Abstract
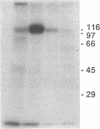
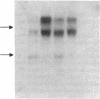
We have previously reported that the amino acid sequence of the common acute lymphoblastic leukemia antigen (CALLA, CD10) translated from a normal human kidney cDNA clone is identical to that of neutral endopeptidase (NEP, EC 3.4.24.11). In this study, we show that by flow cytometry, a monoclonal antibody (135A3) produced against rabbit NEP reacted selectively with leukemia and melanoma cell lines expressing CALLA on their surface. A glycoprotein of apparent Mr 100,000 was immunoprecipitated from surface labeled NALM-1 leukemia or Mel-1477 melanoma cells with monoclonal antibodies to NEP (135A3) or CALLA (44C10). mRNAs hybridizing to a NEP-specific probe were present in CALLA+ leukemia and melanoma cell lines, but absent from CALLA- lines. NEP enzymatic activity was detected on intact cells from CALLA+ lines, but not CALLA- lines. The activity was blocked by two selective inhibitors of NEP, thiorphan and phosphoramidon. CALLA antigen purified from the NALM-6 leukemic cell line by affinity to 44C10-IgG Sepharose retained a peptidase activity that was completely blocked by thiorphan and phosphoramidon. Thus the CALLA antigen present at the surface of leukemia and melanoma cell lines is an enzymatically active neutral endopeptidase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benchetrit T., Bissery V., Mornon J. P., Devault A., Crine P., Roques B. P. Primary structure homologies between two zinc metallopeptidases, the neutral endopeptidase 24.11 ("enkephalinase") and thermolysin, through clustering analysis. Biochemistry. 1988 Jan 26;27(2):592–596. doi: 10.1021/bi00402a014. [DOI] [PubMed] [Google Scholar]
- Bowes M. A., Kenny A. J. An immunohistochemical study of endopeptidase-24.11 and aminopeptidase N in lymphoid tissues. Immunology. 1987 Feb;60(2):247–253. [PMC free article] [PubMed] [Google Scholar]
- Bowes M. A., Kenny A. J. Endopeptidase-24.11 in pig lymph nodes. Purification and immunocytochemical localization in reticular cells. Biochem J. 1986 Jun 15;236(3):801–810. doi: 10.1042/bj2360801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. P., Martin P. J., Ledbetter J. A., Hansen J. A. Granulocytes and cultured human fibroblasts express common acute lymphoblastic leukemia-associated antigens. Blood. 1983 Apr;61(4):718–725. [PubMed] [Google Scholar]
- Carrel S., Accolla R. S., Carmagnola A. L., Mach J. P. Common human melanoma-associated antigen(s) detected by monoclonal antibodies. Cancer Res. 1980 Jul;40(7):2523–2528. [PubMed] [Google Scholar]
- Carrel S., Buchegger F., Heumann D., Girardet C., Barras C., Losa G., Mach J. P., von Fliedner V. Detection of the common acute lymphoblastic leukemia antigen in the serum of leukemia patients. J Clin Invest. 1984 Nov;74(5):1882–1885. doi: 10.1172/JCI111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel S., De Tribolet N., Gross N. Expression of HLA-DR and common acute lymphoblastic leukemia antigens on glioma cells. Eur J Immunol. 1982 Apr;12(4):354–357. doi: 10.1002/eji.1830120418. [DOI] [PubMed] [Google Scholar]
- Carrel S., Salvi S., Giuffrè L., Isler P., Cerottini J. C. A novel 90-kDa polypeptide (Tp90) possibly involved in an antigen-independent pathway of T cell activation. Eur J Immunol. 1987 Jun;17(6):835–841. doi: 10.1002/eji.1830170616. [DOI] [PubMed] [Google Scholar]
- Carrel S., Schmidt-Kessen A., Mach J. P., Heumann D., Girardet C. Expression of common acute lymphoblastic leukemia antigen (CALLA) on human malignant melanoma cell lines. J Immunol. 1983 May;130(5):2456–2460. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossman J., Neckers L. M., Leonard W. J., Greene W. C. Polymorphonuclear neutrophils express the common acute lymphoblastic leukemia antigen. J Exp Med. 1983 Mar 1;157(3):1064–1069. doi: 10.1084/jem.157.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin D., Sassi A., Roques B. P. A highly sensitive fluorometric assay for "enkephalinase," a neutral metalloendopeptidase that releases tyrosine-glycine-glycine from enkephalins. Anal Biochem. 1984 Aug 15;141(1):62–69. doi: 10.1016/0003-2697(84)90425-1. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Todd R. F., 3rd Immunologic classification of leukemia and lymphoma. Blood. 1986 Jul;68(1):1–31. [PubMed] [Google Scholar]
- Gafford J. T., Skidgel R. A., Erdös E. G., Hersh L. B. Human kidney "enkephalinase", a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983 Jun 21;22(13):3265–3271. doi: 10.1021/bi00282a035. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Bowes M. A., Buck P., Kenny A. J. An immunoradiometric assay for endopeptidase-24.11 shows it to be a widely distributed enzyme in pig tissues. Biochem J. 1985 May 15;228(1):119–126. doi: 10.1042/bj2280119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Matsas R., Kenny A. J. A monoclonal antibody to kidney endopeptidase-24.11. Its application in immunoadsorbent purification of the enzyme and immunofluorescent microscopy of kidney and intestine. Biochem J. 1983 Aug 15;214(2):377–386. doi: 10.1042/bj2140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrè L., Schreyer M., Mach J. P., Carrel S. Cyclic AMP induces differentiation in vitro of human melanoma cells. Cancer. 1988 Mar 15;61(6):1132–1141. doi: 10.1002/1097-0142(19880315)61:6<1132::aid-cncr2820610613>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G., Rapson N. T., Lister T. A. Antisera to acute lymphoblastic leukemia cells. Clin Immunol Immunopathol. 1975 May;4(1):67–84. doi: 10.1016/0090-1229(75)90041-0. [DOI] [PubMed] [Google Scholar]
- Hokland P., Rosenthal P., Griffin J. D., Nadler L. M., Daley J., Hokland M., Schlossman S. F., Ritz J. Purification and characterization of fetal hematopoietic cells that express the common acute lymphoblastic leukemia antigen (CALLA). J Exp Med. 1983 Jan 1;157(1):114–129. doi: 10.1084/jem.157.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz R., Hozier J., LeBien T., Minowada J., Gajl-Peczalska K., Kubonishi I., Kersey J. Characterization of a leukemic cell line of the pre-B phenotype. Int J Cancer. 1979 Feb;23(2):174–180. doi: 10.1002/ijc.2910230206. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte M., Iturbe S., Quackenbush E. J. A glycoprotein of molecular weight 85,000 on human cells of B-lineage: detection with a family of monoclonal antibodies. Mol Immunol. 1985 Feb;22(2):113–124. doi: 10.1016/s0161-5890(85)80005-5. [DOI] [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quackenbush E. J., Jongeneel C. V., McInnes R. R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988 Oct 1;168(4):1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Lorkowski G., Zijderhand-Bleekemolen J. E., Erdös E. G., von Figura K., Hasilik A. Neutral endopeptidase-24.11 (enkephalinase). Biosynthesis and localization in human fibroblasts. Biochem J. 1987 Dec 1;248(2):345–350. doi: 10.1042/bj2480345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa G. A., Heumann D., Carrel S., von Fliedner V., Mach J. P. Characterization of membrane vesicles circulating in the serum of patients with common acute lymphoblastic leukemia. Lab Invest. 1986 Nov;55(5):573–579. [PubMed] [Google Scholar]
- Malfroy B., Kuang W. J., Seeburg P. H., Mason A. J., Schofield P. R. Molecular cloning and amino acid sequence of human enkephalinase (neutral endopeptidase). FEBS Lett. 1988 Feb 29;229(1):206–210. doi: 10.1016/0014-5793(88)80828-7. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schofield P. R., Kuang W. J., Seeburg P. H., Mason A. J., Henzel W. J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack R. T., Nelson R. D., LeBien T. W. Structure/function studies of the common acute lymphoblastic leukemia antigen (CALLA/CD10) expressed on human neutrophils. J Immunol. 1986 Aug 1;137(3):1075–1082. [PubMed] [Google Scholar]
- Metzgar R. S., Borowitz M. J., Jones N. H., Dowell B. L. Distribution of common acute lymphoblastic leukemia antigen in nonhematopoietic tissues. J Exp Med. 1981 Oct 1;154(4):1249–1254. doi: 10.1084/jem.154.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R. A., Sutherland R., Greaves M. F. The biochemical characterization of a cell surface antigen associated with acute lymphoblastic leukemia and lymphocyte precursors. J Immunol. 1981 May;126(5):2024–2030. [PubMed] [Google Scholar]
- Pierart M. E., Najdovski T., Appelboom T. E., Deschodt-Lanckman M. M. Effect of human endopeptidase 24.11 ("enkephalinase") on IL-1-induced thymocyte proliferation activity. J Immunol. 1988 Jun 1;140(11):3808–3811. [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohemopoietic differentiation antigens. J Exp Med. 1983 Jan 1;157(1):155–172. doi: 10.1084/jem.157.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush E. J., Gougos A., Baumal R., Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. J Immunol. 1986 Jan;136(1):118–124. [PubMed] [Google Scholar]
- Quackenbush E. J., Letarte M. Identification of several cell surface proteins of non-T, non-B acute lymphoblastic leukemia by using monoclonal antibodies. J Immunol. 1985 Feb;134(2):1276–1285. [PubMed] [Google Scholar]
- Ritz J., Nadler L. M., Bhan A. K., Notis-McConarty J., Pesando J. M., Schlossman S. F. Expression of common acute lymphoblastic leukemia antigen (CALLA) by lymphomas of B-cell and T-cell lineage. Blood. 1981 Sep;58(3):648–652. [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Ronco P., Geniteau M., Poujeol P., Melcion C., Verroust P., Vandewalle A. Characterization of monoclonal antibodies to rabbit renal cortical cells. Am J Physiol. 1986 Mar;250(3 Pt 1):C506–C516. doi: 10.1152/ajpcell.1986.250.3.C506. [DOI] [PubMed] [Google Scholar]
- Ronco P., Pollard H., Galceran M., Delauche M., Schwartz J. C., Verroust P. Distribution of enkephalinase (membrane metalloendopeptidase, E.C. 3.4.24.11) in rat organs. Detection using a monoclonal antibody. Lab Invest. 1988 Feb;58(2):210–217. [PubMed] [Google Scholar]
- Ryan D. H., Chapple C. W., Kossover S. A., Sandberg A. A., Cohen H. J. Phenotypic similarities and differences between CALLA-positive acute lymphoblastic leukemia cells and normal marrow CALLA-positive B cell precursors. Blood. 1987 Sep;70(3):814–821. [PubMed] [Google Scholar]
- Shipp M. A., Richardson N. E., Sayre P. H., Brown N. R., Masteller E. L., Clayton L. K., Ritz J., Reinherz E. L. Molecular cloning of the common acute lymphoblastic leukemia antigen (CALLA) identifies a type II integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4819–4823. doi: 10.1073/pnas.85.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. The hydrolysis of alpha-human atrial natriuretic peptide by pig kidney microvillar membranes is initiated by endopeptidase-24.11. Biochem J. 1987 Apr 1;243(1):183–187. doi: 10.1042/bj2430183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauc M., Chatelet F., Verroust P., Vandewalle A., Poujeol P., Ronco P. Characterization of monoclonal antibodies specific for rabbit renal brush-border hydrolases: application to immunohistological localization. J Histochem Cytochem. 1988 May;36(5):523–532. doi: 10.1177/36.5.2895788. [DOI] [PubMed] [Google Scholar]