Abstract
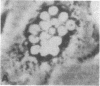
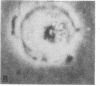
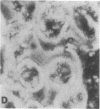
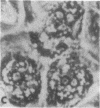
Transplantation studies have suggested that peripheral blood mononuclear cells contain precursors for osteoclasts. Thus we tested the capacity of peripheral blood monocytes to form osteoclasts in long-term culture. We have reported previously that mononuclear cells from feline, baboon, and human marrow form osteoclast-like cells in long term cultures. Further, the formation of these cells is increased in response to bone resorption stimulatory agents such as PTH, interleukin 1, and transforming growth factor alpha. We now report that these cells show characteristic cytoplasmic contraction with calcitonin and form resorption lacunae when cultured on sperm whale dentine. Thus, these bone marrow-derived multinucleated cells fulfill the functional criteria for osteoclasts. Although cultured peripheral blood monocytes can be induced to form multinucleated cells with 1,25-dihydroxyvitamin D3, these cells did not show similar responses to the osteotropic factors as multinucleated cells formed in the bone marrow cultures multinucleated cells. These results indicate that osteoclasts or cells closely related to osteoclasts form in long-term human bone marrow cultures. In contrast, few mononuclear cells in the peripheral blood appear capable of forming osteoclasts under the culture conditions used in these experiments.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Shiina Y., Miyaura C., Tanaka H., Hayashi T., Kanegasaki S., Saito M., Nishii Y., DeLuca H. F., Suda T. Activation and fusion induced by 1 alpha,25-dihydroxyvitamin D3 and their relation in alveolar macrophages. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7112–7116. doi: 10.1073/pnas.81.22.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N. N., Jones S. J., Boyde A. Monocyte-enriched cells on calcified tissues. Anat Embryol (Berl) 1984;170(2):169–175. doi: 10.1007/BF00319002. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Teitelbaum S. L., Reitsma P., Hall A., Pegg L. E., Trial J., Kahn A. J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde A., Ali N. N., Jones S. J. Optical and scanning electron microscopy in the single osteoclast resorption assay. Scan Electron Microsc. 1985;(Pt 3):1259–1271. [PubMed] [Google Scholar]
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Horton M. A. Failure of cells of the mononuclear phagocyte series to resorb bone. Calcif Tissue Int. 1984 Sep;36(5):556–558. doi: 10.1007/BF02405365. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Magnus C. J. Calcitonin alters behaviour of isolated osteoclasts. J Pathol. 1982 Jan;136(1):27–39. doi: 10.1002/path.1711360104. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., McSheehy P. M., Thomson B. M., Fuller K. The effect of calcium-regulating hormones and prostaglandins on bone resorption by osteoclasts disaggregated from neonatal rabbit bones. Endocrinology. 1985 Jan;116(1):234–239. doi: 10.1210/endo-116-1-234. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Revell P. A., Fuller K., Athanasou N. A. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984 Mar;66:383–399. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- Dewhirst F. E., Stashenko P. P., Mole J. E., Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol. 1985 Oct;135(4):2562–2568. [PubMed] [Google Scholar]
- Gowen M., Mundy G. R. Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol. 1986 Apr 1;136(7):2478–2482. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. On the histogenesis of the cells in fracture callus. Electron microscopic autoradiographic observations in parabiotic rats and studies on labeled monocytes. Virchows Arch B Cell Pathol. 1973 Mar 30;12(4):318–329. [PubMed] [Google Scholar]
- Heath J. K., Saklatvala J., Meikle M. C., Atkinson S. J., Reynolds J. J. Pig interleukin 1 (catabolin) is a potent stimulator of bone resorption in vitro. Calcif Tissue Int. 1985 Jan;37(1):95–97. doi: 10.1007/BF02557686. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Ibbotson K. J., D'Souza S. M., Ng K. W., Osborne C. K., Niall M., Martin T. J., Mundy G. R. Tumor-derived growth factor increases bone resorption in a tumor associated with humoral hypercalcemia of malignancy. Science. 1983 Sep 23;221(4617):1292–1294. doi: 10.1126/science.6577602. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Harrod J., Gowen M., D'Souza S., Smith D. D., Winkler M. E., Derynck R., Mundy G. R. Human recombinant transforming growth factor alpha stimulates bone resorption and inhibits formation in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2228–2232. doi: 10.1073/pnas.83.7.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson K. J., Twardzik D. R., D'Souza S. M., Hargreaves W. R., Todaro G. J., Mundy G. R. Stimulation of bone resorption in vitro by synthetic transforming growth factor-alpha. Science. 1985 May 24;228(4702):1007–1009. doi: 10.1126/science.3859011. [DOI] [PubMed] [Google Scholar]
- Jones S. J., Boyde A., Ali N. N., Maconnachie E. Variation in the sizes of resorption lacunae made in vitro. Scan Electron Microsc. 1986;(Pt 4):1571–1580. [PubMed] [Google Scholar]
- MacDonald B. R., Takahashi N., McManus L. M., Holahan J., Mundy G. R., Roodman G. D. Formation of multinucleated cells that respond to osteotropic hormones in long term human bone marrow cultures. Endocrinology. 1987 Jun;120(6):2326–2333. doi: 10.1210/endo-120-6-2326. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr The origin of osteoclasts: evidence, clinical implications and investigative challenges of an extra-skeletal source. J Oral Pathol. 1983 Aug;12(4):226–256. doi: 10.1111/j.1600-0714.1983.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Osdoby P., Martini M. C., Caplan A. I. Isolated osteoclasts and their presumed progenitor cells, the monocyte, in culture. J Exp Zool. 1982 Dec 30;224(3):331–344. doi: 10.1002/jez.1402240306. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Simmons H. A., Sandberg A. L., Canalis E. Direct stimulation of bone resorption by epidermal growth factor. Endocrinology. 1980 Jul;107(1):270–273. doi: 10.1210/endo-107-1-270. [DOI] [PubMed] [Google Scholar]
- Roodman G. D., Ibbotson K. J., MacDonald B. R., Kuehl T. J., Mundy G. R. 1,25-Dihydroxyvitamin D3 causes formation of multinucleated cells with several osteoclast characteristics in cultures of primate marrow. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8213–8217. doi: 10.1073/pnas.82.23.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes R. G., Lam K. W., Dodd R. C., Gray T. K., Cohen M. S. Acid phosphatase activity in mononuclear phagocytes and the U937 cell line: monocyte-derived macrophages express tartrate-resistant acid phosphatase. Blood. 1986 Mar;67(3):729–734. [PubMed] [Google Scholar]
- Stern P. H., Krieger N. S., Nissenson R. A., Williams R. D., Winkler M. E., Derynck R., Strewler G. J. Human transforming growth factor-alpha stimulates bone resorption in vitro. J Clin Invest. 1985 Nov;76(5):2016–2019. doi: 10.1172/JCI112202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., MacDonald B. R., Hon J., Winkler M. E., Derynck R., Mundy G. R., Roodman G. D. Recombinant human transforming growth factor-alpha stimulates the formation of osteoclast-like cells in long-term human marrow cultures. J Clin Invest. 1986 Oct;78(4):894–898. doi: 10.1172/JCI112677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Mundy G. R., Roodman G. D. Recombinant human interferon-gamma inhibits formation of human osteoclast-like cells. J Immunol. 1986 Dec 1;137(11):3544–3549. [PubMed] [Google Scholar]
- Tanaka H., Abe E., Miyaura C., Shiina Y., Suda T. 1 alpha,25-dihydroxyvitamin D3 induces differentiation of human promyelocytic leukemia cells (HL-60) into monocyte-macrophages, but not into granulocytes. Biochem Biophys Res Commun. 1983 Nov 30;117(1):86–92. doi: 10.1016/0006-291x(83)91544-9. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Levine L. Epidermal growth factor stimulates prostaglandin production and bone resorption in cultured mouse calvaria. Biochem Biophys Res Commun. 1978 Dec 14;85(3):966–975. doi: 10.1016/0006-291x(78)90638-1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Singer F. R., Roberts A. B., Derynck R., Winkler M. E., Levine L. Alpha and beta human transforming growth factors stimulate prostaglandin production and bone resorption in cultured mouse calvaria. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4535–4538. doi: 10.1073/pnas.82.13.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G. Congenital osteopetrosis in mice cured by parabiotic union with normal siblings. Endocrinology. 1972 Oct;91(4):916–920. doi: 10.1210/endo-91-4-916. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Osteopetrosis cured by temporary parabiosis. Science. 1973 May 25;180(4088):875–875. doi: 10.1126/science.180.4088.875. [DOI] [PubMed] [Google Scholar]
- Zambonin Zallone A., Teti A., Primavera M. V. Monocytes from circulating blood fuse in vitro with purified osteoclasts in primary culture. J Cell Sci. 1984 Mar;66:335–342. doi: 10.1242/jcs.66.1.335. [DOI] [PubMed] [Google Scholar]