Abstract
Oligoclonal and cloned T lines from peripheral blood or thymuses of patients with myasthenia gravis (MG) were selected for reactivity against nicotinic acetylcholine receptors (AChR) from Torpedo california, or against a recombinant fusion peptide, X4, representing the extracellular portion of the mouse AChR alpha-chain. All cell lines expressed the CD4 membrane phenotype, and their antigen reactivity was blocked by antibodies against monomorphic HLA DR/DP determinants. Using a panel of fusion proteins of different, overlapping mouse AChR alpha-chain sequences, a major T cell epitope was localized between amino acid positions 85 and 142. This determinant was distinct from the humoral main immunogenic region, which has been identified on the sequence 61-76. The response pattern of uncloned T lines from three patients with different HLA haplotypes suggests, however, that in any one MG patient T lymphocytes may recognize more than one autoantigenic epitope on the AChR alpha-chain, and that the T lymphocyte response profiles vary among individual patients.
Full text
PDF
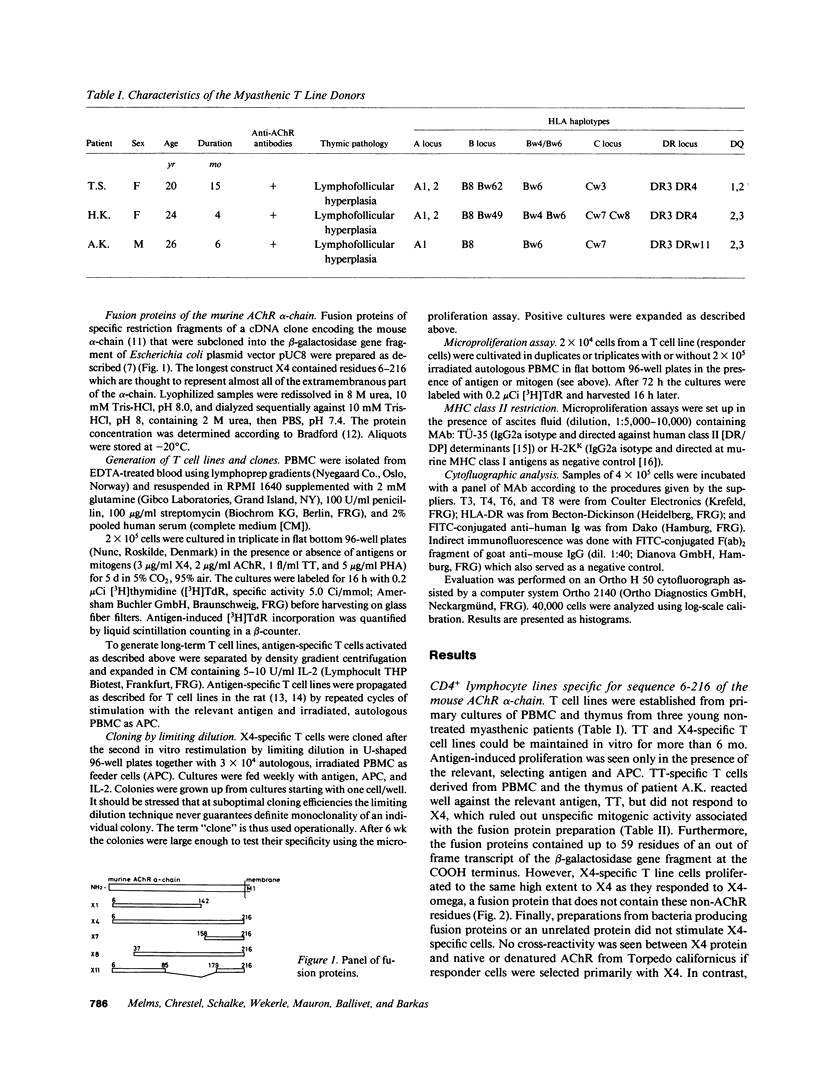

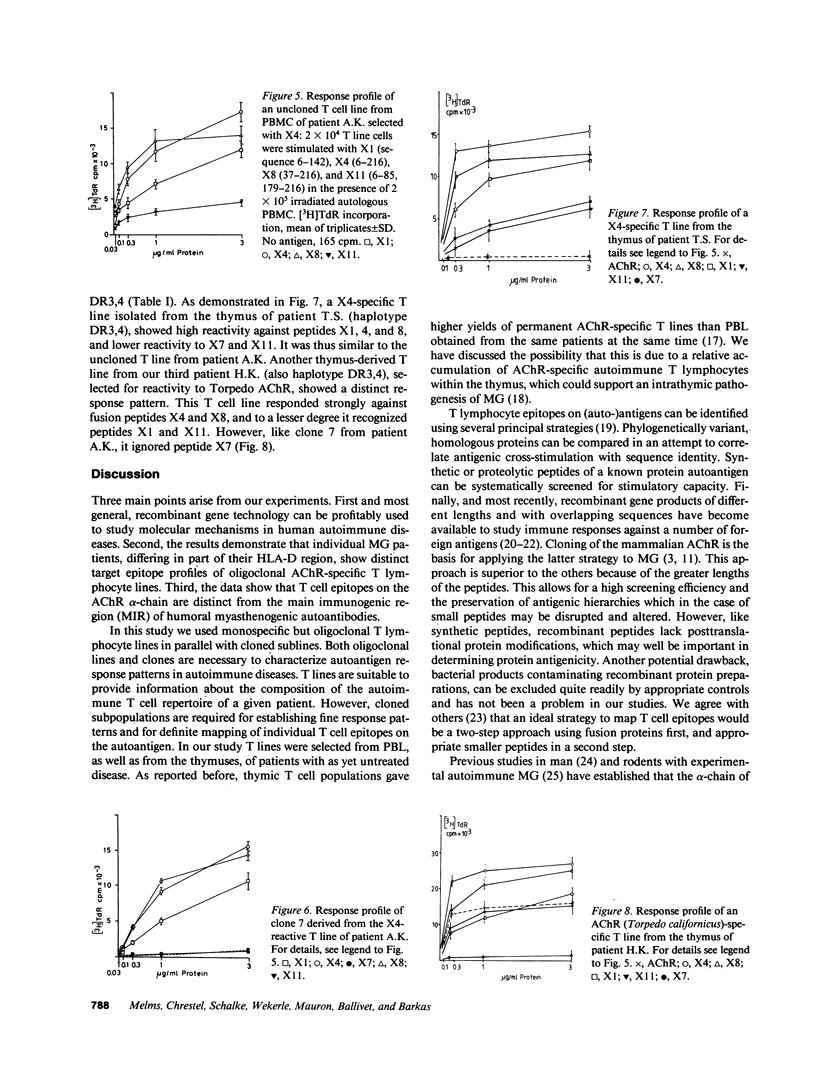


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkas T., Gabriel J. M., Mauron A., Hughes G. J., Roth B., Alliod C., Tzartos S. J., Ballivet M. Monoclonal antibodies to the main immunogenic region of the nicotinic acetylcholine receptor bind to residues 61-76 of the alpha subunit. J Biol Chem. 1988 Apr 25;263(12):5916–5920. [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Boulter J., Luyten W., Evans K., Mason P., Ballivet M., Goldman D., Stengelin S., Martin G., Heinemann S., Patrick J. Isolation of a clone coding for the alpha-subunit of a mouse acetylcholine receptor. J Neurosci. 1985 Sep;5(9):2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Christadoss P., Lindstrom J. M., Melvold R. W., Talal N. Mutation at I-A beta chain prevents experimental autoimmune myasthenia gravis. Immunogenetics. 1985;21(1):33–38. doi: 10.1007/BF00372239. [DOI] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Lindstrom J. Specificity of the T cell immune response to acetylcholine receptor in experimental autoimmune myasthenia gravis. Response to subunits and synthetic peptides. J Immunol. 1988 Mar 15;140(6):1830–1837. [PubMed] [Google Scholar]
- Gotch F., McMichael A., Smith G., Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J Exp Med. 1987 Feb 1;165(2):408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt G. C., Sommer N., Rothbard J., Willcox H. N., Newsom-Davis J. A juxta-membrane epitope on the human acetylcholine receptor recognized by T cells in myasthenia gravis. J Clin Invest. 1988 Oct;82(4):1295–1300. doi: 10.1172/JCI113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R., Conti-Tronconi B., Kalies I., Bertrams J., Toyka K. V. Genetic restriction of autoreactive acetylcholine receptor-specific T lymphocytes in myasthenia gravis. J Immunol. 1985 Oct;135(4):2393–2399. [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Miner L. L., Walgrave S. L., Conti-Tronconi B. M. Amphipathic segment of the nicotinic receptor alpha subunit contains epitopes recognized by T lymphocytes in myasthenia gravis. J Clin Invest. 1988 Mar;81(3):657–660. doi: 10.1172/JCI113369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Tzartos S. J., Carson W., Conti-Tronconi B. M. Human T-helper lymphocytes in myasthenia gravis recognize the nicotinic receptor alpha subunit. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5379–5383. doi: 10.1073/pnas.84.15.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Autoimmune response to acetylcholine receptors in myasthenia gravis and its animal model. Adv Immunol. 1979;27:1–50. doi: 10.1016/s0065-2776(08)60261-8. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Campbell M., Nave B. Specificities of antibodies to acetylcholine receptors. Muscle Nerve. 1978 Mar-Apr;1(2):140–145. doi: 10.1002/mus.880010206. [DOI] [PubMed] [Google Scholar]
- Livingstone A. M., Fathman C. G. The structure of T-cell epitopes. Annu Rev Immunol. 1987;5:477–501. doi: 10.1146/annurev.iy.05.040187.002401. [DOI] [PubMed] [Google Scholar]
- Melms A., Schalke B. C., Kirchner T., Müller-Hermelink H. K., Albert E., Wekerle H. Thymus in myasthenia gravis. Isolation of T-lymphocyte lines specific for the nicotinic acetylcholine receptor from thymuses of myasthenic patients. J Clin Invest. 1988 Mar;81(3):902–908. doi: 10.1172/JCI113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Naeim F., Keesey J. C., Herrmann C., Jr, Lindstrom J., Zeller E., Walford R. L. Association of HLA-B8, DRw3, and anti-acetylcholine receptor antibodies in myasthenia gravis. Tissue Antigens. 1978 Nov;12(5):381–386. doi: 10.1111/j.1399-0039.1978.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Ratnam M., Nguyen D. L., Rivier J., Sargent P. B., Lindstrom J. Transmembrane topography of nicotinic acetylcholine receptor: immunochemical tests contradict theoretical predictions based on hydrophobicity profiles. Biochemistry. 1986 May 6;25(9):2633–2643. doi: 10.1021/bi00357a052. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Watters D., Maelicke A. Molecular forms and hydrodynamic properties of acetylcholine receptor from electric tissue. Eur J Biochem. 1981 Oct;119(2):215–223. doi: 10.1111/j.1432-1033.1981.tb05597.x. [DOI] [PubMed] [Google Scholar]
- Schalke B. C., Klinkert W. E., Wekerle H., Dwyer D. S. Enhanced activation of a T cell line specific for acetylcholine receptor (AChR) by using anti-AChR monoclonal antibodies plus receptors. J Immunol. 1985 Jun;134(6):3643–3648. [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. A simple assay for the study of solubilized acetylcholine receptors. Anal Biochem. 1973 Apr;52(2):349–354. doi: 10.1016/0003-2697(73)90036-5. [DOI] [PubMed] [Google Scholar]
- Svejgaard A., Platz P., Ryder L. P. HLA and disease 1982--a survey. Immunol Rev. 1983;70:193–218. doi: 10.1111/j.1600-065x.1983.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Tami J. A., Urso O. E., Krolick K. A. T cell hybridomas reactive with the acetylcholine receptor and its subunits. J Immunol. 1987 Feb 1;138(3):732–738. [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet. 1977 Mar 26;1(8013):678–680. doi: 10.1016/s0140-6736(77)92118-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Barkas T., Juillerat M., Schwendimann B., Wekerle H. T cell epitopes in experimental autoimmune myasthenia gravis of the rat: strain-specific epitopes and cross-reaction between two distinct segments of the alpha chain of the nicotinic acetylcholine receptor (Torpedo californica). Eur J Immunol. 1988 Apr;18(4):551–557. doi: 10.1002/eji.1830180410. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Heinig J., Müller C., Götz H., Thinnes F. P., Uchańska-Ziegler B., Wernet P. Analysis by sequential immunoprecipitations of the specificities of the monoclonal antibodies TU22,34,35,36,37,39,43,58 and YD1/63.HLK directed against human HLA class II antigens. Immunobiology. 1986 Mar;171(1-2):77–92. doi: 10.1016/S0171-2985(86)80019-5. [DOI] [PubMed] [Google Scholar]