Abstract
Objective
To test the hypothesis that NF-E2–related factor 2 (Nrf2) expression plays an antiatherogenic role by its vascular antioxidant and anti-inflammatory properties.
Methods and Results
Nrf2 is an important transcription factor that regulates the expression of phase 2 detoxifying enzymes and antioxidant genes. Its expression in vascular cells appears to be an important factor in the protection against vascular oxidative stress and inflammation. We developed Nrf2 heterozygous (HET) and homozygous knockout (KO) mice on an apolipoprotein (apo) E–null background by sequential breeding, resulting in Nrf2−/−, apoE−/− (KO), Nrf2−/+, apoE−/− (HET) and Nrf2+/+, and apoE−/− wild-type littermates. KO mice exhibited decreased levels of antioxidant genes with evidence of increased reactive oxygen species generation compared with wild-type controls. Surprisingly, KO males exhibited 47% and 53% reductions in the degree of aortic atherosclerosis compared with HET or wild-type littermates, respectively. Decreased atherosclerosis in KO mice correlated with lower plasma total cholesterol in a sex-dependent manner. KO mice also had a decreased hepatic cholesterol content and a lower expression of lipogenic genes, suggesting that hepatic lipogenesis could be reduced. In addition, KO mice exhibited atherosclerotic plaques characterized by a lesser macrophage component and decreased foam cell formation in an in vitro lipid-loading assay. This was associated with a lower rate of cholesterol influx, mediated in part by decreased expression of the scavenger receptor CD36.
Conclusion
Nrf2 expression unexpectedly promotes atherosclerotic lesion formation in a sex-dependent manner, most likely by a combination of systemic metabolic and local vascular effects.
Keywords: atherosclerosis, cytokines, lipoproteins, reactive oxygen species, foam cell formation, lipogenesis, Nrf2
Atherosclerosis is a chronic inflammatory disease with both genetic and environmental components,1,2 characterized by the deposition of lipids in the artery wall and the infiltration of inflammatory cells, such as monocytes and lymphocytes. Vascular oxidative stress is thought to be an important element in its pathogenesis. Indeed, oxidative modifications of low-density lipoprotein (LDL) in the subendothelial space with subsequent generation of foam cells is 1 of the earliest events in atherogenesis.3 In addition, endothelial cells, macrophages, and smooth muscle cells are all sources of reactive oxygen species (ROS).4–6 Therefore, the interplay between pro-oxidant and antioxidant factors is likely to be of key importance,7 although this notion has been questioned on the basis that oxidative modifications could be consequential, rather than causal, to atherosclerotic lesion formation.8
Vascular cells express an extensive repertoire of antioxidant genes and phase 2 detoxifying enzymes regulated by a transcription factor, NF-E2–related factor 2 (Nrf2). Nrf2 is a member of the Cap and Collar subfamily of basic leucine zipper transcription factors that act through the formation of a heterodimer with 1 of the small Maf proteins9 and bind to a cis-acting enhancer sequence, known as the antioxidant response element,10–13 in the 5′-flanking regions of its target genes.12 Although the regulation of Nrf2 activity is not completely understood, it appears that its subcellular distribution plays a key role.14 Under oxidative stress caused by electrophilic agents, Nrf2 detaches from its repressor Keap 1 and escapes proteosomal degradation to translocate into the nucleus, resulting in enhanced transcriptional activation of its targets.11,12
Cumulative evidence supports the overall antioxidant role of Nrf2 expression. Nrf2 knockout (KO) mice exhibit decreased expression of antioxidant genes, resulting in increased tissue oxidative stress and toxicity in the liver,15,16 lungs,17 and brain18; and greater activation of the innate immune response.19 Nrf2 antioxidant functions may also be important in vascular diseases.20–22 Indeed, expression levels of Nrf2-regulated heme oxygenase-1 (HO-1) and glutathione peroxidase-1 play a protective role in atherogenesis,23,24 paralleling the antioxidant and anti-inflammatory effects of HO-1 demonstrated in other models of vascular inflammation, such as immediate allograft rejection25 and ischemia reperfusion.26 Therefore, we hypothesized that Nrf2 expression plays an antiatherogenic role by its vascular antioxidant and anti-inflammatory properties.
In the present study, we used Nrf2 homozygous and heterozygous KO mice in the apolipoprotein (apo) E–null background to test our hypothesis. Surprisingly, total absence of Nrf2 expression resulted in decreased atherosclerotic lesions, in agreement with a report from Sussan et al,27 but in a sex-dependent manner. However, partial Nrf2 deficiency did not influence atherogenesis. We conclude that total, but not partial, Nrf2 deficiency protects against atherosclerosis, likely by a combination of systemic metabolic and local vascular effects that outweigh protection against suspected atherogenic factors. In addition to previously known effects of Nrf2 on the expression of antioxidant genes and CD36, the results in this study uncover novel effects of Nrf2 on plasma lipoprotein and glucose levels, hepatic lipid content, lipogenic gene expression, and macrophage lipid accumulation and deposition in aortic lesions.
Methods
We developed Nrf2 heterozygous and homozygous KO mice on an apoE-null background by sequential breeding. Mice were used to evaluate atherosclerotic lesion formation and peritoneal macrophage functions. Details on reagents, animals and diet, histological and immunohistochemistry results, lipid and Malondialdehyde (MDA) measurements, lipid-loading and cholesterol efflux and influx assays, real-time quantitative PCR analysis, flow cytometry, and statistical analysis are provided in the supplemental material (available online at http://atvb.ahajournals.org).
Results
Expression of Nrf2 Leads to Increased Atherosclerosis
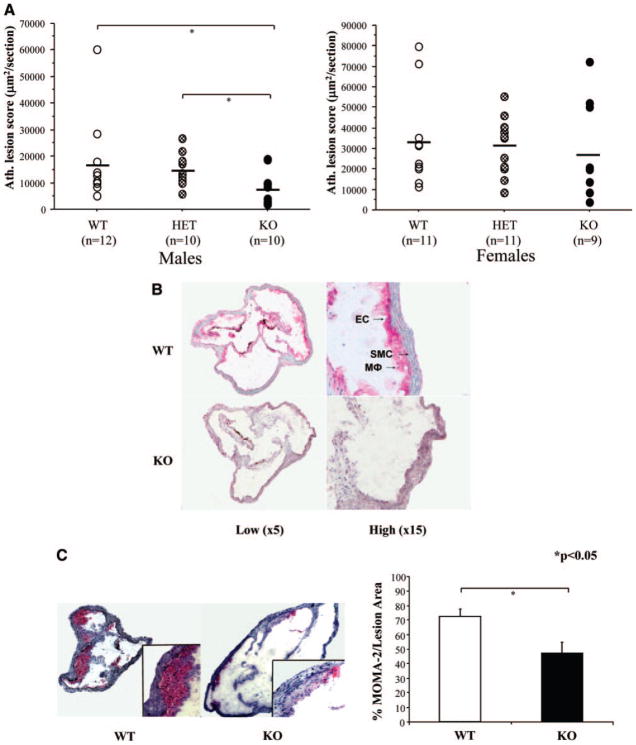
We used Nrf2−/−, apoE−/− (KO), Nrf2−/+, apoE−/− (HET) and Nrf2+/+, and apoE−/− wild-type (WT) mice to assess the effect of Nrf2 expression levels on atherogenesis. HET mice exhibited 50% of WT Nrf2 mRNA levels in the liver (supplemental material). Decreased or absent Nrf2 expression led to dose-dependent effects on the hepatic expression levels of Nrf2-regulated antioxidant genes, such as NADPH–quinone oxidoreductase-1 and catalase, and trends (P<0.2) toward increased hepatic lipid peroxidation (supplemental Figure I). We assessed aortic atherosclerosis in sex-matched 14-week-old mice of all 3 genotypes when fed a chow diet. Surprisingly, KO males exhibited a 47% reduction in atherosclerotic lesion size (7998±2037 μm2 per section) compared with HET males (14 963±1934 μm2 per section) (P=0.03) and a 53% reduction when compared with WT controls (16 992±4261 μm2 per section) (P=0.045) (Figure 1A). Likewise, KO females showed an 18.5% decrease in atherosclerotic lesion size (26 659±8387 μm2 per section) compared with WT littermates (32 727±6772 μm2 per section), although this was not statistically significant (Figure 1A). There were no differences between WT and HET mice. Atherosclerotic plaques were of an early stage (fatty streaks). Total lack of Nrf2 appeared to affect the development of more advanced lesions; we also observed suggestive differences in chow-fed 54-week-old KO male mice that displayed a 26% decrease in aortic atherosclerosis scores (468 250±70 478) compared with WT littermates (630 139±72 674) (P=0.15) (supplemental Figure II).
Figure 1.
Lack of Nrf2 protects against atherosclerosis. A, Aortic atherosclerosis was scored as the lesional area, averaged over 25 serial sections of the aortic root and expressed as μm2/section in 14-week-old KO, HET, and WT mice. B, Immunohistochemistry for Nrf2, performed in aortas of 14-week-old WT and KO male animals. Arrows show positive Nrf2 staining in endothelial cells, smooth muscle cells, and macrophages (Mφs). Photomicrographs were taken from ×20 digital scans at low (×5, left) and high (×15, right) magnifications. C, Immunohistochemistry for MOMA-2 shows the macrophage composition of atherosclerotic plaques developed by WT and KO male mice. Photomicrographs were taken with a light microscopy digital camera (large panel, ×25 magnification; insert, ×400 magnification). The percentage of macrophage area over total lesional area was estimated over 5 to 7 mice per genotype. Values shown are mean±SEM. *P<0.05.
Nrf2 expression was strongly upregulated in all main cell types involved in atherosclerotic lesions, such as endothelial cells, macrophages, and smooth muscle cells of WT mice, as judged by Nrf2 immunohistochemical analysis (Figure 1B). Compared with WT mice, KO mice exhibited a 35% reduction in the macrophage-stained area of their atherosclerotic lesions, as determined by MOMA-2 immunohistochemistry results (Figure 1C). Thus, absence of Nrf2 expression not only led to reduced atherosclerotic lesion size but also altered composition of the plaques.
Nrf2 Expression Influences Macrophage Lipid Loading
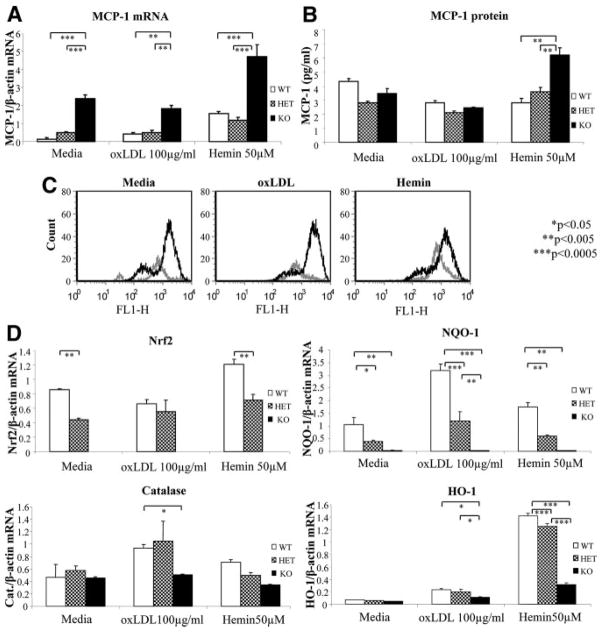
We explored whether KO macrophages had lower expression levels of chemotactic factors, such as monocyte chemotactic protein (MCP)-1, or exhibited reduced lipid loading, which could explain the decreased macrophage contribution to atherosclerotic plaques by less monocyte migration or foam cell formation. Thioglycollate-elicited peritoneal macrophages were harvested from KO, HET, and WT mice and were stimulated with oxidized LDL (oxLDL), hemin or left untreated with media alone (baseline). Hemin was chosen as an alternative stimulus that is known to induce oxidative stress and activate Nrf2.
Compared with male WT macrophages, male KO cells displayed higher levels of MCP-1 mRNA (Figure 2A) and protein expression (Figure 2B) and higher levels of murine homologue for IL-8 mRNA (supplemental Figure IIIA), which paralleled increased MCP-1 mRNA expression in the liver (supplemental Figure IIIB); there was also a borderline significant trend toward greater plasma protein levels in male mice (23.6 versus 16.6 pg/mL; n=5 to 6 per group; P=0.059). Increased MCP-1 expression correlated with greater generation of ROS, as evaluated by 2′,7′-dichlorofluorescin fluorescence (Figure 2C), which was consistent with a previous report in HO-1−/− versus HO-1+/+ macrophages.28 More oxidative stress in KO macrophages was associated with lower expression levels of Nrf2-regulated antioxidant genes, NADPH– quinone oxidoreductase-1, catalase, and HO-1 (Figure 2D). Therefore, in agreement with our determinations in the liver, male Nrf2 KO macrophages exhibited evidence of greater oxidative stress and proinflammatory factors compared with WT. Interestingly, these effects were much less pronounced or even absent in female cells and livers (supplemental Figure IV).
Figure 2.
Nrf2 KO macrophages display increased oxidative stress and MCP-1 expression. A, mRNA levels of MCP-1 quantified by quantitative PCR. B, MCP-1 protein levels determined by ELISA (pg/mL). C, ROS generation of WT (gray) and KO (black) macrophages that were left untreated (media) or stimulated with oxLDL (100 μg/mL) or hemin (50 μmol/L) for 2 hours. DCF fluorescence was assessed by flow cytometry. D, mRNA levels of Nrf2, HO-1, NADPH–quinone oxidoreductase-1, and catalase were assessed by quantitative PCR and normalized by β-actin. For A, B, and D, peritoneal macrophages of WT, HET, and KO male mice were left untreated (media only) or treated with oxLDL (100 μg/mL) or hemin (50 μmol/L) for 4 hours. Values shown are mean±SEM of triplicate wells from 1 representative experiment. *P<0.05, **P<0.005, and ***P<0.0005.
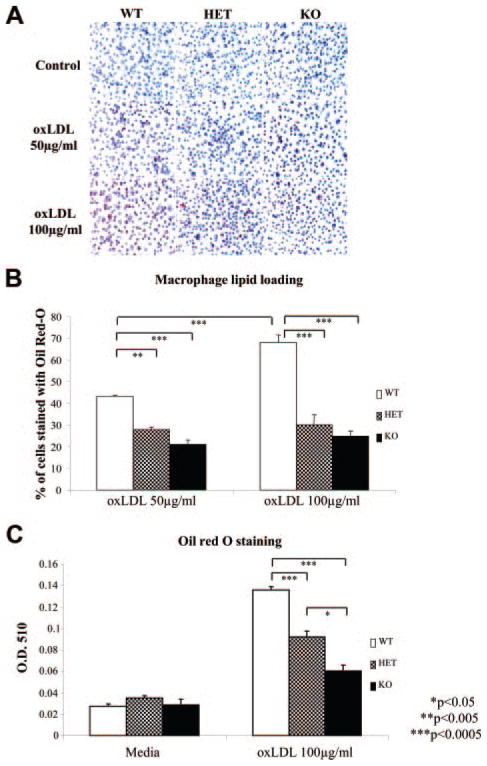
We also assessed the influence of Nrf2 on foam cell formation. KO peritoneal macrophages exhibited significantly lower lipid loading (P<0.05) than WT controls, both from female (Figure 3A and 3B) and male (Figure 3C) mice. HET macrophage lipid loading was intermediate between WT and KO cells (Figure 3C), in apparent dissociation of the aortic atherosclerosis outcomes.
Figure 3.
Nrf2 and lipid loading. A, Photographs of oil red O–stained macrophages. B, Percentages of stained cells over the total number of cells. C, Oil red O absorbance. Peritoneal macrophages from WT, HET, and KO female (A and B) and male (C) mice were incubated with oxLDL (50 or 100 μg/mL) for 48 (A and B) or 24 (C) hours. Values shown are mean±SEM. *P<0.005, and **P<0.0005.
Nrf2 Expression Affects Cholesterol Transport and Lipid Metabolism
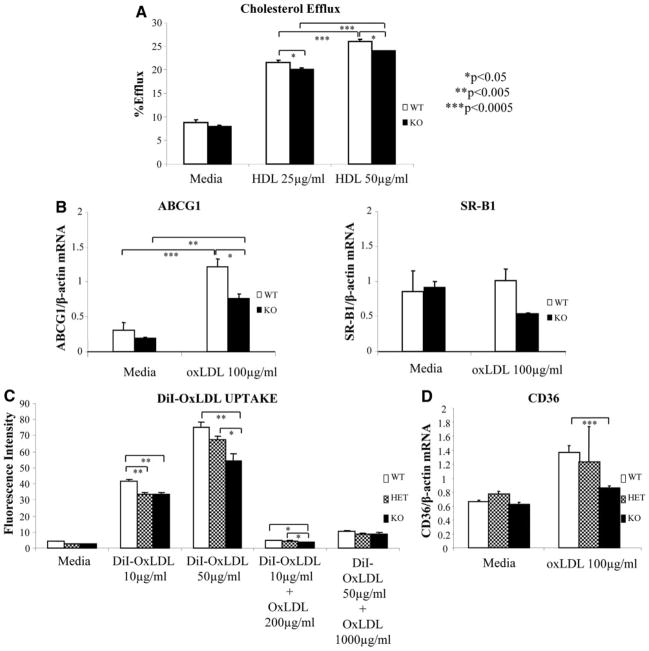
We evaluated cholesterol efflux in 3H-cholesterol–loaded peritoneal macrophages harvested from KO and WT mice in the presence of high-density lipoprotein (HDL) as an acceptor. Nrf2-depleted macrophages showed less cholesterol efflux compared with WT macrophages (P<0.05) (Figure 4A). This correlated with decreased expression of ATP-binding cassette, subfamily G, member 1 and a trend (P=0.09) toward decreased expression of scavenger receptor B1, genes that are both involved in cholesterol transport (Figure 4B). There were no significant differences in the levels of ATP-binding cassette, subfamily A, member 1 (data not shown).
Figure 4.
Cholesterol transport. A, Cholesterol efflux was determined in triplicate in wells of peritoneal macrophages from WT and KO male mice, loaded with 3H-cholesterol overnight and incubated with media as a control or HDL (25 and 50 μg/mL) for 6 hours. The degree of efflux is expressed as the radioactivity in the media divided by the sum of radioactivity in the media plus the cells. B, mRNA levels of scavenger receptor B1 and ATP-binding cassette, subfamily G, member 1 (ABCG1) were quantified by quantitative PCR in macrophages of WT and KO male mice after no treatment (media) or after 4 hours of treatment with oxLDL (100 μg/mL) and normalized against β-actin. C, Cholesterol influx was assessed in quadruplicate in wells of peritoneal macrophages from WT and KO male mice after 24-hour incubation with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine–oxLDL (10 and 50 μg/mL) in the presence or absence of 20-fold excess of nonlabeled competitor (oxLDL) or media alone. Fluorescence intensity was quantified using a fluorescence plate reader (excitation, 530 nm; emission, 590 nm). D, mRNA levels of CD36 quantified in peritoneal macrophages of WT, HET, and KO male mice, as in B. Values shown are mean±SEM from 1 representative experiment. *P<0.05, **P<0.005, and ***P<0.0005.
Next, we evaluated lipid influx by assessing 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI)–oxLDL and DiI–acetylated (Ac) LDL uptake. KO macrophages exhibited a lesser degree of uptake compared with WT controls (Figure 4C and supplemental Figure V). HET macrophages displayed cholesterol uptake that was intermediate between WT and KO cells (Figure 4C). Decreased cholesterol influx exhibited by KO macrophages correlated with decreased CD36 mRNA expression levels after stimulation with oxLDL (Figure 4D), an important scavenger receptor involved in macrophage uptake of both oxLDL and AcLDL.29 On the contrary, there were no significant differences in the expression of the oxLDL (lectinlike) receptor 1 (LOX1) scavenger receptor and there was an increase in scavenger receptor A mRNA and protein levels that would not explain the decreased lipid uptake (data not shown). We concluded that the degree of reduction in cholesterol influx must outweigh the reduction in cholesterol efflux, resulting in lower lipid loading in vitro, which could be responsible, at least in part, for the decreased macrophage-stained area of atherosclerotic plaques in KO mice.
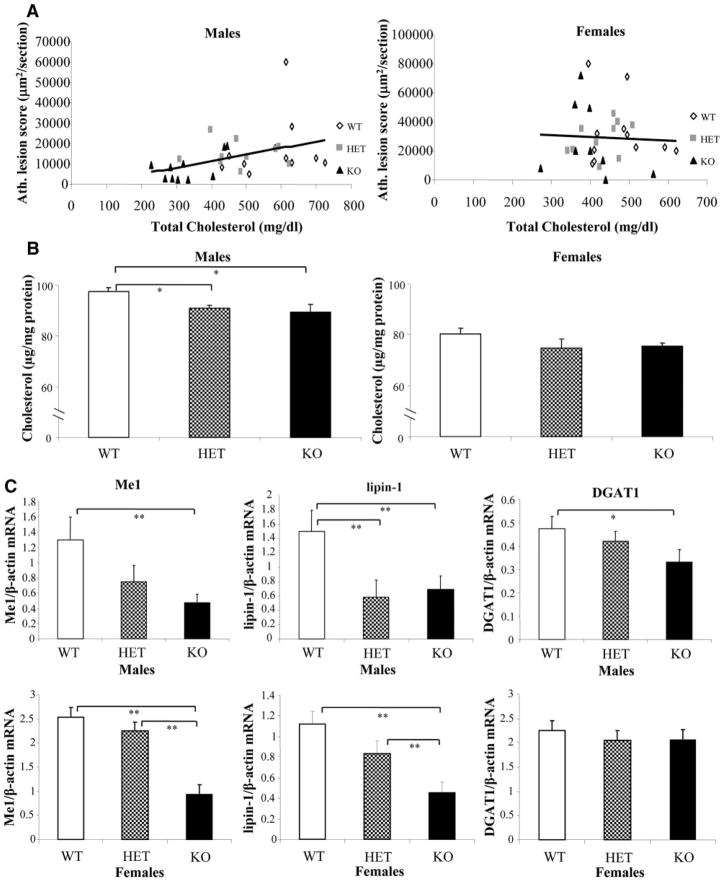
The alterations in cholesterol transport at the cellular level were accompanied by significant changes in plasma lipoprotein levels in a manner akin to the aortic atherosclerosis outcomes. Thus, 14-week-old male and female KO mice exhibited lower levels of plasma total cholesterol because of a substantial reduction in the non-HDL cholesterol fractions (P<0.05) (Table). Similar results were seen in male KO mice at the age of 54 weeks (P<0.05) (supplemental Table I). Male, but not female, total plasma cholesterol and non-HDL/HDL ratios correlated positively with the aortic atherosclerotic scores (Figure 5A and supplemental Figure VI). Interestingly, male KO mice had lower levels of LDL and intermediate-density lipoprotein particles than female KO counterparts, as judged by fast protein liquid chromatographic analysis (supplemental Figure VII); male KO mice also had a lower hepatic cholesterol content (Figure 5B). KO mice displayed lower levels of unesterified cholesterol, free fatty acids, and glucose (Table) and downregulation of hepatic genes involved in lipogenesis and gluconeogenesis. Thus, compared with WT controls, KO male livers displayed reductions in the mRNA levels of malic enzyme 1 (Me1) (P<0.05), lipin-1 (P<0.05), and diacylglycerol acyl transferase (DGAT1) (P=0.056) (Figure 5C) and trends toward decreased levels of fatty acyl synthase (P=0.14) and glucose 6-phosphate dehydrogenase (P=0.2) (supplemental Figure VIII). Female mice also displayed significant reductions in hepatic Me1 and lipin-1 (P<0.05) but not in DGAT1 mRNA levels (Figure 5C). No changes were noted in genes involved in lipid catabolism, such as acyl coenzyme A oxidase or carnitine palmitoyl transferase.
Table.
Lipid Profile in Males and Females*
| Type | Triglycerides | Total Cholesterol | HDL Cholesterol | Non–HDL Cholesterol | Unesterified Cholesterol | Free Fatty Acids | Glucose |
|---|---|---|---|---|---|---|---|
| Males (aged 14 wk) | |||||||
| Nrf2+/+ (n=10) | 41±7 | 577±32 | 12±1 | 551±32 | 291±18 | 80±5 | 256±8 |
| Nrf2−/+ (n=10) | 23±5 | 506±35† | 11±1 | 495±34† | 257±22† | 71±4† | 246±12† |
| Nrf2−/− (n=10) | 35±6 | 331±23‡ | 11±1 | 321±24‡ | 142±11‡ | 44±2‡ | 185±8‡ |
| Females (aged 14 wk) | |||||||
| Nrf2+/+ (n=11) | 10±2 | 477±23 | 8±1 | 469±24 | 239±17 | 68±4 | 235±9 |
| Nrf2−/+ (n=10) | 10±3† | 429±18 | 10±1 | 419±17 | 199±7§ | 56±4†§ | 220±7† |
| Nrf2−/− (n=9) | 19±4‡ | 401±26‡ | 8±1 | 392±25‡ | 174±12‡ | 45±3‡ | 175±7‡ |
Data are given as mean±SE (mg/dL).
P<0.05, HET vs KO.
P<0.05, WT vs KO.
P<0.05, WT vs HET.
Figure 5.
Nrf2 and lipid metabolism. A, Correlation between aortic atherosclerotic scores and total plasma cholesterol in 14-week-old KO (n=10), HET (n=10), and WT (n=11) male mice (r=0.47, P=0.008) and 14-week-old KO (n=9), HET (n=10), and WT (n=11) female mice (r=−0.005, P=0.98). B, Total cholesterol content in livers of WT, HET, and KO male and female mice (n=8 to 10 per group), normalized by liver total protein. C, mRNA levels of Me1, lipin-1, and DGAT1 were quantified by quantitative PCR in livers of WT, HET, and KO (n=10 per group) male and female mice and normalized against β-actin. Values shown are mean±SEM. *P≤0.05, and **P<0.05.
Discussion
Our study reveals that total, but not partial, Nrf2 deficiency protects against atherosclerosis in a sex-dependent manner. We generated Nrf2 KO mice in the apoE-null background to test our hypothesis that Nrf2 deficiency would result in greater atherosclerosis because of increased tissue oxidative stress and vascular inflammation. These mice were fed a chow diet to more closely mimic human lipoprotein levels. However, contrary to our hypothesis, chow-fed male KO mice exhibited decreased atherosclerotic lesion formation with decreased macrophage content. Our results are in agreement with those of Sussan et al,27 who used other Nrf2−/− and apoE−/− mice fed a high-fat diet. Several differences in the experimental designs may be responsible for several contrasting effects, shown in detail in supplemental Table II. In contrast to this previous work, Nrf2 effects on atherosclerotic lesions, inflammation, and plasma lipoproteins were sex dependent and most prominent in males in our study (supplemental Table III).
To gain a better understanding of the mechanism(s) by which Nrf2 deficiency protects against atherosclerosis in males, we examined several factors known to contribute to the disease and compared their effect in males versus females and in heterozygotes versus homozygotes. Of all factors evaluated, the levels of plasma lipoproteins most closely resembled the sex- and genotype-dependent pattern exhibited by atherosclerotic lesion formation (Table). Our study strongly suggests an important role for decreased cholesterol levels in the decreased atherosclerosis noted in male KO mice. KO mice exhibited markedly lower plasma total and non-HDL cholesterol compared with HET and WT littermates, effects that were most pronounced in males and that resulted in positive correlations between aortic atherosclerotic scores and both plasma total cholesterol (Figure 5A) and non-HDL/HDL cholesterol ratios (supplemental Figure VI) in males but not in females. Moreover, KO mice exhibited lower hepatic cholesterol content than WT controls, which was also more pronounced and significant in males (P=0.007) than in females (P=0.17) (Figure 5B).
In both sexes, Nrf2 deficiency led to reduced expression of Me1 and lipin-1, indicating that Nrf2 may have a role in the regulation of lipogenic genes (Figure 5C). Me1 participates in the regeneration of pyruvate from malate back to the mitochondria and assists with the release of acetyl–coenzyme A and NADPH from the mitochondria into the cytosol. Decreased Me1 could lead to lower NADPH content, resulting in decreased lipogenesis and gluconeogenesis, with lower levels of total cholesterol and glucose, as shown by Me1−/− mice.30 Lipin-1 is a phosphatase enzyme that converts phosphatidate to diacylglycerol during triglyceride and biosynthesis of phospholipids.31 However, the fact that both enzymes were reduced in KO livers from both sexes makes it less likely that they are responsible for the sex-dependent effects on atherosclerosis. Interestingly, hepatic DGAT1, responsible for the final step of triglyceride synthesis, was reduced in male mice only. Although changes in DGAT1 expression cannot explain the increased hepatic triglyceride content observed in KO mice (supplemental Figure IX), the apparent dissociation between liver and plasma triglyceride levels could respond to sex-specific effects on the release of triglycerides to the circulating lipoproteins. In addition, DGAT1 expression levels could play a role in the sex-dependent effects noted on hepatic MCP-1 levels. Indeed, DGAT1 protects against the lipotoxic effects of fatty acids in skeletal muscle32 and macrophages,33 leading to reduced inflammation. Thus, lower DGAT1 levels in male, but not in female, KO livers could have resulted in decreased protection against fatty acid toxicity and increased expression of inflammatory markers, such as MCP-1, which occurred only in male KOs.
We also explored whether changes to macrophages might explain the decrease in atherosclerosis. Male KO mice, compared with WT controls, exhibited evidence of greater ROS generation and expression of proinflammatory cytokines in macrophages and the liver (Figure 2 and supplemental Figure III), consistent with previous studies.19,34 These findings were less pronounced or even absent in females (supplemental Figure IV and Table III). However, these results did not explain the surprising decrease in atherosclerosis in male mice because increases in cytokines and ROS generation have been associated with increased atherosclerosis in several mouse models.
The lower macrophage area in KO atherosclerotic plaques raised the possibilities of decreased monocyte migration and/or retention in the aorta, decreased inflammatory/immune status, and accelerated removal of lesional macrophages by apoptosis or macrophage emigration. However, our data suggest that none of these are likely to be responsible for the reduced atherosclerosis in male KO mice. Thus, male KO macrophages exhibited increased MCP-1 expression (Figure 2) and Nrf2−/− mice have developed strong inflammatory and immune responses against both endogenous18 and exogenous19 stimuli. Although KO peritoneal macrophages were more susceptible to ROS-inducing conditions, such as a treatment with cadmium chloride (supplemental Figure X), we did not observe significant differences in the degree of apoptosis in atherosclerotic lesions from KO versus WT mice by the TUNEL assay (data not shown), making it less likely that differences in the degree of apoptosis accounted for the plaque composition changes noted in vivo. Alternatively, KO mice could have exhibited a greater degree of macrophage efflux from their atherosclerotic plaques than WT littermates, as reported in a robust model of plaque regression35; however, this may be less likely to occur in early fatty streaks under conditions of increased ROS formation, as observed in KO mice. Last, the decreased macrophage-stained area in KO lesions could have been the result of decreased size of lesional macrophages because of decreased lipid loading. Indeed, Nrf2 deficiency resulted in decreased cholesterol influx in macrophages, which correlated with lower CD36 expression. This was consistent with similar reports.27,36 However, because Nrf2 effects on lipid loading were sex independent and HET macrophages exhibited an intermediate degree of lipid loading between KO and WT cells (Figure 3), it is clear that Nrf2 effects on CD36 expression and cholesterol influx can only partly explain its overall effects on atherogenesis. Instead, a significant upregulation of scavenger receptor A in KO macrophages was observed. This scavenger receptor A protein could be less active, perhaps by posttranslational modifications, such as phosphorylation,37 or its upregulation may be offset by the downregulation of CD36 or other pathways mediating decreased cholesterol influx. Thus, changes to macrophages in KO mice did not explain the difference in atherosclerosis between males and females.
Although both the present study and the study by Sussan et al27 show a decrease in atherosclerosis in Nrf2-null mice, effects on cholesterol levels were not seen in their study and there was no evidence of a preferential effect on male mice. One possible reason for this difference is the feeding of a high-fat diet that strongly elevates plasma cholesterol levels, which might have obscured the role of Nrf2 in the regulation of lipid metabolism. The study by Sussan et al emphasized an effect of Nrf2 KO on macrophage cholesterol influx. We also observed this effect, but it was not sex specific, as previously discussed. An important caveat in our studies of peritoneal macrophages and liver is that, although they are informative, they did not provide a direct assessment of changes occurring in the vascular wall. Thus, it remains possible that other elements beyond those analyzed herein may contribute to the observed outcomes.
In conclusion, Nrf2 expression results in the promotion of atherosclerosis in a sex-dependent manner, likely by a combination of systemic metabolic and local vascular effects. Our study revealed important new effects of Nrf2 that may involve the regulation of lipid metabolism, particularly plasma cholesterol levels. These effects may need to be considered when using antioxidant therapies that elevate Nrf2 expression.
Supplementary Material
Acknowledgments
We thank Janet Danciger, MS, Ladan Vakili, MD, and Sarada Charugundla, MS, “Essential Laboratory Services” Core, UCLA Atherosclerosis Research Unit, for providing oxLDL and determining levels of plasma lipids; and Judith Berliner, PhD, for input and constructive review of the manuscript.
Sources of Funding
This work was supported by National Institute of Environmental Health Sciences grant RO1 ES016959 (Dr Araujo); National Heart, Blood and Lung Institute grant HL30568 (Dr Lusis); Harold Amos Medical Faculty Development Program Award 049595 from the Robert Wood Johnson Foundation (Dr Araujo); and American Heart Association grant 0665006Y (Dr Araujo).
Footnotes
Disclosures
None.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in athero-genesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 4.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 5.Segal AW, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993;18:43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- 6.Matsubara T, Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986;127:207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- 7.Levonen AL, Vahakangas E, Koponen JK, Yla-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation. 2008;117:2142–2150. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 8.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 9.Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 10.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithio-lethiones through the Keap1-Nrf2 pathway: identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 11.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemo-preventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 14.Numazawa S, Yoshida T. Nrf2-dependent gene expressions: a molecular toxicological aspect. J Toxicol Sci. 2004;29:81–89. doi: 10.2131/jts.29.81. [DOI] [PubMed] [Google Scholar]
- 15.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 16.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18:261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 19.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharavi NM, Gargalovic PS, Chang I, Araujo JA, Clark MJ, Szeto WL, Watson AD, Lusis AJ, Berliner JA. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arterioscler Thromb Vasc Biol. 2007;27:1346–1353. doi: 10.1161/ATVBAHA.107.141283. [DOI] [PubMed] [Google Scholar]
- 21.Levonen AL, Inkala M, Heikura T, Jauhiainen S, Jyrkkanen HK, Kansanen E, Maatta K, Romppanen E, Turunen P, Rutanen J, Yla-Herttuala S. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler Thromb Vasc Biol. 2007;27:741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- 22.Jyrkkanen HK, Kansanen E, Inkala M, Kivela AM, Hurttila H, Heinonen SE, Goldsteins G, Jauhiainen S, Tiainen S, Makkonen H, Oskolkova O, Afonyushkin T, Koistinaho J, Yamamoto M, Bochkov VN, Yla-Herttuala S, Levonen AL. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ Res. 2008;103:e1–e9. doi: 10.1161/CIRCRESAHA.108.176883. [DOI] [PubMed] [Google Scholar]
- 23.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 24.Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, de Haan JB. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 25.Araujo JA, Meng L, Tward AD, Hancock WW, Zhai Y, Lee A, Ishikawa K, Iyer S, Buelow R, Busuttil RW, Shih DM, Lusis AJ, Kupiec-Weglinski JW. Systemic rather than local heme oxygenase-1 overexpression improves cardiac allograft outcomes in a new transgenic mouse. J Immunol. 2003;171:1572–1580. doi: 10.4049/jimmunol.171.3.1572. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchihashi S, Livhits M, Zhai Y, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Basal rather than induced heme oxygenase-1 levels are crucial in the antioxidant cytoprotection. J Immunol. 2006;177:4749–4757. doi: 10.4049/jimmunol.177.7.4749. [DOI] [PubMed] [Google Scholar]
- 27.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS ONE. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih DM, Agarwal A, Lusis AJ, Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100:1703–1711. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 29.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 30.Zhong H, Beaulaurier J, Lum PY, Molony C, Yang X, Macneil DJ, Weingarth DT, Zhang B, Greenawalt D, Dobrin R, Hao K, Woo S, Fabre-Suver C, Qian S, Tota MR, Keller MP, Kendziorski CM, Yandell BS, Castro V, Attie AD, Kaplan LM, Schadt EE. Liver and adipose expression associated SNPs are enriched for association to type 2 diabetes. PLoS Genet. 2010;6:e1000932. doi: 10.1371/journal.pgen.1000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reue K, Dwyer JR. Lipin proteins and metabolic homeostasis. J Lipid Res. 2009;50:S109–S114. doi: 10.1194/jlr.R800052-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, Farese RV., Jr DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120:756–767. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 35.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 37.Heider H, Wintergerst ES. Mimicking phosphorylation at Ser-48 strongly reduces surface expression of human macrophage scavenger receptor class A: implications on cell motility. FEBS Lett. 2001;505:185–190. doi: 10.1016/s0014-5793(01)02819-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.