Abstract
Mutations in human copper-zinc superoxide dismutase (SOD1) cause an inherited form of amyotrophic lateral sclerosis (ALS). Inclusions enriched in pathogenic SOD1 accumulate in the spinal cords of transgenic mice expressing these proteins, but endogenous mouse SOD1 is not found as a component of these aggregates. In the accompanying paper, Karch and colleagues analyze aggregation propensities of human/mouse SOD1 chimeras in cell culture and identify two sequence elements in the human enzyme that seem to enhance its aggregation relative to the mouse enzyme. Here, we report the first structure of mouse SOD1 along with those of SOD1 chimeras in which residues 1-80 come from human SOD1 and residues 81-153 come from mouse SOD1 and vice versa. Taken together, the structural and cell-based data suggest a model in which residues Q42 and Q123 in mouse SOD1 modulate nonnative SOD1-SOD1 intermolecular interactions at edge strands in the SOD1 Greek key β-barrel.
Keywords: Copper-zinc superoxide dismutase, amyotrophic lateral sclerosis, X-ray crystallography, protein aggregation
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal neurodegenerative disorder characterized by the loss of upper and lower motor neurons [1]. The discovery of a link between dominant mutations in human copper-zinc superoxide dismutase (SOD1) and some familial ALS (fALS) patients [2, 3] generated excitement because the structure and action of SOD1 are known, and it was anticipated that this knowledge might facilitate an understanding of the molecular basis for how pathogenic SOD1 mutations exert toxic effects in motor neurons and might also illuminate novel avenues of therapeutic intervention. Because inherited and non-inherited forms of ALS are similar clinically, it is also possible that the underlying defects in motor neuron function arising from the two forms of the disease might be related, and therapeutic agents identified as effective for SOD1-linked ALS might also prove useful for the more prevalent sporadic forms of the disease. Although a large number of pathogenic SOD1 proteins have been studied intensely by many laboratories over years (reviewed in [4-7]), the precise molecular mechanism(s) through which these proteins exert their toxic effects remain to be delineated.
Although ALS is a human disease, the development of transgenic mouse models that retain many of the pathological features found in human ALS have been extremely valuable. Mutant human SOD1 genes that have been introduced into mice include A4V [8], G37R [9], H46R [10], G85R [11], G93A [12], L126Z [8, 13], L126del (introduces a stop codon at position 131) [14], and Gins127ins (introduces a stop codon at position 133) [15]. The accumulation of insoluble forms of mutant SOD1 is observed in all of these mouse models as the disease progresses, leading to the suggestion that SOD1-linked ALS is a protein misfolding disease. However, there is debate as to whether the SOD1-containing aggregates, their soluble precursors, or a combination of the two, represent the noxious species [16, 17].
Studies in transgenic mice and in cell culture reveal that, when co-expressed with human pathogenic SOD1 mutants, human wild type SOD1, but not endogenous mouse wild type SOD1, co-aggregates with the pathogenic human SOD1 variants expressed in these systems. For example, in mouse models expressing the pathogenic human SOD1 variants G37R, G93A, H46R/H48Q, wild type mouse SOD1 was not detected in the detergent insoluble fraction of spinal cord lysates using Western blot or shotgun mass spectrometry proteomic analyses [18, 19]. In contrast, the human wild type SOD1 protein co-expressed in mice with the pathogenic SOD1 variants A4V, G85R, G93A, and L126Z was found co-aggregated in the detergent insoluble fractions of spinal cord lysates and the presence of human wild type SOD1 in these animals appeared to exacerbate the toxicity of the pathogenic SOD1 variants as evidenced by an accelerated disease course [8, 20]. In recent studies in cell culture, Prudencio and colleagues demonstrated that human (but not mouse) wild type SOD1 co-aggregates with the pathogenic SOD1 variants [21]. These differences in the aggregation propensity of human and mouse wild type SOD1 in these mouse and cell culture models is somewhat unexpected given that the two proteins are 84% identical in amino acid sequence.
The three-dimensional (3D) structures of homodimeric SOD1 proteins from yeast [22-24], frog [25], cow [26, 27], and human [28-30] have been determined and they are all conserved. In mammals, 112 of 153 residues conserved across species and 70 residues are invariant across eukaryotic phyla [31]. Sixty-one of the pathogenic mutations occur at residues conserved in mammals, with 49 occurring at positions that are “extremely conserved” [31]. Given the high degree of sequence and structural conservation found among eukaryotic SOD1 proteins, the structure of mouse SOD1 is also anticipated to be similar to those of known structure in which the SOD1 monomer folds as an eight-stranded Greek key β-barrel, binds one catalytic copper ion and one structurally important zinc ion, and harbors an intrasubunit disulfide bond.
In an effort to understand the molecular basis for how amino acid sequence differences in mouse and human SOD1 affect their aggregation propensities in mouse and cell culture models, we determined the crystal structures of wild type mouse SOD1 and two human/mouse chimeras in which residues 1-80 of human SOD1 were fused to residues 81-153 of mouse SOD1 and vice versa, were determined, refined, and compared. Analysis of these structures, together with previously determined structures of pathogenic SOD1 variants in the context of the aggregation data coming from the accompanying paper by Karch and colleagues [32], suggests a model in which amino acid residues Q42 and Q123 in mouse SOD1 modulate non-native SOD1-SOD1 intermolecular interactions that have been observed to give rise to higher order assemblies in pathogenic human SOD1 proteins [33, 34].
Materials and Methods
Materials
Pfu DNA polymerase and deoxyribonucleotides were purchased from Stratagene. Restriction enzymes (NcoI and SalI), RNAse, and DNA ligase were obtained from New England Biolabs. XL-1 Blue competent cells and BL-21 DE3 star cells were obtained from Invitrogen. Luria-Bertani (LB) media, sodium chloride, tris-HCl, ampicillin and sodium acetate were obtained from Fischer Scientific. The tris, HEPES and the PEG reagents used for crystallization came from Fluka. Protease inhibitor cocktails and the DNAase used in SOD1 purification were obtained from Sigma. Crystallization screening kits and crystal growth trays were purchased from Qiagen. His Hi-Trap HP, Hi-Trap Q and Sephacryl 100 columns were purchased from GE Healthcare. Precast 4-12 % gradient SDS PAGE and 12 % native gels were obtained from Invitrogen. Unless otherwise noted in the text, all solutions were prepared using deionized water passed through a Millipore ultra-purification system.
SOD1 Cloning, Expression, and Purification
DNA fragments encoding wild type mouse and human/mouse chimeric constructs were generated by the polymerase chain reaction (PCR) from plasmids generously supplied by Dr. David Borchelt, University of Florida. Wild type mouse SOD1 and the two chimeric SOD1 constructs (designated mouse-human and human-mouse SOD1, depending which species corresponds to the N-terminal 80 amino acids) were cloned into a pkA8H vector, derived from pkM265 [35], which contains an inducible LacZ promoter, and an N-terminal 8x-His tag that can be released from the target protein via a tobacco etch virus (TEV) protease cleavage site. SOD1 proteins were expressed in Escherichia coli strain BL21 DE3. Cells containing these expression plasmids were grown in LB media at 37 °C to an OD600 of 0.5-0.7. After induction with isopropyl-D-thiogalactopyranoside (IPTG), the cells were transferred to 22 °C for an additional 8 h before being harvested. This temperature shift results in an increased levels of recombinant SOD1 proteins in the soluble fraction. Cleared cell lysates were loaded onto a Ni2+-NTA His-Trap high performance column and eluted using a linear gradient from 0-250 mM imidazole. After purification, the 8x-His tag was removed from the SOD1 constructs using tobacco etch virus (TEV) protease produced in-house and engineered to contain its own noncleavable 6x-His tag. After digestion overnight at room temperature, TEV protease was removed from the sample by a final pass through the nickel column. The resulting SOD1 protein was concentrated, dialyzed into 50 mM Tris pH 8.0, and loaded onto a Source Q column by applying a linear gradient of 10-500 mM sodium chloride. Purified SOD1 proteins were dialyzed into 50 mM Tris pH 8.0 and 100 mM sodium chloride before crystallization trials. All purified SOD1 proteins were characterized by SDS PAGE gels, electrospray ionization mass spectrometry and inductively coupled plasma mass spectrometry (ICP-MS). ICP-MS was performed at the Chemical Analysis Laboratory at the University of Athens, Georgia.
Crystallization, Data Collection, Structure Determination, and Refinement
All crystals were grown at room temperature using the hanging drop vapor diffusion method with protein concentrations of 14 mg/mL for wild type mouse SOD1 and 18 mg/mL for human-mouse and mouse-human SOD1 chimeras. (Please note that these chimeric SOD1 proteins are designated N-H/C-M and N-M/C-H chimeras, respectively, in the accompanying paper by Karch et al. [32]). Pure protein in 50 mM Tris, pH 8.0, and 100 mM sodium chloride was mixed with an equal volume of reservoir solution containing 0.1 M Tris, pH 8.5 and 30% (w/v) poly ethylene glycol (PEG) 1000 for wild type mouse SOD1, 0.1 M sodium HEPES, pH 7.5 and 25% (w/v) PEG 8000 for the human-mouse SOD1 chimera, and 0.1 M sodium acetate, pH 4.6 and 25% PEG 4000 (w/v) for the mouse-human SOD1 chimera. Wild type mouse SOD1 and human-mouse SOD1 chimera crystals grew within one month while crystals of the mouse-human SOD1 chimera grew after approximately 2 months. The hexagonal plate C2221 crystal form and the bi-pyramidal P61 crystal form each contain three SOD1 dimers per asymmetric unit (AU), while the pyramidal P21 crystal form contains six dimers in the AU. Crystals suitable for single crystal diffraction experiments were soaked in a cryoprotectant solution consisting of mother liquor made 15% (v/v) in ethylene glycol before flash cooling in liquid nitrogen immediately prior to data collection. Native diffraction data for the C2221 and the P21 forms were taken to resolutions of 2.40 Å and 2.20 Å, respectively, at the X-ray Crystallography Core Laboratory at the University of Texas Health Science Center at San Antonio using a Rigaku MicroMax 007HF X-ray generator equipped with Rigaku HTC imaging plate detectors and VariMax high flux optics. Diffraction data from the P61 crystal form to 2.45 Å resolution were taken at the Advanced Photon Source, Argonne, IL, microfocus beam line ID-24-E. All diffraction data were processed using HKL2000 [36]. Five percent of the reflections were selected as a function of resolution bins using DATAMAN [37] and set aside during refinement for cross validation [38].
The three structures were determined by molecular replacement using the program MOLREP [39] with the structure of human G37R SOD1 (Protein Data Bank entry 1AZV [40]) as the search model. Rigid-body refinement using monomers as individual rigid elements was followed by iterative cycles of positional and atomic displacement parameter (B-factor) refinement including simulated annealing using PHENIX [41]. No stereochemical restraints were applied to the metal-ligand distances or bond angles. Due to the moderate resolution and the fact that there are 6 dimers in the AU, non-crystallographic symmetry restraints were imposed on the model of the mouse-human SOD1 chimera. XTRIAGE, a subroutine incorporated in the PHENIX suite, detected minor pseudo-merohedral twinning in the C2221 and P21 forms. The models were subsequently refined in PHENIX by applying the twinning operators 1/2h+1/2k, 3/2h-1/2k,-l for the C2221 form and l,-k,h for the P21 form. The final twinning fractions of the C2221 and the P21 forms refined to values of 0.14 and 0.12 respectively. All three models were adjusted manually into SIGMA-A-weighted electron density maps [42] using the program COOT [43]. Structure validation was performed with the Structural Analysis and Verification Server (SAVS) which incorporates Procheck [44] and Whatcheck [45]. A Ramachandran plot generated with Procheck reveals that the current models exhibit excellent geometries with no residues in disallowed regions. Atomic coordinates and diffraction data have been deposited in the Protein Data Bank with codes 3GTT for wild-type mouse SOD1, 3GTV for the human-mouse SOD1 chimera, and 3GTW for the mouse-human SOD1 chimera.
Figure Preparation
All figures were prepared using PyMOL (DeLano, W.L. The PyMOL Molecular Graphics System, 2002, DeLano Scientific, San Carlos, CA, USA. http://www.pymol.org).
Results
Crystal Structures of Mouse SOD1 and Human/Mouse SOD1 Chimeras
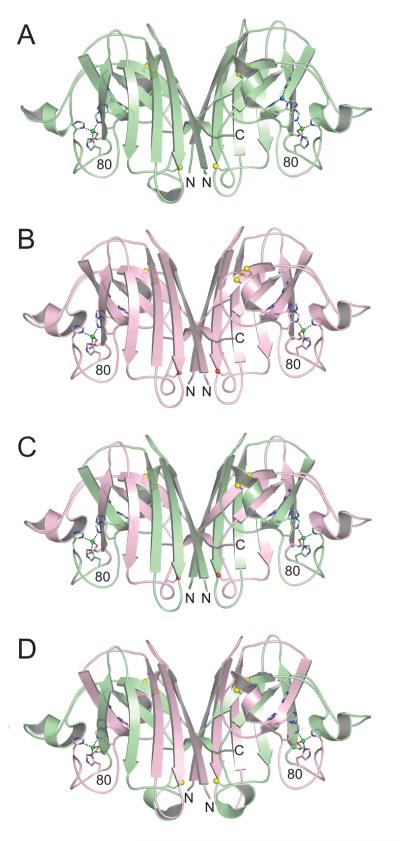
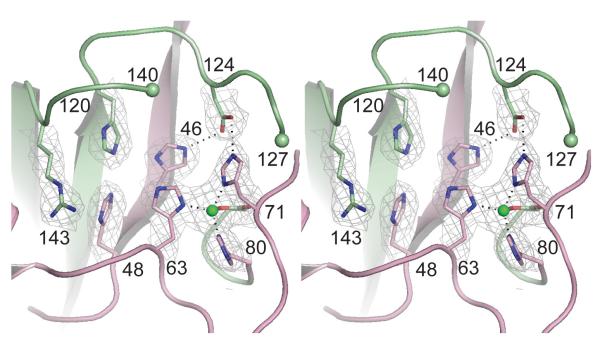
Crystal structures of wild type mouse SOD1, the human (residue 1-80)/mouse (81-153) SOD1 chimera, and the mouse (residue 1-80)/human (81-153) SOD1 chimera were determined and refined in space groups C2221, P21 and P61 to resolutions of 2.40, 2.20 and 2.45 Å, respectively. The X-ray diffraction data and protein structure refinement statistics are summarized in Table 1. In all three structures, each SOD1 monomer folds as an eight-stranded Greek key β-barrel in which the β-strands are interspersed by seven loop elements. Figure 1 shows the overall similarity in the structures of human wild type SOD1 (pdb code 2c9v, [30]), mouse wild type SOD1, the human/mouse SOD1 chimera, and the mouse/human SOD1 chimera SOD1. The color-coding indicates the amino acid residues/structural elements coming from the human and mouse SOD1 proteins that are fused to form the chimeric SOD1 proteins. Figure 2 shows that the copper-binding sites, formed by the side chains of H46, H48, H63, and H120, are unoccupied in all subunits of all three structures. Figure 2 also reveals that the zinc-binding sites, formed by the side chains of H63, H71, H80 and D83, coordinate zinc ions in a distorted tetrahedral geometry in all subunits of all three structures. The metal occupancies observed in these structures are consistent with the metal analyses obtained using ICP-MS.
Table 1.
X-ray diffraction data and refinement statistics for the SOD1 structures determined in this study.
| Mouse WT | Human/Mouse Chimera |
Mouse/Human Chimera |
|
|---|---|---|---|
| PDB deposition code | 3GTT | 3GTV | 3GTW |
| Data collection | |||
| Space Group | C2221 | P21 | P61 |
| Cell Dimensions | |||
| a, b, c (Å) | 112.5, 194.5, 149.8 | 112.6, 144.0, 112.6 | 127.5,127.5,102.5 |
| α, β, γ (°) | 90, 90, 90 | 90, 119.8, 90 | 90, 90, 120 |
| Resolution (Å) | 50.0-2.40 (2.49-2.40)* | 50.0-2.20 (2.28-2.20) | 50.0-2.45 (2.54-2.45) |
| λ (Å) | 1.54 | 1.54 | 0.98 |
| Rsym (on I) (%) | 10.5 (46.3) | 11.1 (47.6) | 10.3 (55.0) |
| I/ σI | 11.5 (2.8) | 8.9 (2.5) | 14.5 (3.3) |
| Completeness (%) | 99.9 (100.0) | 94.0 (90.0) | 98.7 (99.7) |
| Redundancy | 4.2 (4.0) | 3.4 (3.2) | 5.0 (5.0) |
| Refinement | |||
| Resolution (Å) | 50.0-2.40 | 50.0-2.02 | 50.0-2.45 |
| No. reflections | 64,174 | 1,48,404 | 34,219 |
| Rwork/ Rfree (%) | 15.82/19.69 | 18.38/23.05 | 19.53/26.49 |
| Number of dimers/AU | 3 | 6 | 3 |
| Number of atoms | |||
| Protein | 6654 | 13,397 | 6591 |
| Metal ions | 6 (Zn) | 12 (Zn) | 6 (Zn) |
| Water | 721 | 1808 | 145 |
| Stereochemistry | |||
| Bond lengths (Å) | 0.006 | 0.008 | 0.009 |
| Bond angles (°) | 1.076 | 1.171 | 1.178 |
Values in parentheses represent the highest resolution bin.
Figure 1.
Structures of human wild type SOD1, mouse wild type SOD1, human (1-80)/mouse (81-153) chimeric SOD1, and mouse (1-80)/human (81-153) chimeric SOD1 in equivalent orientations. A) Human wild type SOD1 homodimer (pdb code 2C9V [30]). The copper and zinc ions are shown as blue and green spheres, respectively. The side chain sulfur atoms of C57, C111, and C146 are shown as yellow spheres. B) Mouse wild type SOD1. The copper-binding sites are devoid of copper, but the zinc-binding sites contain zinc (see text and Figure 2). The side chain oxygen atoms of S111 are shown as red spheres. C) Human (1-80)/mouse (81-153) chimeric SOD1. The color coding is as in panels A) and B), with amino acid residues corresponding to human SOD1 in green and mouse SOD1 in pink. D) Mouse (1-80)/human (81-153) chimeric SOD1. The color coding is as in previous panels.
Figure 2.
Divergent stereo view of the metal-binding sites in subunit A of the mouse (1-80)/human (81-153) chimeric SOD1 structure. The 2.45 Å resolution SIGMA-A-weighted electron density, with coefficients 2mFo-DFc is contoured at 1.2 σ. Zinc ions are shown as green spheres and zinc-ligand bonds and hydrogen bonds between D124 and the nonliganding imidazole nitrogen atoms of copper ligand H46 and H71 are shown as dotted lines. The copper-binding site is empty (see text). This metal-binding site is representative of what is observed in all three structures reported here.
Comparison of Human and Mouse Wild Type SOD1 Structures
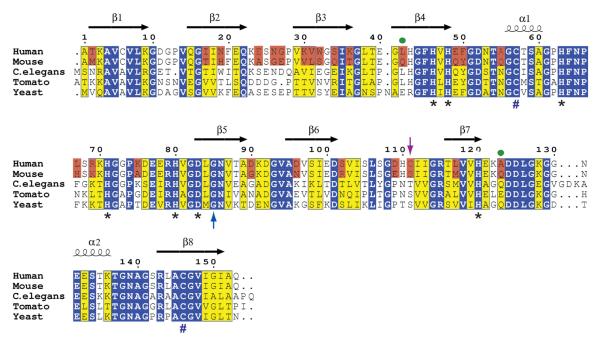
The overall fold of the mouse SOD1 structure is nearly identical to that of human SOD1, with the monomeric subunits coming from the two species aligning with an average root mean square (rms) deviation of only for backbone atoms. This degree of overall structural similarity is not surprising given that only 25 out of 153 amino acids differ between mouse and human SOD1. These differences in amino acid sequence are shown in Figures 3 and 4. A comparison of the active site and the disulfide loop regions of mouse and human SOD1 reveal that they are also conserved, both in terms of sequence and in terms of side chain orientation.
Figure 3.
Structure-based amino acid sequence alignment of SOD1 proteins from human, mouse, worm, tomato and yeast. The initial alignment of these sequences using BLAST , was subsequently modified using the program ESPript [61]. Secondary structural elements are indicated as calculated in DSSP [62] for the structure of wild type human SOD1 (pdb code 2C9V [30]). Human and mouse SOD1 are 84% in sequence, differing at 25 of 153 amino acids, which are boxed in pink. Residues that are invariant in all five sequences are boxed in blue. Homologous residues are boxed in yellow. Asterisks (*) denote amino acid residues that serve as ligands to copper and zinc ions in the holoenzyme. Pound signs (#) represent the positions of cysteine residues that form the intrasubunit disulfide bond. The blue upward arrow highlights the position of G85 and the purple downward arrow highlights the positions of C111 and S111 (see text). Green dots highlight residues in mouse SOD1 that are observed to modulate its aggregation in a cell-based assay [32] (see text).
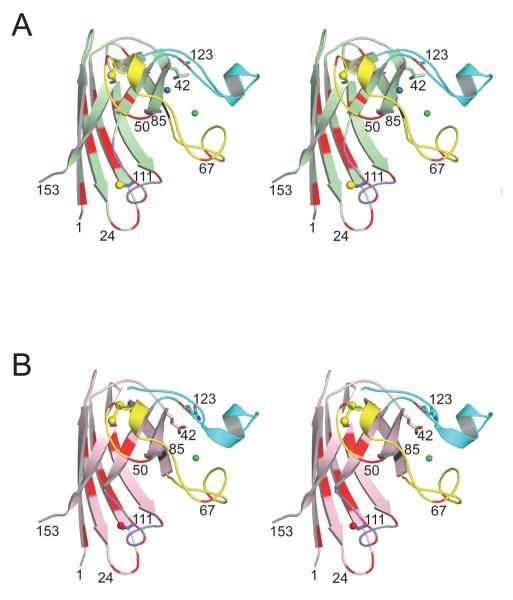
Figure 4.
Divergent stereo views of the human and mouse SOD1 monomers indicating the positions of their side chain differences in their 3D structures. A) Human SOD1 (pdb code 2C9V [30]). The zinc loop (loop IV, residues 49-83), the Greek key loop (loop VI, residues 101-115), and the electrostatic loop (loop VII, residues 121-142) are shown in yellow, magenta, and cyan, respectively. The copper and zinc ions are shown as blue and green spheres, respectively. Sulfur atoms coming from Cys57, Cys111, and Cys146 are shown as yellow spheres. The side chains of residues 42 and 123 are shown as sticks (see text and Figure 5). B) Mouse SOD1. The color coding is as in panel A), except that the red sphere represents the side chain oxygen atom of Ser111.
Novel Side Chain Interactions in Wild Type Mouse SOD1
The Mouse wild type SOD1 structure reveals several side chain interactions that are absent in the human SOD1 structure. For example, hydrogen bonding interactions are observed between the side chain amide moieties of Q42 in β-strand 4 and Q123 in β-strand 7 as well as between Q34 in β-strand 3 and N96 in β-strand 6. The side chain hydroxyl of Y50 located at the homodimeric interface of mouse SOD1 accepts a hydrogen bond from the carboxyl oxygen of E49. In addition, the guanidium nitrogen atoms of R102 donate hydrogen bonds to the side chain oxygen atoms of E27 and E100. Unlike human SOD1, mouse SOD1 possesses two methionine residues. Mouse SOD1 residue M2 forms weak hydrogen bonds with the side chain hydroxyl moieties of S25 and S107. Mouse SOD1 residue M117, which is a Val in human SOD1, is buried in a hydrophobic pocket formed by residues coming from between β-strands 4 and 7.
Discussion
Co-Aggregation of Mouse and Human Wild Type SOD1 with Mutant SOD1
As shown in Figures 3 and 4, mouse and human wild type SOD1 are 84% identical in amino acid sequence, differing at only 25 of 153 amino acids. Given this high degree of sequence identity, it might be expected that mouse SOD1 would be prone to co-aggregate with mutant human SOD1 in transgenic mice and in cell culture. However, immunoblot and/or shotgun mass spectrometry proteomic analyses of the detergent insoluble fractions coming from spinal cord lysates of transgenic mice expressing the pathogenic human SOD1 variants G37R, G93A, and H46R/H48Q, revealed that endogenous mouse SOD1 was not a component of these aggregates [18, 19]. In contrast, human wild type SOD1, when co-expressed in mice with the pathogenic SOD1 variants A4V, G85R, G93A, and L126Z, was found co-aggregated with the pathogenic SOD1 variants in the detergent insoluble fractions of spinal cord lysates coming from these animals [8, 20]. In addition, the presence of human wild type SOD1 seemed to exacerbate the toxicity of the pathogenic SOD1 variants as evidenced by an accelerated disease course [8, 20]. These results were mirrored in cell culture experiments by Prudencio and colleagues in which mouse and human wild type SOD1 was co-expressed with pathogenic human SOD1 variants and human wild type SOD1 was found to co-aggregate with the mutant SOD1 variants while mouse wild type SOD1 was not [46] These results suggest that there must be sequence differences in mouse and human SOD1 that are responsible for the modulation of the co-aggregation of these proteins with human pathogenic SOD1 variants in these systems.
Structures of Mouse and Human/Mouse SOD1 Chimeras
In an effort to better understand the similarities and differences between mouse and human SOD1 and their different aggregation behaviors in the cell culture and murine models, and because the structure of mouse wild type SOD1 was previously unknown, we determined the structures of wild–type mouse SOD1 and two human/mouse chimeras in which residues 1-80 of human SOD1 were fused to residues 81-153 of mouse SOD1 and vice versa. Figure 1 shows that these three structures are very similar in their overall fold to the human SOD1 structures characterized earlier, an observation that correlates well with the high degree of sequence identity between mouse and human SOD1. As anticipated from the sequence alignment shown in Figure 3, all the critical features required for maintaining the structural integrity and proper functioning of the SOD1 enzyme are conserved (Figure 4).
The copper-binding sites in the three SOD1 structures determined in this study are completely devoid of copper (Figure 2), which likely occurs because these proteins were expressed in a bacterial expression system in the absence of exogenously added copper salts. It should also be noted that the E. coli expression system used in this study lacks the copper chaperone for SOD1 (CCS), a helper protein found in most eukaryotes that inserts the catalytic copper ion into newly translated SOD1 proteins and oxidizes the SOD1 intrasubunit bond [47, 48]. Because the structural integrity of the copper-binding sites in these structures are maintained, if copper were to be incorporated into these sites, these enzymes would be anticipated to be fully active. It should be stressed, however, the absence of copper ion in the copper-binding site does not have a significant effect on the overall 3D structure of SOD1, as it has been previously demonstrated that the binding of zinc exerts the largest effect by inducing order in the zinc loop (loop IV, residues 49-83) and the electrostatic loop (loop VII, residues 121-142) [49, 50]. Indeed, SOD1 proteins harboring a metal ion in the copper-binding site and possessing an empty zinc-binding site still demonstrate conformational disorder in their zinc and electrostatic loop elements and remain capable of forming higher order filamentous structures [34] (see below).
Specific Residues in Mouse SOD1 Protect Against Co-Aggregation in Cell Culture
In the accompanying paper, Karch and colleagues studied the aggregation propensities of chimeric SOD1 constructs containing stretches of human and mouse sequences in a highly aggregating G85R SOD1 background in an effort to identify structural elements that might modulate the aggregation of these proteins [32]. They found that the G85R mutation in a chimeric SOD1 protein in which residues 1-80 were of human origin and residues 81-153 were of mouse origin was not very prone to aggregation. However, if residues 109-123 of this chimeric protein were restored to the human SOD1 sequence, the G85R mutation induced rapid aggregation (see Figures 5 and 7 in [32]). Subsequent mutational analyses suggested that there exists crosstalk between the human Greek key loop element (109-123) and residues in β-strand 4 (42-50) and that C111 also modulates the aggregation propensity of SOD1 [32].
Model for Protection of Mouse SOD1 From Co-Aggregation with Mutant Human SOD1
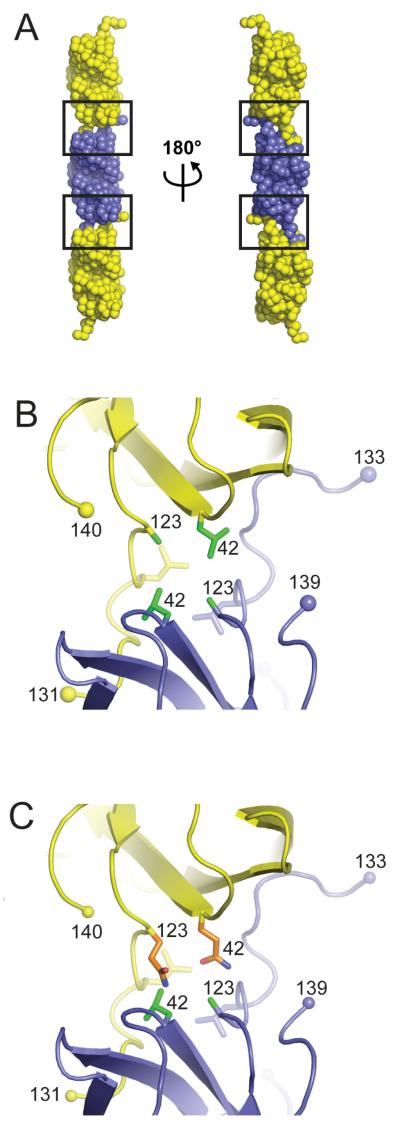
Earlier structural work from our laboratory revealed that the metal-deficient human SOD1 variants H46R and S134N can engage in non-native SOD1-SOD1 protein-protein interactions that give rise to higher order filamentous arrays [33, 34]. Recently, non-native intermolecular SOD1-SOD1 interactions nearly identical to those observed for the H46R and S134N variants have also been observed in the pathogenic human SOD1 variant H80R, which contains a metal ion in the copper-binding site but possesses an ablated zinc-binding site [34]. As shown in Figure 5A, these higher order arrays of SOD1 are formed by reciprocal interactions between residues of the electrostatic loop coming from one SOD1 subunit with the edge strands of the Greek key β-barrel from a neighboring SOD1 subunit, resulting in a new, robust interface that has been referred to as a “gain-of-interaction interface”, which has been proposed to underlie the “toxic gain-of-function” ascribed to pathogenic SOD1 in SOD1-linked ALS [33, 34, 51].
Figure 5.
Tthe gain-of-interaction interfaces observed in crystal structures of metal deficient pathogenic SOD1 proteins H46R [33], H80R [34], S134N [33] and in human SOD1 proteins engineered to possess an ablated zinc-binding site [51, 53]. A) The metal-deficient SOD1 proteins assemble end-to-end, with residues of the electrostatic loop (loop VII) of each protein making contact with the exposed edges of β-strands 5 and 6 as well as the depression between these strands. The left and right filament in this panel are related as shown. The gain-of-interaction interfaces are boxed. B) Reciprocal apolar interactions between residues L42, A123 (both in green), and L126 at the gain-of-interaction interface coming from SOD1 subunits in adjacent dimers in the filamentous arrays. This panel is an enlargement of the boxed region shown in the same orientation as the leftmost filament in panel A). C) Mouse SOD1 residues Q42 and Q123 (both in orange) are not compatible with the gain-of-interaction interface found in human pathogenic SOD1 proteins and would destabilize the gain-of-interaction interface due to steric clashes and differences in polarity relative to human SOD1 residues L42 and A123 (both in green).
The absence of metal ions bound at the zinc-binding site is a prerequisite for these non-native intermolecular SOD1-SOD1 interactions, as the lack of a metal ion at this site results in conformational flexibility of the zinc loop (loop IV, residues 49-83) and electrostatic loop (loop VII, residues 121-142) elements. In this context, it is notable that the highly aggregating G85R SOD1 mutation used in the Karch et al study alluded to above [32], which is immediately adjacent to the zinc ligand D83, results in a SOD1 protein with a dramatically lowered affinity for zinc [52]. The conformational flexibility in these loop elements as a consequence of metal ion deficiency exposes the edges of β-strands 5 and 6 to the solvent, permitting the close approach of two SOD1 proteins and allowing residues 124-139 of the electrostatic loop to adopt an extended conformation in which they reach out to interact with the edge strands of the two β-sheets in the SOD1 Greek key β-barrel of a neighboring SOD1 protein in the crystal lattice [33, 34, 51, 53]. This close packing of the β-barrels cannot occur when the proteins possess zinc and electrostatic loops in their well-ordered, metal-bound conformations, as superposition of holoSOD1 dimers onto mutant dimers comprising the filamentous arrays shown in Figure 5 reveals that there exists steric and electrostatic repulsion from zinc loop residues D76 and E77, precluding formation of the gain-of-interaction interface [33]. These observations, together with observations that pathogenic SOD1 proteins isolated from the soluble fraction of spinal cord lysates of transgenic mice expressing these proteins tend to be metal-deficient, disulfide-reduced, or both [16, 17], have led to the suggestion that immature pathogenic SOD1 variants that fail to be fully posttranslationally modified may represent a noxious species in SOD1-linked ALS [7, 34, 54-57].
As shown in Figure 5B, the heart of this non-native gain-of-interaction interface between pathogenic human SOD1 mutants in the filamentous arrays is an apolar core formed by reciprocal interactions around a pseudo-dyad by human SOD1 residues L42, A123, and L126 coming from each participating subunit. The solvent accessible surface area buried at this interface is greater than 600 Å2 per polypeptide, approximately the same that is buried per polypeptide at the naturally occurring, robust homodimeric interface in wild type SOD1 [33]. As shown in Figure 5C, when the corresponding mouse SOD1 residues Q42 and Q123 are substituted for their L42 and A123 human counterparts in one subunit of the gain-of-interaction interface, they are predicted to disrupt the hydrophobic core of this interface, thereby inhibiting this mode of mouse SOD1-human SOD1 interaction. This model, while speculative, offers a rationalization of the robust aggregation endowed upon the human/mouse chimera observed in the Karch et al. study when human residues 109-123 are reinserted into the mouse C-terminal section of the human-mouse chimeric SOD1 protein and it also offers an explanation for the partial protection against aggregation through the insertion of residues 42-50 from mouse into the human N-terminal section of the strongly aggregating construct [see Figures 5 and 7 of Karch et al. [32]]. We and others have suggested that the non-native interactions found at the gain-of-interaction interface shown in Figure 5 might initiate pathogenic SOD1 self-association, and these initial interactions may or may not persist in the insoluble aggregates, which have been demonstrated to become crosslinked via C111 at an as yet undetermined stage of the aggregation process [51, 58, 59]. The role of C111 in pathogenic SOD1 aggregation is discussed at length in references [32] and [59].
Although murine SOD1 G86R is pathogenic and aggregates when overexpressed in transgenic mice [60], it should be stressed that the structural model presented here pertains only to the protection against coaggregation of mouse SOD1 with the human enzyme and does not address the self-aggregation of mouse G86R. Indeed, Figure 6 in the accompanying paper by Karch and colleagues [32] reveals that mouse G86R SOD1 is considerably less aggregation-prone than human G85R SOD1, which in turn suggests that there are differences between mouse and human SOD1 self-aggregation that we currently do not fully understand.
In summary, the three new structures determined in this study, together with differences in mouse and human SOD1 amino acid sequence, were analyzed in the context of the aggregation data presented in the accompanying paper by Karch et al. [32] and in the context of the structures of previously determined pathogenic SOD1 proteins that are observed to assemble into higher order filamentous arrays [33]. The results of this analysis permit the aggregation data to be rationalized, and lead us to present a structure-based model in which amino acid residues Leu42 and Ala123 in human SOD1 play a key role in human pathogenic SOD1 aggregation.
Acknowledgements
†This work was supported by grants from the NIH-NINDS R01-NS39112 (to PJH), P01-NS04913 (to JSV, DRB, and PJH). SVS was supported in part by the William and Ella Owens Medical Research Foundation and the Judith and Jean Pape Adams Charitable Foundation. Support for the X-ray Crystallography Core Laboratory by the UTHSCSA Executive Research Committee and the San Antonio Cancer Institute is also gratefully acknowledged. We thank Celeste Karch, Mercedes Prudencio, and David Borchelt for continued collaboration and invaluable insight into the biological aspects of aggregation of SOD1 and the SOD1 chimera structures described in this paper.
Abbreviations
- ALS
amyotrophic lateral sclerosi
- SOD1
copper-zinc superoxide dismutase
- 3D
three-dimensional
- LB
Luria-Bertani media
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- PEG
polyethylene glycol
- SDS PAGE
sodium dodecyl sulfate polyacrylamide electrophoresis
- PCR
polymerase chain reaction
- TEV
tobacco etch virus
- IPTG
isopropyl-D-thiogalactopyranoside
- ICP-MS
inductively coupled plasma mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Atomic coordinates and structure factors have been deposited in the Protein Data Bank (entries 3GTT, 3GTV, and 3GTW).
References
- [1].Rowland LP, Shneider NA. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- [2].Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, Warner C, Deng G, Soriano E, Smyth C, Parge HE, Ahmed A, Roses AD, Hallewell RA, Pericak-Vance MA, Siddique T. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- [3].Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- [4].Bruijn LI, Miller TM, Cleveland DW. Ann. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- [5].Hart PJ. Curr. Opin. Chem. Biol. 2006;10:131–138. doi: 10.1016/j.cbpa.2006.02.034. [DOI] [PubMed] [Google Scholar]
- [6].Valentine JS. Ann. Rev. Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- [7].Seetharaman SV, Prudencio M, Karch C, Holloway SP, Borchelt DR, Hart PJ. Exp. Biol. Med. 2009;234:1140–1154. doi: 10.3181/0903-MR-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deng H-X, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O’Halloran TV, Siddique T. Proc. Natl. Acad. Sci. USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- [10].Sasaki S, Nagai M, Aoki M, Komori T, Itoyama Y, Iwata M. J. Neuropathol. Exp. Neurol. 2007;66:517–524. doi: 10.1097/01.jnen.0000263868.84188.3b. [DOI] [PubMed] [Google Scholar]
- [11].Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- [12].Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- [13].Wang J, Xu G, Li H, Gonzales V, Fromholt D, Karch C, Copeland NG, Jenkins NA, Borchelt DR. Hum. Mol. Genet. 2005;14:2335–2347. doi: 10.1093/hmg/ddi236. [DOI] [PubMed] [Google Scholar]
- [14].Watanabe Y, Yasui K, Nakano T, Doi K, Fukada Y, Kitayama M, Ishimoto M, Kurihara S, Kawashima M, Fukuda H, Adachi Y, Inoue T, Nakashima K. Mol. Brain Res. 2005;135:12–20. doi: 10.1016/j.molbrainres.2004.11.019. [DOI] [PubMed] [Google Scholar]
- [15].Jonsson PA, Ernhill K, Andersen PM, Bergemalm D, Brannstrom T, Gredal O, Nilsson P, Marklund SL. Brain. 2004;127:73–88. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- [16].Jonsson PA, Karin SG, Peter MA, Thomas B.n.m., Mikael L, Mikael O. Brain. 2006;129:451–464. [Google Scholar]
- [17].Zetterstrom P, Heather GS, Daniel B, Jonsson PA, Karin SG, Peter MA. Proc. Natl. Acad. Sci. USA. 2007;104:14157–14162. doi: 10.1073/pnas.0700477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Hum. Mol. Genet. 2003;12:2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- [19].Lambrechts D, Poesen K, Fernandez-Santiago R, Al-Chalabi A, Del Bo R, Van Vught PW, Khan S, Marklund SL, Brockington A, van Marion I, Anneser J, Shaw C, Ludolph AC, Leigh NP, Comi GP, Gasser T, Shaw PJ, Morrison KE, Andersen PM, Van den Berg LH, Thijs V, Siddique T, Robberecht W, Carmeliet P. J. Med. Genet. 2009;46:840–846. doi: 10.1136/jmg.2008.058222. [DOI] [PubMed] [Google Scholar]
- [20].Wang L, Deng HX, Grisotti G, Zhai H, Siddique T, Roos RP. Hum. Mol. Genet. 2009;18:1642–1651. doi: 10.1093/hmg/ddp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Prudencio M, Durazo A, Whitelegge JP, Borchelt DR. J. Neurochem. 2009;108:1009–1018. doi: 10.1111/j.1471-4159.2008.05839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Djinovic K, Gatti G, Coda A, Antolini L, Pelosi G, Desideri A, Falconi M, Marmocchi F, Rotilio G, Bolognesi M. J. Mol. Biol. 1992;225:791–809. doi: 10.1016/0022-2836(92)90401-5. [DOI] [PubMed] [Google Scholar]
- [23].Hart PJ, Balbirnie MM, Ogihara NL, Nersissian AM, Weiss MS, Valentine JS, Eisenberg D. Biochemistry. 1999;38:2167–2178. doi: 10.1021/bi982284u. [DOI] [PubMed] [Google Scholar]
- [24].Ogihara NL, Parge HE, Hart PJ, Weiss MS, Goto JJ, Crane BR, Tsang J, Slater K, Roe JA, Valentine JS, Eisenberg D, Tainer JA. Biochemistry. 1996;35:2316–2321. doi: 10.1021/bi951930b. [DOI] [PubMed] [Google Scholar]
- [25].Djinovic-Carugo K, Battistoni A, Carri MT, Polticelli F, Desideri A, Rotilio G, Coda A, Wilson KS, Bolognesi M. Acta Crystallogr. 1996;D52:176–188. doi: 10.1107/S0907444995007608. [DOI] [PubMed] [Google Scholar]
- [26].Rypniewski WR, Mangani S, Bruni B, Orioli PL, Casati M, Wilson KS. J. Mol. Biol. 1995;251:282–296. doi: 10.1006/jmbi.1995.0434. [DOI] [PubMed] [Google Scholar]
- [27].Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC. J. Mol. Biol. 1982;160:181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- [28].Banci L, Bertini I, Cantini F, D’Amelio N, Gaggelli E. J. Biol. Chem. 2006;281:2333–2337. doi: 10.1074/jbc.M506497200. [DOI] [PubMed] [Google Scholar]
- [29].Parge HE, Hallewell RA, Tainer JA. Proc. Natl. Acad. Sci. USA. 1992;89:6109–6113. doi: 10.1073/pnas.89.13.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Strange RW, Antonyuk SV, Hough MA, Doucette PA, Valentine JS, Hasnain SS. J. Mol. Biol. 2006;356:1152–1162. doi: 10.1016/j.jmb.2005.11.081. [DOI] [PubMed] [Google Scholar]
- [31].Wang J, Xu G, Borchelt DR. J. Neurochem. 2006;96:1277–1288. doi: 10.1111/j.1471-4159.2005.03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karch C, Borchelt DR. Arch. Biochem. Biophys. 2010;XX:XX–YY. doi: 10.1016/j.abb.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Elam JS, Taylor AB, Strange R, Antonyuk S, Doucette PA, Rodriguez JA, Hasnain SS, Hayward LJ, Valentine JS, Yeates TO, Hart PJ. Nat. Struct. Biol. 2003;10:461–467. doi: 10.1038/nsb935. [DOI] [PubMed] [Google Scholar]
- [34].Seetharaman SV, Winkler DD, Taylor AB, Cao X, Whitson LJ, Doucette PA, Valentine JS, Schirf V, Demeler B, Carroll MC, Culotta VC, Hart PJ. Biochemistry. 2010;49:5714–5725. doi: 10.1021/bi100314n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Melcher K. Anal. Biochem. 2000;277:109–120. doi: 10.1006/abio.1999.4383. [DOI] [PubMed] [Google Scholar]
- [36].Otwinowski Z, Minor W. Meth. Enzymol. 1997;276:306–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- [37].Kleywegt GJ, Jones TA. Acta Crystallogr. 1996;D 52:826–828. doi: 10.1107/S0907444995014983. [DOI] [PubMed] [Google Scholar]
- [38].Brünger AT. In: Meth Enzymol. Carter CWJ, Sweet RM, editors. Academic Press; New York: 1997. pp. 366–396. [Google Scholar]
- [39].Vagin AA, Teplyakov A. J. Appl. Cryst. 1997;30:1022–1025. [Google Scholar]
- [40].Hart PJ, Liu H, Pellegrini M, Nersissian AM, Gralla EB, Valentine JS, Eisenberg D. Protein Sci. 1998;7:545–555. doi: 10.1002/pro.5560070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. Acta Crystallogr. 2002;D58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- [42].Read RJ. Acta Crystallogr. 1986;A42:140–149. [Google Scholar]
- [43].Emsley P, Cowtan K. Acta Crystallogr. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- [44].Laskowski RA, McArthur MW, Moss DS, Thornton JM. J. Appl. Crystallog. 1993;26:283–291. [Google Scholar]
- [45].Vriend G. J. Mol. Graph. 1990;8:52–56. 29. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- [46].Prudencio M, Hart PJ, Borchelt DR, Andersen PM. Hum. Mol. Genet. 2009;18:3217–3226. doi: 10.1093/hmg/ddp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. J. Biol. Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- [48].Furukawa Y, Torres AS, O’Halloran TV. Embo J. 2004;23:2872–2881. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Potter SZ, Zhu H, Shaw BF, Rodriguez JA, Doucette PA, Sohn SH, Durazo A, Faull KF, Gralla EB, Nersissian AM, Valentine JS. J. Am. Chem. Soc. 2007;129:4575–4583. doi: 10.1021/ja066690+. [DOI] [PubMed] [Google Scholar]
- [50].Strange RW, Antonyuk S, Hough MA, Doucette PA, Rodriguez JA, Hart PJ, Hayward LJ, Valentine JS, Hasnain SS. J. Mol. Biol. 2003;328:877–891. doi: 10.1016/s0022-2836(03)00355-3. [DOI] [PubMed] [Google Scholar]
- [51].Nordlund A, Leinartaite L, Saraboji K, Aisenbrey C, Grobner G, Zetterstrom P, Danielsson J, Logan DT, Oliveberg M. Proc. Natl. Acad. Sci. USA. 2009;106:9667–9672. doi: 10.1073/pnas.0812046106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cao X, Antonyuk SV, Seetharaman SV, Whitson LJ, Taylor AB, Holloway SP, Strange RW, Doucette PA, Valentine JS, Tiwari A, Hayward LJ, Padua S, Cohlberg JA, Hasnain SS, Hart PJ. J. Biol. Chem. 2008;283:16169–16177. doi: 10.1074/jbc.M801522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Roberts BR, Tainer JA, Getzoff ED, Malencik DA, Anderson SR, Bomben VC, Meyers KR, Karplus PA, Beckman JS. J. Mol. Biol. 2007;373:877–890. doi: 10.1016/j.jmb.2007.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Furukawa Y, O’Halloran TV. Antiox. Redox. Sig. 2006;8:847–867. doi: 10.1089/ars.2006.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Furukawa Y, Kaneko K, Yamanaka K, O’Halloran TV. N. Nukina J. Biol. Chem. 2008;283:24167–24176. doi: 10.1074/jbc.M802083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Winkler D, Schuermann J, Cao X, Holloway S, Borchelt D, Carroll M, Proescher J, Culotta V, Hart P. Biochemistry. 2009;48:3436–3477. doi: 10.1021/bi8021735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Winkler DD, Prudencio M, Karch C, Borchelt DR, Hart PJ. In: Protein Misfolding Diseases: Current and Emerging Prinicples. Dobson CM, Kelly JW, Ramirez-Alvarado M, editors. John Wiley & Sons, Inc.; Hoboken: 2009. [Google Scholar]
- [58].Elam JS, Malek K, Rodriguez JA, Doucette PA, Taylor AB, Hayward LJ, Cabelli DE, Valentine JS, Hart PJ. J. Biol. Chem. 2003;278:21032–21039. doi: 10.1074/jbc.M300484200. [DOI] [PubMed] [Google Scholar]
- [59].Karch CM, Borchelt DR. J. Biol. Chem. 2008;283:13528–13537. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW. Proc. Natl. Acad. Sci. USA. 1995;92:689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gouet P, Robert X, Courcelle E. Nucleic Acids Res. 2003;31:3320–3323. doi: 10.1093/nar/gkg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hooft RW, Vriend G, Sander C, Abola EE. Nature. 1996;381:272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]