Abstract
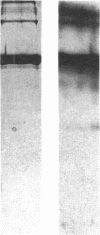
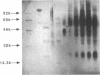
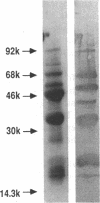
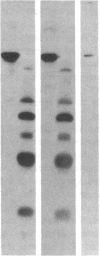
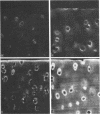
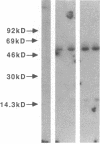
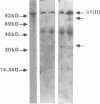
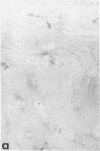
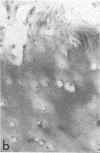
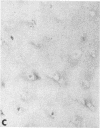
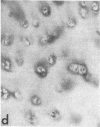
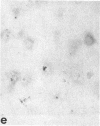
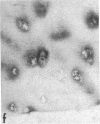
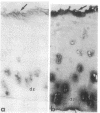
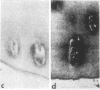
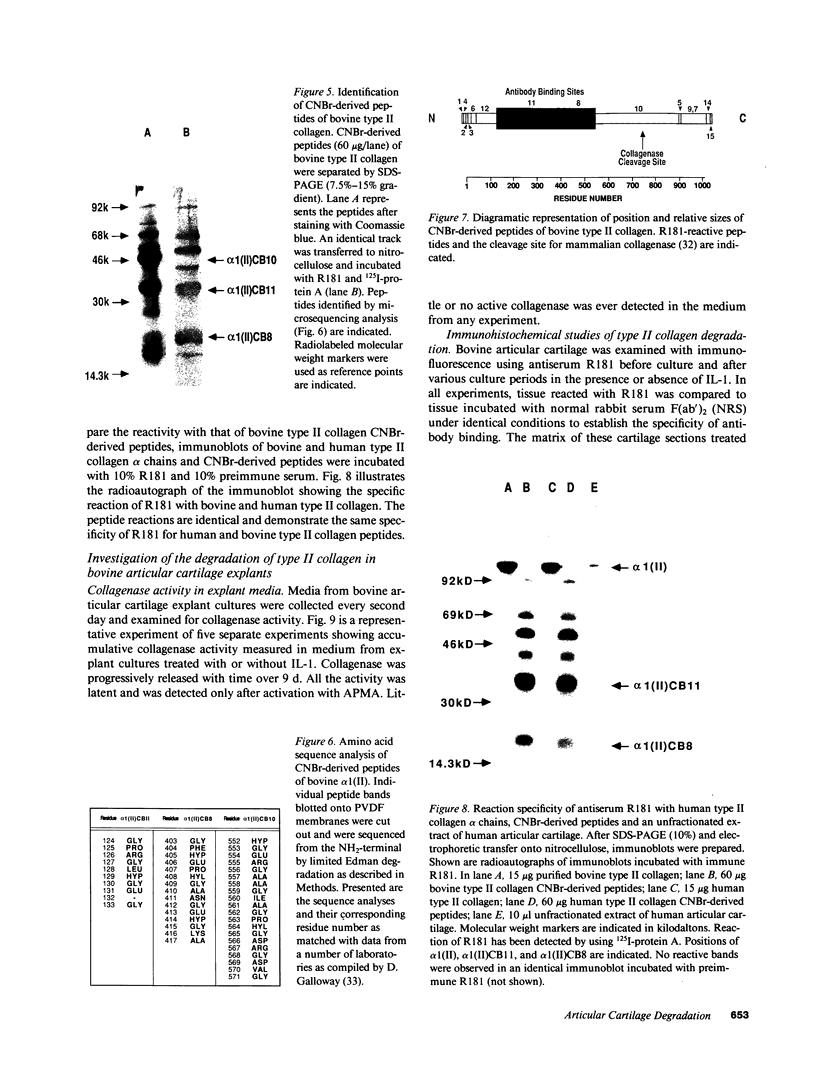
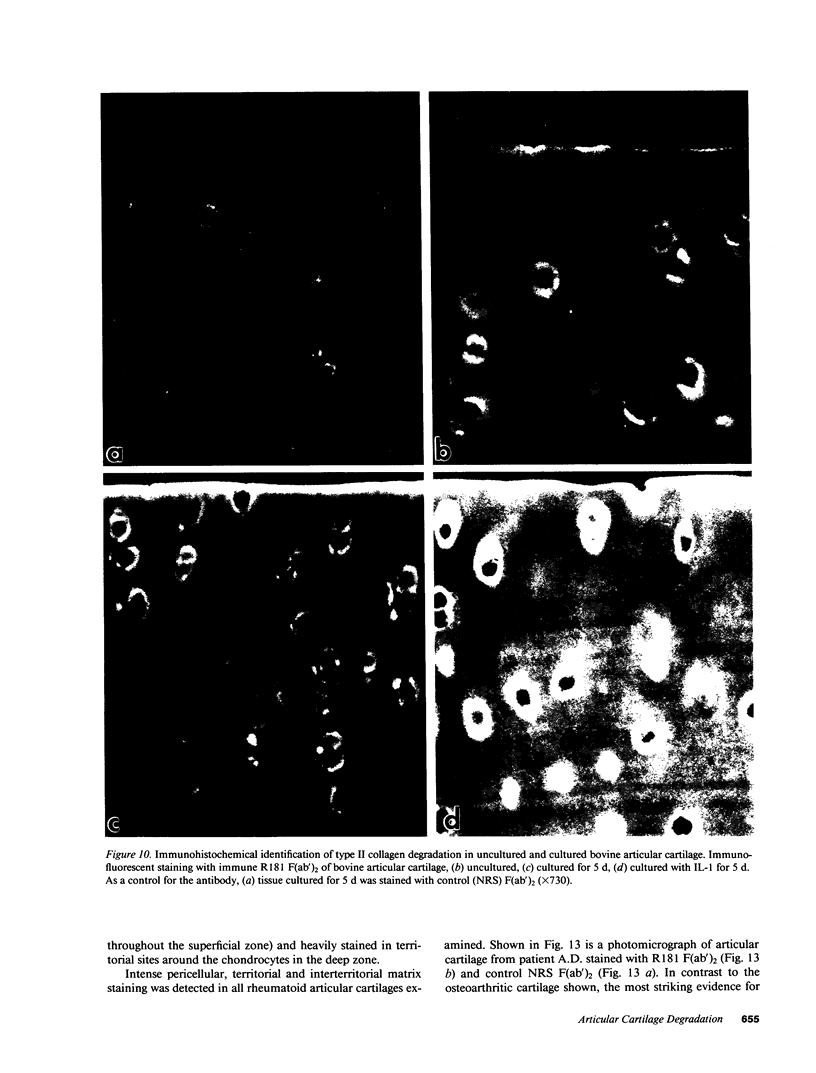
Articular cartilage destruction and loss of function in arthritic diseases involves proteolytic degradation of the connective tissue matrix. We have investigated the degradation of cartilage collagen by developing immunochemical methods that permit the identification and analysis of type II collagen degradation in situ. Previously, a technique to specifically identify type II collagen degradation in situ in articular cartilage did not exist. These methods utilize a polyclonal antiserum (R181) that specifically reacts with unwound alpha-chains and CNBr-derived peptides, alpha 1(II)CB11 and alpha 1(II)CB8, of human and bovine type II collagens. The experimental approach is based on the fact that when fibrillar collagens are cleaved the helical collagen molecule unwinds, exposing hidden epitopes. Here we demonstrate the use of R181 in studying type II collagen degradation in bovine articular cartilage that has been cultured with or without IL-1 and in human normal, rheumatoid, and osteoarthritic articular cartilages. Compared to cartilages either freshly isolated or cultured without IL-1, bovine cartilage cultured with IL-1 for 3-5 d showed an increase in both pericellular and intercellular immunohistochemical staining. Extracts of these cartilages contained type II collagen alpha chains that were increased in amount after culture with IL-1 for 11 d. In addition, culture with IL-1 resulted in the appearance of alpha chain fragments of lower molecular weight. All human arthritic tissues examined showed areas of pronounced pericellular and territorial staining for collagen degradation as compared with non-diseased tissues, indicating that chondrocytes are responsible in part for this degradation as compared with non-diseased tissues. In most cases rheumatoid cartilage was stained most intensely at the articular surface and in the deep and mid-zones, whereas osteoarthritic cartilage usually stained more in the superficial and mid-zones, but less intensely. Distinct patterns of sites of collagen degradation reflect differences in collagen destruction in these diseases, suggesting possible different sources of chondrocyte activation. These experiments demonstrate the application of immunological methods to detect collagen degradation and demonstrate an increase of collagen degradation in human arthritides and in IL-1-treated viable bovine cartilage.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelmann B. C., Kirrane J. The structural basis of cell-mediated immunological reactions of collagen. The species specificity of the cutaneous delayed hypersensitivity reaction. Immunology. 1973 Jul;25(1):123–130. [PMC free article] [PubMed] [Google Scholar]
- Bader D. L., Kempson G. E., Barrett A. J., Webb W. The effects of leucocyte elastase on the mechanical properties of adult human articular cartilage in tension. Biochim Biophys Acta. 1981 Sep 18;677(1):103–108. doi: 10.1016/0304-4165(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Beil W., Timpl R., Furthmayr H. Conformation dependence of antigenic determinants on the collagen molecule. Immunology. 1973 Jan;24(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984 Sep;27(9):968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Champion B. R., Poole A. R. Immunity to homologous cartilage proteoglycans in rabbits with chronic inflammatory arthritis. Coll Relat Res. 1981 Sep;1(5):453–473. doi: 10.1016/s0174-173x(81)80029-5. [DOI] [PubMed] [Google Scholar]
- Champion B. R., Poole A. R. Immunity to homologous type III collagen after partial meniscectomy and sham surgery in rabbits. Arthritis Rheum. 1982 Mar;25(3):274–287. doi: 10.1002/art.1780250305. [DOI] [PubMed] [Google Scholar]
- Champion B. R., Sell S., Poole A. R. Immunity to homologous collagens and cartilage proteoglycans in rabbits. Immunology. 1983 Mar;48(3):605–616. [PMC free article] [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Danscher G., Nörgaard J. O. Light microscopic visualization of colloidal gold on resin-embedded tissue. J Histochem Cytochem. 1983 Dec;31(12):1394–1398. doi: 10.1177/31.12.6631001. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Horsfield P., Fell H. B., Barratt M. E. Breakdown of proteoglycan and collagen induced in pig articular cartilage in organ culture. Ann Rheum Dis. 1975 Aug;34(4):303–311. doi: 10.1136/ard.34.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Characterisation of the major CNBr-Derived peptides of porcine type II collagen. Connect Tissue Res. 1975;3(2):165–170. doi: 10.3109/03008207509152175. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Wu J. J., Apone S. A growing family of collagens in articular cartilage: identification of 5 genetically distinct types. J Rheumatol. 1987 May;14(Spec No):25–27. [PubMed] [Google Scholar]
- Fell H. B., Barratt M. E. The role of soft connective tissue in the breakdown of pig articular cartilage cultivated in the presence of complement-sufficient antiserum to pig erythrocytes. I. Histological changes. Int Arch Allergy Appl Immunol. 1973;44(3):441–468. doi: 10.1159/000230951. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Barratt M. E., Welland H., Green R. The capacity of pig articular cartilage in organ culture to regenerate after breakdown induced by complement-sufficient antiserum to pig erythrocytes. Calcif Tissue Res. 1976 Apr 13;20(1):3–21. doi: 10.1007/BF02546393. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Karmilowicz M. J., Ruby P. L., Beeman A. M., Borges K. A., Brinckerhoff C. E. Cloning of a complementary DNA for rabbit proactivator. A metalloproteinase that activates synovial cell collagenase, shares homology with stromelysin and transin, and is coordinately regulated with collagenase. Arthritis Rheum. 1987 Nov;30(11):1254–1264. doi: 10.1002/art.1780301108. [DOI] [PubMed] [Google Scholar]
- Fisher W. D., Golds E. E., van der Rest M., Cooke T. D., Lyons H. E., Poole A. R. Stimulation of collagenase secretion from rheumatoid synovial tissue by human collagen peptides. J Bone Joint Surg Am. 1982 Apr;64(4):546–557. [PubMed] [Google Scholar]
- Golds E. E., Poole A. R. Connective tissue antigens stimulate collagenase production in arthritic diseases. Cell Immunol. 1984 Jun;86(1):190–205. doi: 10.1016/0008-8749(84)90372-1. [DOI] [PubMed] [Google Scholar]
- Golds E. E., Stephen I. B., Esdaile J. M., Strawczynski H., Poole A. R. Lymphocyte transformation to connective tissue antigens in adult and juvenile rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, systemic lupus erythematosus, and a nonarthritic control population. Cell Immunol. 1983 Nov;82(1):196–209. doi: 10.1016/0008-8749(83)90153-3. [DOI] [PubMed] [Google Scholar]
- Gowen M., Mundy G. R. Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol. 1986 Apr 1;136(7):2478–2482. [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. A mechanism for cartilage destruction in rheumatoid arthritis. Trans Assoc Am Physicians. 1970;83:267–276. [PubMed] [Google Scholar]
- Harris E. D., Jr, Glauert A. M., Murley A. H. Intracellular collagen fibers at the pannus-cartilage junction in rheumatoid arthritis. Arthritis Rheum. 1977 Mar;20(2):657–665. doi: 10.1002/art.1780200204. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K., Larsson E., Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985 Sep;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Jasin H. E., Dingle J. T. Human mononuclear cell factors mediate cartilage matrix degradation through chondrocyte activation. J Clin Invest. 1981 Sep;68(3):571–581. doi: 10.1172/JCI110290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas P. W. The use of a chrome alum-gelatin (subbing) solution as a general adhesive for paraffin sections. Stain Technol. 1971 May;46(3):121–124. doi: 10.3109/10520297109067835. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Dingle J. T., Mallia A. K., Goodman D. S. The localization of retinol-binding protein in rat liver by immunofluorescence microscopy. J Cell Sci. 1975 Nov;19(2):379–394. doi: 10.1242/jcs.19.2.379. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Tang L. H., Choi H., Rosenberg L. Localization of proteoglycan monomer and link protein in the matrix of bovine articular cartilage: An immunohistochemical study. J Histochem Cytochem. 1980 Jul;28(7):621–635. doi: 10.1177/28.7.6156200. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J., Townsend Y. Pig interleukin 1. Purification of two immunologically different leukocyte proteins that cause cartilage resorption, lymphocyte activation, and fever. J Exp Med. 1985 Oct 1;162(4):1208–1222. doi: 10.1084/jem.162.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. G., Telser A. G., Veis A. Semiquantitative determination of cyanogen bromide peptides of collagen in SDS-polyacrylamide gels. Anal Biochem. 1976 Jan;70(1):251–257. doi: 10.1016/s0003-2697(76)80065-6. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J., Burleigh M. C. The degradation of articular collagen by neutrophil proteinases. Biochim Biophys Acta. 1977 Aug 11;483(2):386–397. doi: 10.1016/0005-2744(77)90066-3. [DOI] [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]
- Thomson B. M., Mundy G. R., Chambers T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugai K., Ishikawa H., Hirohata K., Shirane H. Interaction of polymorphonuclear leukocytes with immune complexes trapped in rheumatoid articular cartilage. Arthritis Rheum. 1983 Dec;26(12):1434–1441. doi: 10.1002/art.1780261204. [DOI] [PubMed] [Google Scholar]
- Ugai K., Ziff M., Jasin H. E. Interaction of polymorphonuclear leukocytes with immune complexes trapped in joint collagenous tissues. Arthritis Rheum. 1979 Apr;22(4):353–364. doi: 10.1002/art.1780220407. [DOI] [PubMed] [Google Scholar]
- Witter J., Roughley P. J., Webber C., Roberts N., Keystone E., Poole A. R. The immunologic detection and characterization of cartilage proteoglycan degradation products in synovial fluids of patients with arthritis. Arthritis Rheum. 1987 May;30(5):519–529. doi: 10.1002/art.1780300506. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]