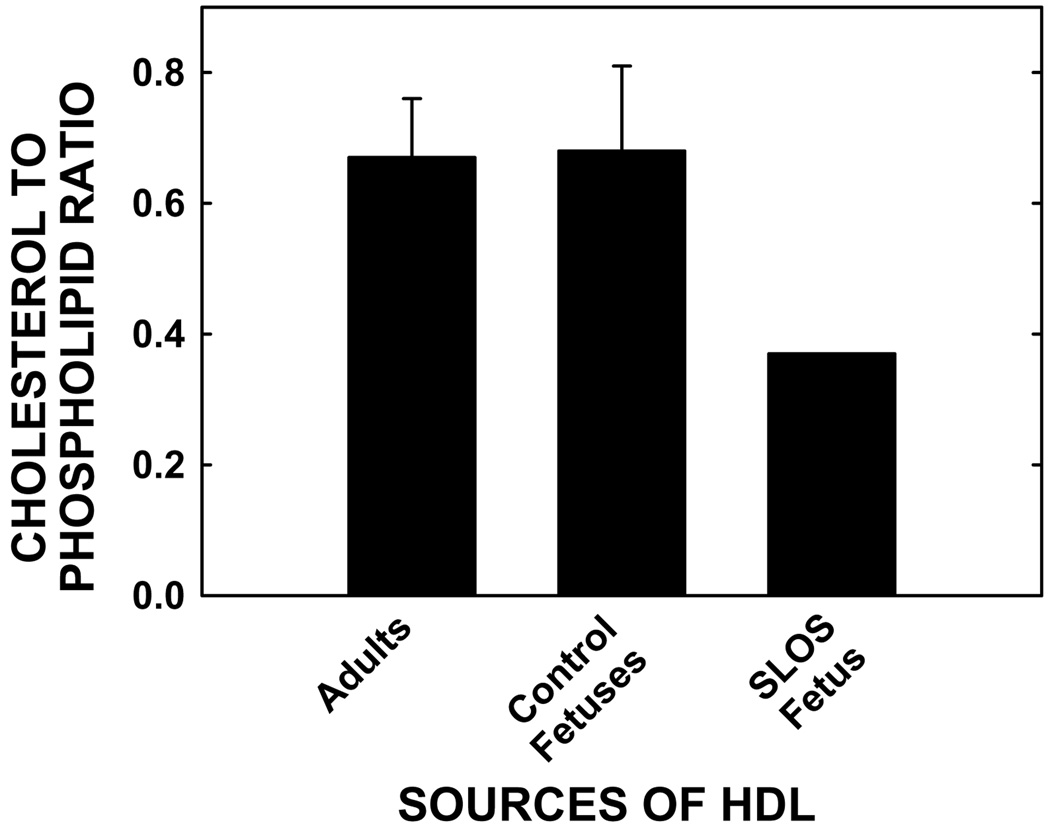
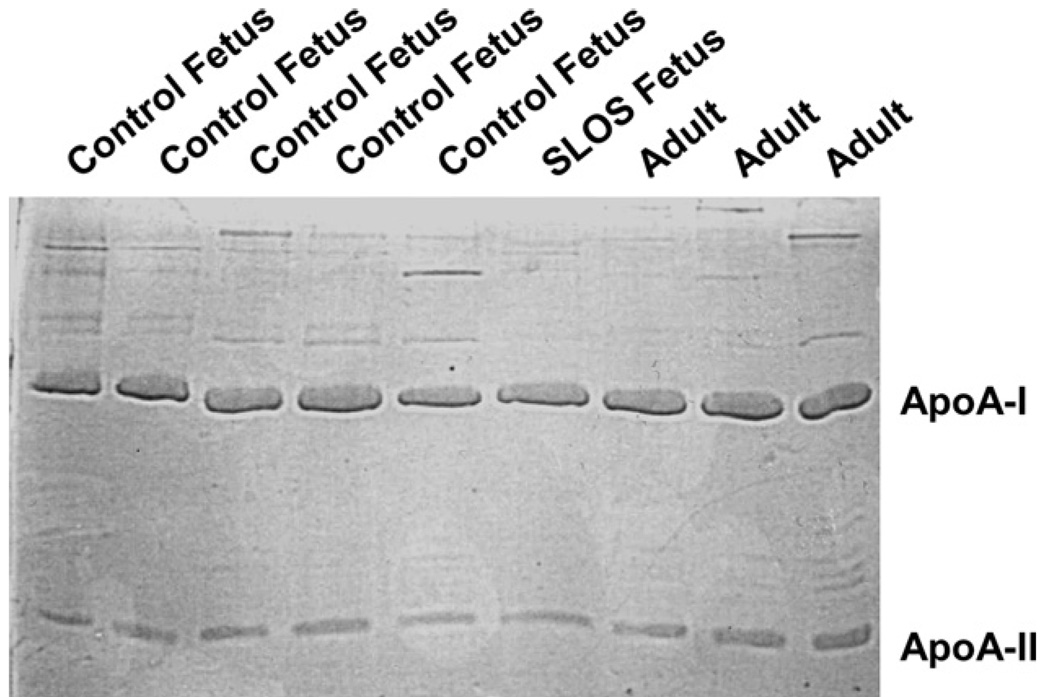
Plasma cholesterol concentrations of the afflicted fetus were low (14.1 mg/dl) as compared to previously reported fetal plasma cholesterol concentrations obtained similarly from cord blood (56–69 mg/dl) [41–43]. Substantial plasma levels of 7-DHC (4.5 mg/dl) and 8-DHC (5.2 mg/dl) were measured in the SLOS fetus; sterol precursors are essentially undetectable in cord blood from unaffected fetuses (data not shown). Since HDL is an excellent acceptor of cellular cholesterol [35], HDL was isolated and characterized and used as a acceptor for cholesterol efflux. HDL particles were sized by gel chromatography and band densities converted to a figure (Fig. 1). Normal adult HDL exhibited two bands. The majority of the protein stained had an apparent hydrodynamic diameter of about 11.5 nm (band 1) with a minor shoulder at 10.8 nm. There was also a band at the bottom of the gel (band 5) which likely represents apoproteins that were stripped off the particles during the electrophoresis run. Normal fetal HDL also exhibited bands 1 and 5, but in addition, there was a peak with a diameter of about 10.4 nm (band 2) that may or may not correspond to the shoulder seen in the adult HDL sample. Additionally, fetal HDL exhibited a strong band at about 7.8 nm that was not observed in the adult preparation (band 4). Interestingly, SLOS fetal HDL particles ran predominantly as the particles corresponding to band 4. There were no detectable particles in the large band (band 1) in the SLOS HDL as noted for the normal adult and fetal HDL. In addition to some band 5 staining, the SLOS sample also exhibited particles that were about 9.8 nm in diameter (band 3). As might be expected in smaller particles, the cholesterol to phospholipid ratio was significantly less in the fetal SLOS HDL as compared to the ratio in adult and control fetal HDL (Fig. 2). It should be noted that 7- and 8-DHC were not measurable in HDL from the SLOS fetus due to the small amount of sample available for this assay. Thus, the ratio of sterol to phospholipid could change if 7- and 8-DHC were present but undetectable. There were no obvious differences in the levels of apoA-I and apoA-II, the major apolipoproteins in HDL, in all samples, regardless of genotype or age (Fig. 3).
Fig 1.
Sizing of HDL particles. HDL particles were isolated from one random unaffected (control) fetus, from an SLOS fetus, and from one random adult. HDL was run on a native polyacrylamide gel, and the gel was stained with Coomassie Blue and densities determined. The densities were plotted, and sizes of HDL as compared to standards of known sizes are presented in graph form. The five major HDL peaks are labeled as well.
Fig 2.
Cholesterol to phospholipid masses in HDL from different sources. HDL particles were isolated from control fetuses, an SLOS fetus, and adults, and ratios of cholesterol to phospholipid masses were calculated. Data are presented as means ± 1 SD (n = 3–5).
Fig 3.
Apolipoprotein composition of HDL from different sources. HDL was isolated from control fetuses, an SLOS fetus, and adults. Equal amounts of protein were run on a polyacrylamide gel, and proteins stained with Coomassie Blue.
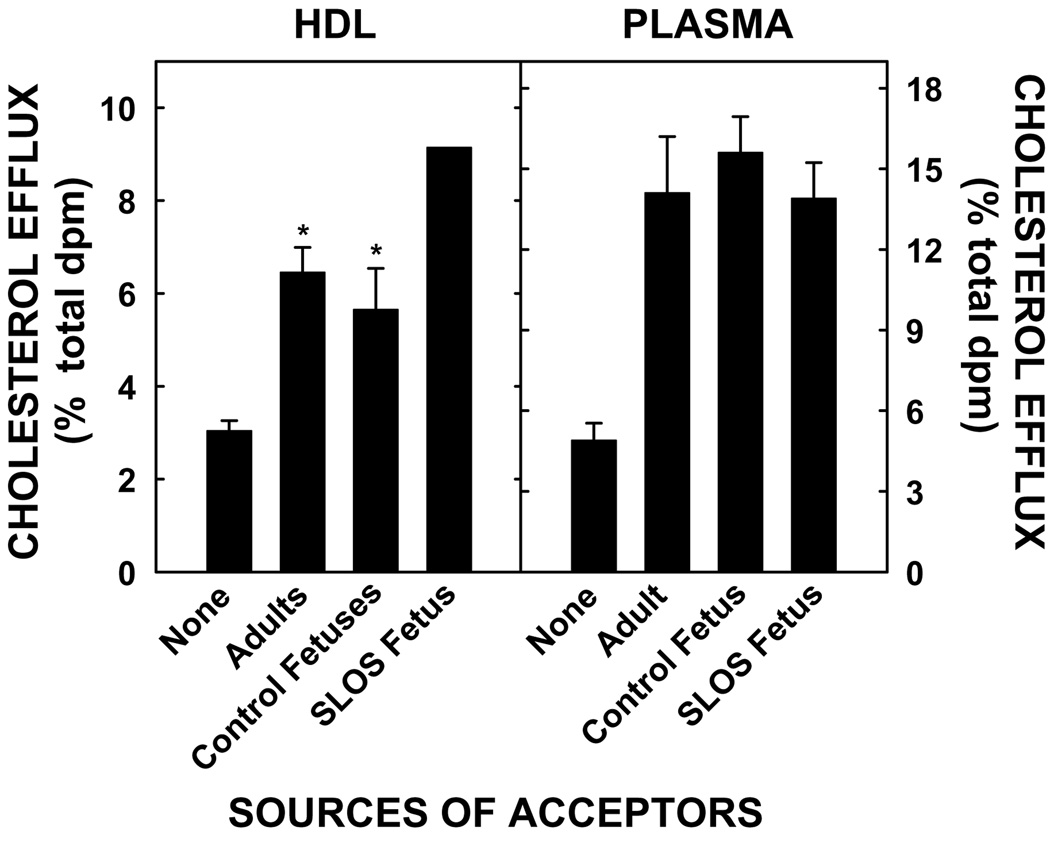
In addition to analyzing HDL size and composition, the ability of HDL from SLOS fetuses to efflux cholesterol from BeWo cells was compared to the ability of HDL from control fetuses and adults to efflux cholesterol. As demonstrated previously, there was ≈3% maternal-derived cholesterol in the basolateral media even in the absence of acceptors, suggesting some secretion of cholesterol (Fig. 4, left panel). The addition of phospholipid vesicles to the basolateral side (our positive control) caused efflux to increase ≈2-fold (5.6 ± 0.9%) (data not shown). The HDL from adults and unaffected fetuses effluxed very similar amounts of cholesterol (≈6%). Strikingly, HDL from the SLOS fetus effluxed ≈50% more cholesterol as compared to the HDL from adults as well as unaffected fetuses. When plasma was used as an acceptor instead of HDL, the amount of cholesterol effluxed was similar between all groups (Fig. 4, right panel). Though HDL from 5 unaffected fetuses, 1 affected fetus, and 3 adults were used for efflux, plasma from 1 unaffected fetus, one SLOS fetus, and one adult were used for efflux. Only one random sample of the unaffected fetal controls was used due to the small volume of cord blood obtained from the other four subjects and the need to use the whole sample for HDL isolation. The plasmas used for the unaffected fetus and the adult were chosen because their HDL effluxed cholesterol very close to the mean for efflux for that particular group. We have shown previously that efflux to different fetal plasma samples does not vary much [40].
Fig 4.
Efflux of cholesterol from the basolateral surface of BeWo cells to different acceptors. The apical side of cells were labeled with [3H]cholesterol for 24 h. After labeling, cells were washed and incubated with SFM (none) or HDL (11.5 µg phospholipid/ml) from 3 adults, one SLOS fetus, and 5 control fetuses or plasma (10%) from one of each type of subject. An aliquot of the basolateral media was counted, and the data are presented as the amount of [3H]cholesterol effluxed as a percentage of cellular [3H]cholesterol at time 0. Using HDL as an acceptor, data are presented as means of different samples ± 1 SD for the different subjects. *Significant differences (P < 0.05) between acceptors and SFM (none) are shown. Using plasma as an acceptor, data are presented as means ± 1 SD for triplicate assays for one control fetus, one SLOS fetus, and one adult.
Discussion
Cholesterol is essential for in utero development. There are two sources of fetal cholesterol, that synthesized de novo and that obtained from exogenous sources [30,52]. We have shown previously that fetal HDL is able to efflux cholesterol from trophoblasts [40] and as such could be a significant source of exogenous sterol during development [30]. The ability to obtain exogenous cholesterol could have a great impact upon fetuses that are cholesterol deficient, such as those with inborn errors in sterol synthesis, or those with reduced uterine blood flow and nutrient delivery, as occurs during intrauterine growth restriction (IUGR). Since HDL from sterol-deficient fetuses may be of abnormal composition and possibly low concentration [53,54], the question arises: Is plasma, and specifically HDL, from sterol-deficient fetuses able to efflux cholesterol? We had a unique opportunity to determine if HDL from a cholesterol-deficient SLOS fetus was able to act as a cholesterol acceptor from trophoblasts. It was not only capable, but surprisingly, it was actually more effective compared on an equal phospholipid basis with the normal fetal or adult HDL. These are significant findings for fetuses diagnosed in utero with SLOS or other inborn errors of sterol metabolism or with IUGR, and possibly for fetuses with marginal growth rates because they suggest that if ways can be devised to increase placental cholesterol, then more of that will eventually make it to the fetal circulation.
What makes the SLOS fetal HDL a better acceptor of cellular cholesterol than fetal control or adult HDL? Several properties of HDL impact upon how well these particles can efflux cellular cholesterol. One of the key predictors for the ability of HDL to efflux cholesterol is the ratio of cholesterol to phospholipid [36]. Since cholesterol efflux by diffusional pathways, such as those mediated by SR-BI, ABCG1, or simple diffusion, are subject to mass action, the equilibrium is shifted in favor of a phospholipid-rich acceptor particle such as HDL. Once the cholesterol to phospholipid ratio increases to a level that is comparable to the donor membrane, the driving force for a net movement of cholesterol mass decreases. Thus, the observed lower cholesterol to phospholipid ratio observed in the SLOS fetal HDL provides the most likely explanation for the increased cholesterol efflux. Alternatively, HDL from the SLOS fetus was smaller than the other sources of HDL, even the fetal control HDL. It has been suggested previously that smaller HDL particles may be more efficient cholesterol acceptors than larger particles, which is consistent with our results [55,56]. The observed differences in cholesterol efflux were probably not due to differences in the apolipoprotein content of the SLOS HDL particles since there were no gross differences in the SDS gel protein profiles for the different samples. However, this does not preclude the possibility that differences in some of the more minor protein constituents of HDL could have an effect. Finally, the SLOS HDL particles could have a modified, more accepting structure due to the likely presence of 7- and 8-DHC; previous studies have shown that HDL will carry about the same percentage of 7- and 8-DHC as that in the whole plasma [53]. Since 7- and 8-DHC can affect membrane structure and function [57,58], it is possible that they can also affect the ability to accept cholesterol from exogenous sources.
It should be noted that the properties of HDL which make them better acceptors of cholesterol are somewhat dependent upon the mechanism responsible for the efflux. There are four major processes by which cholesterol is effluxed from cells: ABCA1-mediated, SR-BI-mediated, ABCG1-mediated, and simple diffusion down a concentration gradient [59,60]. Our previous studies suggest that ABCA1 is not the primary mechanism of cholesterol efflux from trophoblasts since apoA-I does not efflux trophoblast cholesterol [40]. Likewise, the process does not appear to be mediated by SR-BI since Wadsak et al. [61] demonstrated that SR-BI is on the apical side of trophoblasts. Since HDL and phospholipid vesicles appear to be the preferred acceptors and ABCG1 is expressed in the placenta [62,63], we hypothesize that the efflux is mediated through ABCG1 [64] or through simple diffusion. Thus, the ratio of cholesterol to phospholipid may have the greatest impact on enhanced efflux of the SLOS HDL.
While we have shown that the SLOS fetal HDL particles are better acceptors than HDL from adults or unaffected fetuses, what does this mean in vivo for the SLOS fetus? The cholesterol concentration of cord blood plasma of the SLOS fetus was low (14.1 mg/dl), ≈25% of concentrations documented in unaffected fetuses [41–43]. Interestingly, SLOS fetal plasma effluxed the same percentage of cholesterol as did plasma from control fetuses and adults. Given that the SLOS fetus likely had significantly lower levels of all lipoproteins in the plasma, including HDL, this supports the idea that HDL from SLOS fetuses is a highly efficient cholesterol acceptor. Fewer SLOS particles could mediate the same amount of cholesterol efflux as a higher number of normal HDL particles.
The ability to obtain exogenous cholesterol from trophoblasts, and possibly the placental endothelial cells as well, is noteworthy for the cholesterol-depleted SLOS fetus because of the key roles cholesterol plays in development. First, cholesterol is a structural component of every single membrane. As such, membrane cholesterol maintains membrane integrity and consequently structure and function of membrane-bound proteins [65,66], including signaling originating in rafts or lipid-rich domains [67,68]. Effects are not found merely from a change in cholesterol concentrations, but also with changes in sterol precursors. Lipid rafts in membranes of fibroblasts of SLOS subjects are enriched with 7- and 8-DHC [57], and the cells consequently have dramatic changes in signaling events and membrane protein activity [58]. Other developmental signaling pathways affected by cellular cholesterol levels are those that occur downstream of the hedgehogs, sonic, Indian, and desert. It appears that cholesterol may be important in not only the activation of the hedgehogs, but in propagation of the signal as well [14–17]. The roles of the hedgehogs throughout development are vast and include forebrain patterning to lung and limb development [15]. In addition to involvement with signaling events, cholesterol is also the precursor for various integrators of metabolism, such as bile acids and oxysterols. These sterols can activate various nuclear receptors, such as FXR, PXR, and LXR [69–71], that are involved in a variety of processes involved in whole body metabolism [72–74].
To summarize, SLOS is a cholesterol deficiency syndrome, with no proven treatment. Clinical trials of cholesterol supplementation in infants and children with SLOS are ongoing. Optimal therapy may require prenatal treatment, however, based on the roles of cholesterol in development. The present study demonstrates that despite profound cholesterol deficiency, SLOS fetal plasma and HDL can efflux cholesterol. In fact, fetal SLOSHDL cholesterol efflux is enhanced compared with controls. Therefore, it is plausible that increased delivery of maternal cholesterol to the SLOS fetus can be achieved, so that future efforts to enhance cholesterol efflux from maternal plasma to the fetus in attempts at ameliorating the SLOS phenotype are warranted. It should be noted that a change in cholesterol transport could also affect fetuses unaffected by SLOS since maternal cholesterol concentrations are directly related to growth rates of unaffected fetuses [75–77]. This is important to fetuses that are either small or large for their gestational age since abnormal growth rates throughout development, from the fetal to neonatal periods, can affect the development of diseases later in life [78–84].
Acknowledgments
The authors thank Leslie Cooperman for collecting the placenta and umbilical cord blood samples from a fetus with SLOS on very short notice, and Diane Brockman for obtaining umbilical cord blood from control fetuses. These studies were supported by Grants HD34089 (L.A.W.), HD39419 (L.A.W.), and HL073980 (R.D.S.) from the National Institutes of Health.
References
- 1.Kelley RI, Hennikam RCM. The Smith–Lemli–Opitz syndrome. J. Med. Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter FD. RSH/Smith–Lemli–Opitz Syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol. Genet. Metab. 2000;71:163–174. doi: 10.1006/mgme.2000.3069. [DOI] [PubMed] [Google Scholar]
- 3.Battaile KP, Steiner RD. Smith–Lemli–Opitz syndrome: the first malformation syndrome associated with defective cholesterol synthesis. Mol. Genet. Metab. 2000;71:154–162. doi: 10.1006/mgme.2000.3020. [DOI] [PubMed] [Google Scholar]
- 4.Irons M, Elias ER, Salen G, Tint GS, Batta AK. Defective cholesterol biosynthesis in Smith–Lemli–Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- 5.Shefer S, Salen G, Batta AK, Honda A, Tint GS, Irons M, Elias ER, Chen TC, Holick MF. Markedly inhibited 7-dehydrocholesterol-delta 7-reductase activity in liver microsomes from Smith–Lemli–Opitz homozygotes. J. Clin. Invest. 1995;96:1779–1785. doi: 10.1172/JCI118223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzky BU, Witsch-Baumgartner M, Erdel M, No Lee J, Paik Y-K, Glossmann H, Utermann G, Moebius FF. Mutations in the delta7-sterol reductase gene in patients with the Smith–Lemli–Opitz syndrome. Proc. Natl. Acad. Sci. USA. 1998;95:8181–8196. doi: 10.1073/pnas.95.14.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wassif CA, Maslen C, Kachilele-Linjewile S, Lin D, Linck LM, Connor WE, Steiner RD, Porter FD. Mutations in the human sterol delta7-reductase gene at 11q12–13 cause Smith–Lemli–Opitz syndrome. Am. J. Hum. Genet. 1998;63:55–62. doi: 10.1086/301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waterham HR, Wijburg FA, Hennekam RC, Vreken P, Poll-The BT, Dorland L, Duran M, Jira PE, Smeitink JA, Wevers RA, Wanders RJ. Smith–Lemli–Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am. J. Hum. Genet. 1998;63:329–338. doi: 10.1086/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaile KP, Battaile BC, Merkens LS, Maslen CL, Steiner RD. Carrier frequency of the common mutation IVS8-1G>C in DHCR7 and estimate of the expected incidence of Smith–Lemli–Opitz syndrome. Mol. Genet. Metab. 2001;72:67–71. doi: 10.1006/mgme.2000.3103. [DOI] [PubMed] [Google Scholar]
- 10.Nowaczyk M, Nakamura L, Eng B, Porter F, Wyae J. Frequency and ethnic distribution of the common DHCR7 mutation in Smith–Lemli–Opitz syndrome. Am. J. Med. Genet. 2001;102:385–386. doi: 10.1002/ajmg.1441. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert-Barnes E, Barnes LA. The Metabolic Basis of Inherited Diseases. New York: McGraw-Hill Book Co.; 2000. [Google Scholar]
- 12.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 13.Shimamura K, Rubenstein JLR. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 14.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 15.Cooper MK, Wassif CA, Krabowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 17.Guy RK. Inhibition of sonic hedgehog autoprocessing in cultured mammalian cells by sterol deprivation. Proc. Natl. Acad. Sci. USA. 2000;97:7307–7312. doi: 10.1073/pnas.97.13.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koide T, Hayata T, Cho KWY. Negative regulation of Hedgehog signaling by the cholesterogenic enzyme 7-dehydrocholesterol reductase. Development. 2006;133:2395–2405. doi: 10.1242/dev.02393. [DOI] [PubMed] [Google Scholar]
- 19.Linck LM, Hayflick SJ, Lin DS, Battalie KP, Ginat S, Burlingame T, Gibson KM, Honda M, Honda A, Salen G, Tint GS, Connor WE, Steiner RD. Fetal demise with Smith–Lemli–Opitz syndrome confirmed by tissue sterol analysis and the absence of measurable 7-dehydrocholesterol delta(7)-reductase activity in chorionic villi. Prenat. Diagn. 2000;20:238–240. [PubMed] [Google Scholar]
- 20.Nowaczyk MJM, Farrell SA, Sirkin WL, Velsher L, Krakowiak PA, Waye JS, Porter FD. Smith–Lemli–Opitz (RHS) syndrome: holoprosencephaly and homozygous IVS8-1G C genotype. Am. J. Med. Genet. 2001;103:75–80. doi: 10.1002/1096-8628(20010915)103:1<75::aid-ajmg1502>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Witsch-Baumgartner M, Gruber M, Kraft HG, Rossi M, Clayton P, Giros M, Haas D, Kelley RI, Krajewska-Walasek M, Utermann G. Maternal apo E genotype is a modifier of the Smith–Lemli–Opitz syndrome. J. Med. Genet. 2004;41:577–584. doi: 10.1136/jmg.2004.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utermann G. Apolipoprotein E polymorphism in health and disease. Am. Heart J. 1987;113:433–440. doi: 10.1016/0002-8703(87)90610-7. [DOI] [PubMed] [Google Scholar]
- 23.Synder SM, Terdiman JF, Caan B, Feingold KR, Hubl ST, Smith RS, Young SG. Relationship of apolipoprotein E phenotypes to hypocholesterolemia. Am. J. Med. 1993;95:480–488. doi: 10.1016/0002-9343(93)90330-r. [DOI] [PubMed] [Google Scholar]
- 24.Mahley RW, Innerarity TL, Rall SCJ, Weisgraber KH. Lipoproteins of special significance in atherosclerosis. Insights provided by studies of type III hyperlipoproteinemia. Ann. NY Acad. Sci. 1985;454:209–221. doi: 10.1111/j.1749-6632.1985.tb11860.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin DS, Pitkin RM, Connor WE. Placental transfer of cholesterol into the human fetus. Am. J. Obstet. Gynecol. 1977;128:735–739. doi: 10.1016/0002-9378(77)90713-x. [DOI] [PubMed] [Google Scholar]
- 26.Pitkin RM, Connor WE, Lin DS. Cholesterol metabolism and placental transfer in the pregnant rhesus monkey. J. Clin. Invest. 1972;51:2584–2592. doi: 10.1172/JCI107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plotz EJ, Kabara JJ, Davis ME, LeRoy GV, Gould RG. Studies on the synthesis of cholesterol in the brain of the human fetus. Am. J. Obstet. Gynecol. 1968;101:534–538. doi: 10.1016/0002-9378(68)90565-6. [DOI] [PubMed] [Google Scholar]
- 28.Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Wiztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of LDL and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napoli C, Wiztum JL, deNigris F, Palumbo G, D’Armiento FP, Palinski W. Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation. 1999;99:2003–2010. doi: 10.1161/01.cir.99.15.2003. [DOI] [PubMed] [Google Scholar]
- 30.Woollett LA. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am. J. Clin. Nutr. 2005;82:1155–1161. doi: 10.1093/ajcn/82.6.1155. [DOI] [PubMed] [Google Scholar]
- 31.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histotropic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 2002;87:2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- 32.Burton GJ, Hempstock J, Jauniaux E. Nutrition of the human fetus during the first trimester-a review. Placenta. 2001;22:S70–S76. doi: 10.1053/plac.2001.0639. [DOI] [PubMed] [Google Scholar]
- 33.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd Collection revisited. Am. J. Obstet. Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 34.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Physiol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlin JB, Johnson WJ, Benedict CR, Chacko GK, Phillips MC, Rothblat GH. Cholesterol flux between cells and high density lipoproteins. J. Biol. Chem. 1984;262:12557–12564. [PubMed] [Google Scholar]
- 36.Davidson WS, Rodrigueza WV, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC. Effects of acceptor particle size on the efflux of cellular free cholesterol. J. Biol. Chem. 1995;270:17106–17113. doi: 10.1074/jbc.270.29.17106. [DOI] [PubMed] [Google Scholar]
- 37.Remaley AT, Stonik JA, Demonsky SJ, Neufeld EB, Bocharov AV, Vishnyakova TG, Eggerman TL, Patterson AP, Duverger NJ, Santamarina-Fojo S, Brewer HB., Jr Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem. Biophys. Res. Commun. 2001;280:818–823. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 38.Smith JD, Miyata M, Ginsberg M, Grigaux C, Shmookler E, Plump AS. Cyclic AMP induces apolipoprotein E binding activity and promotes cholesterol efflux from a macrophage cell line to apolipoprotein acceptors. J. Biol. Chem. 1996;271:30647–30655. doi: 10.1074/jbc.271.48.30647. [DOI] [PubMed] [Google Scholar]
- 39.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 1999;104:R25–R31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid KE, Davidson WS, Myatt L, Woollett LA. The transport of cholesterol across a placental cell monolayer: implications for net transport of sterol from the maternal to fetal circulation. J. Lipid Res. 2003:1909–1918. doi: 10.1194/jlr.M300126-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Vuorio AF, Miettinen TA, Turtola H, Oksanen H, Gylling H. Cholesterol metabolism in normal and heterozygous familial hypercholesterolemic newborns. J. Lab. Clin. Med. 2002;140:35–42. doi: 10.1067/mlc.2002.125214. [DOI] [PubMed] [Google Scholar]
- 42.Parker CRJ, Carr BR, Simpson ER, MacDonald PC. Decline in the concentration of low-density lipoprotein-cholesterol in human fetal plasma near term. Metabolism. 1983;32:919–923. doi: 10.1016/0026-0495(83)90207-x. [DOI] [PubMed] [Google Scholar]
- 43.Nagasaka H, Chiba H, Kikuta H, Akita H, Takahashi Y, Yanai H, Hui S, Fuda H, Fujiwara H, Kobayashi K. Unique character and metabolism of high density lipoprotein (HDL) in fetus. Atherosclerosis. 2002;161:215–223. doi: 10.1016/s0021-9150(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 44.Bick DP, McCorkle D, Stanley WAS, Stern HJ, Staszak PB, Berkovitz GD, Meyers CM, Kelley RI. Prenatal diagnosis of Smith–Lemli–Opitz syndrome in a pregnancy with low maternal serum oestriol and a sex-reversed fetus. Prenat. Diagn. 1999;19:68–71. doi: 10.1002/(sici)1097-0223(199901)19:1<68::aid-pd461>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Craig JE, Savage V, Cowley D, Clague A, Glass IA. Low maternal serum oestriol at mid-trimester may indicate a fetal disorder of cholesterol biosynthesis. Aust. NZ J. Obstet. Gynecol. 1999;39:249–251. doi: 10.1111/j.1479-828x.1999.tb03384.x. [DOI] [PubMed] [Google Scholar]
- 46.Lin DS, Steiner RD, Flavell DP, Connor WE. Intestinal absorption of cholesterol by patients with Smith–Lemli–Opitz syndrome. Pediatr. Res. 2005;57:765–770. doi: 10.1203/01.PDR.0000157723.98422.B5. [DOI] [PubMed] [Google Scholar]
- 47.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 48.Sokoloff L, Rothblat GH. Sterol to phospholipid molar ratios of L cells with qualitative and quantitative variations of cellular sterol. Proc. Soc. Exp. Biol. Med. 1974;146:1166–1172. doi: 10.3181/00379727-146-38267. [DOI] [PubMed] [Google Scholar]
- 49.Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am. J. Physiol. 1997;273:C1596–C1604. doi: 10.1152/ajpcell.1997.273.5.C1596. [DOI] [PubMed] [Google Scholar]
- 50.Knipp GT, Audus KL, Soares MJ. Nutrient transport across the placenta. Adv. Drug Deliv. Rev. 1999;38:41–58. doi: 10.1016/s0169-409x(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 51.Barenholz Y, Gibbes D, Litman BJ, Goll J, Thompson TE, Carlson RD. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977;16:2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- 52.Woollett LA. The origins and roles of cholesterol and fatty acids in the fetus. Curr. Opin. Lipidol. 2001;12:305–312. doi: 10.1097/00041433-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Merkens LS, Connor WE, Linck LM, Lin DS, Flavell DP, Steiner RD. Effects of dietary cholesterol on plasma lipoproteins in Smith–Lemli–Opitz syndrome. Pediatr. Res. 2004;56:726–732. doi: 10.1203/01.PDR.0000141522.14177.4F. [DOI] [PubMed] [Google Scholar]
- 54.Behulova D, Bzduch V, Skodova J, Dello Russo A, Corso GP, Ponec J, Kasaicka A. Serum lipids and apolipoproteins in children with the Smith–Lemli–Opitz syndrome. J. Inherit. Metab. Dis. 2000;23:413–415. doi: 10.1023/a:1005668305864. [DOI] [PubMed] [Google Scholar]
- 55.Ohta T, Saku K, Takata K, Nakamura R, Ikeda Y, Matsuda I. Different effects of subclasses of HDL containing apoA-I but not apoA-II (LpA-I) on cholesterol esterification in plasma and net cholesterol efflux from foam cells. Arterioscler. Thromb. Vasc. Biol. 1995;15:956–962. doi: 10.1161/01.atv.15.7.956. [DOI] [PubMed] [Google Scholar]
- 56.Tricerri MA, Sanchez SA, Arnulphi C, Durbin DM, Gratton E, Jonas A. Interaction of apolipoprotein A–I in three different conformations with palmitoyl oleoyl phosphatidylcholine vesicles. J. Lipid Res. 2002;43:187–197. [PubMed] [Google Scholar]
- 57.Keller RK, Arnold TP, Fliesler SJ. Formation of 7-dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith–Lemli–Opitz syndrome. J. Lipid Res. 2004;45:347–355. doi: 10.1194/jlr.M300232-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tulenko TM, Boeze-Battaglia K, Mason RP, Tint GS, Steiner RD, Connor WE, Labelle EF. A membrane defect in the pathogenesis of the Smith–Lemli–Opitz syndrome. J. Lipid Res. 2006;47:134–143. doi: 10.1194/jlr.M500306-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Leera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 60.Baldan A, Tarr P, Lee R, Edwards PA. ATP-binding cassette transporter G1 and lipid homeostasis. Curr. Opin. Lipidol. 2006;17:227–232. doi: 10.1097/01.mol.0000226113.89812.bb. [DOI] [PubMed] [Google Scholar]
- 61.Wadsack C, Hirschmugl B, Maier A, Hiden U, Desoye G. The placental scavenger receptor class B type-I (SR-BI) undergoes spatio-developmental changes in human pregnancy. Placenta. 2005;26:A49. [Google Scholar]
- 62.Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, Schmitz G. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin. Chem. 2003;49:230–238. doi: 10.1373/49.2.230. [DOI] [PubMed] [Google Scholar]
- 63.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab. Pharmacokinet. 2005;20:452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- 64.Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. 2005;280:30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 65.Rothberg KG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 66.Nezil FA, Bloom M. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys. J. 1992;61:1176–1183. doi: 10.1016/S0006-3495(92)81926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foster LJ, deHoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fielding CJ, Fielding PE. Membrane cholesterol and the regulation of signal transduction. Biochem. Soc. Trans. 2004;32:65–69. doi: 10.1042/bst0320065. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 70.Standinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klassen CD, Brown KK, Reinhard J, Wilson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janowski BA, Willy PJ, Devi TR, Falch JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 72.Repa JJ, Mangelsdorf DJ. Nuclear receptor regulation of cholesterol and bile acid metabolism. Curr. Opin. Biotechnol. 1999;10:557–563. doi: 10.1016/s0958-1669(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 73.Chiang JYL. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Edwards PA, Kast HR, Anisfeld AM. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J. Lipid Res. 2002;43:2–12. [PubMed] [Google Scholar]
- 75.Clausen T, Burski TK, Oyen N, Godang K, Bollerslev J, Henriksen T. Maternal anthropometric and metabolic factors in the first half of pregnancy and risk of neonatal macrosomia in term pregnancies. A prospective study. Eur. J. Endocrinol. 2005;153:887–894. doi: 10.1530/eje.1.02034. [DOI] [PubMed] [Google Scholar]
- 76.Wadsack C, Tabano S, Maier A, Hiden U, Alvino G, Cozzi V, Huttinger M, Schneider WJ, Lang U, Cetin I, Desoye G. Intrauterine growth restriction (IUGR) is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition. Am. J. Physiol. Endocrinol. Metab. 2006;292:E476–E484. doi: 10.1152/ajpendo.00547.2005. [DOI] [PubMed] [Google Scholar]
- 77.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, Muenke M. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120:723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 78.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21:B142–B149. [PubMed] [Google Scholar]
- 79.Samaras TT, Elrick H, Storms LH. Birthweight, rapid growth, cancer, and longevity: a review. J. Natl. Med. Assoc. 2003;95:1170–1183. [PMC free article] [PubMed] [Google Scholar]
- 80.Hediger ML, Overpeck MD, McGlynn A, Kuczmarski RJ, Maurer KR, Davis WW. Growth and fatness at three to six years of age in children born small- or large-for-gestational age. Pediatrics. 1999;104:e33. doi: 10.1542/peds.104.3.e33. [DOI] [PubMed] [Google Scholar]
- 81.Layer AA, Syddall HE, Dennison EM, Gilbody HJ, Duggleby SL, Cooper C, Barker DJ, Phillips DI. Birth weight, weight at 1 y of age, and body composition in older men: findings from the Hertfordshire Cohort Study. Am. J. Clin. Nutr. 2004;80:199–203. doi: 10.1093/ajcn/80.1.199. [DOI] [PubMed] [Google Scholar]
- 82.Barker DJP, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005;353:1348–1350. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 83.Barker DJP. The developmental origins of adult disease. J. Am. Coll Nutr. 2004;23:558S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 84.Barker DJP. The developmental origins of chronic adult disease. Acta Paediatr. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]