Abstract
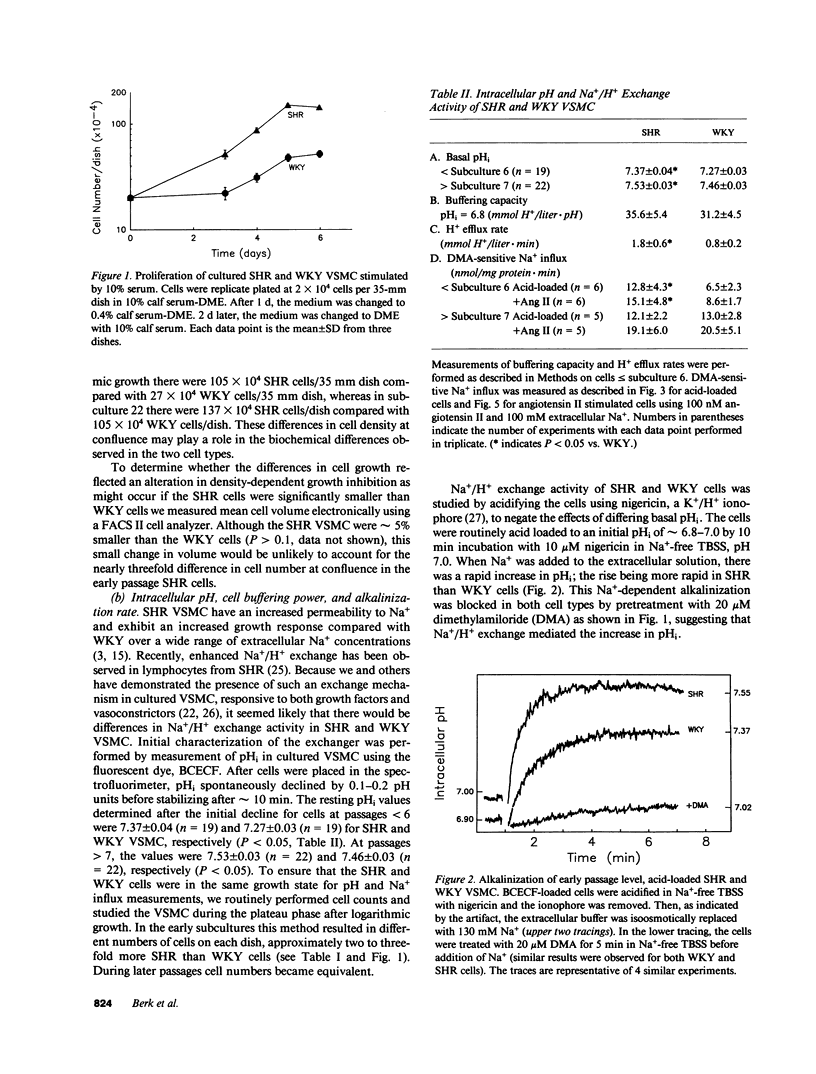
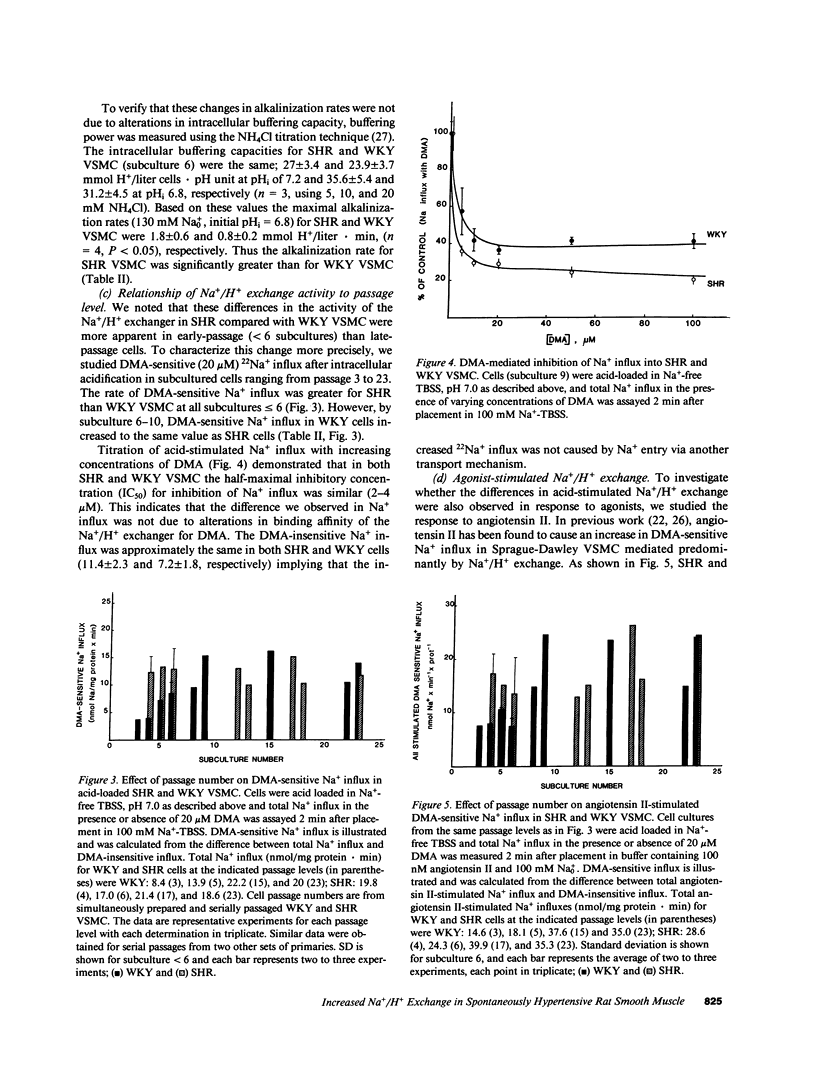
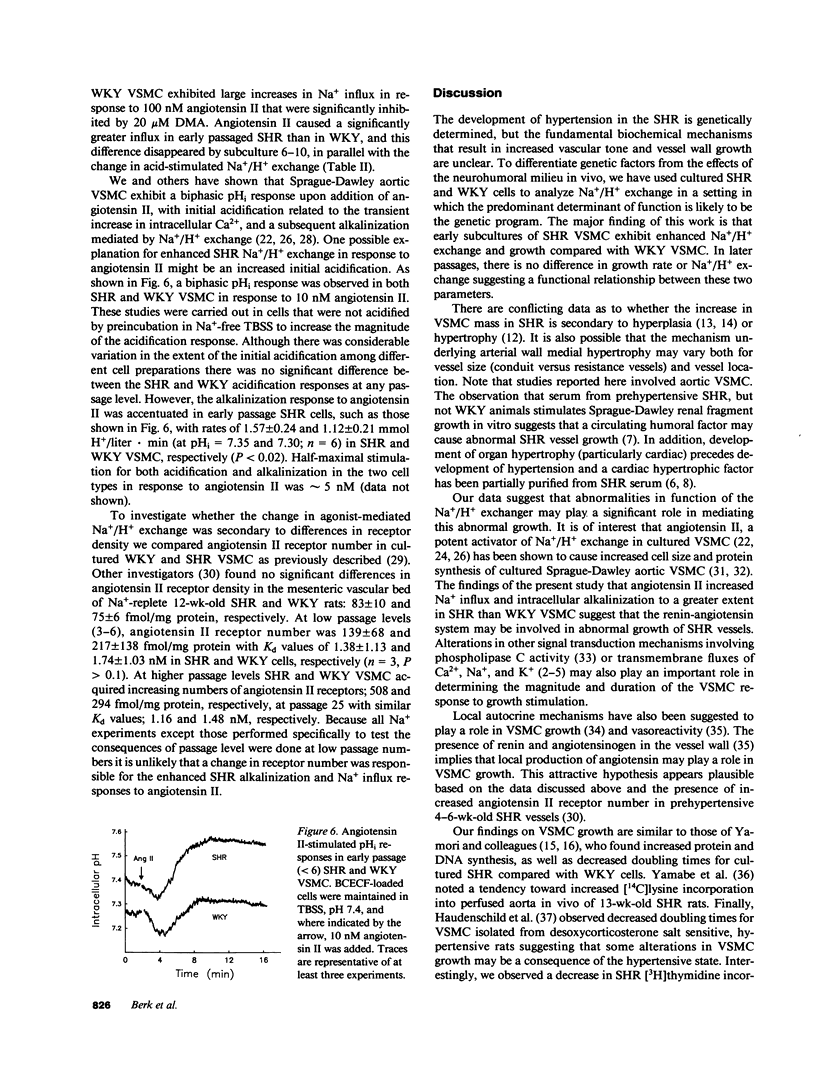
The cellular mechanisms responsible for abnormalities in spontaneously hypertensive rat (SHR) vascular smooth muscle cell (VSMC) growth and vasoreactivity are not defined. Because Na+/H+ exchange, which we have previously demonstrated in cultured VSMC, plays an essential role in mediating growth factor responses, we hypothesized that abnormalities in SHR growth regulation might be reflected in the activity of this transporter. To test this hypothesis, we studied DNA synthesis and Na+/H+ exchange (measured as the rate of amiloride-sensitive intracellular alkalinization or Na+ influx) in early subcultures (less than 6) of aortic VSMC from 12-wk-old SHR and Wistar Kyoto (WKY) animals. Serum-deprived SHR VSMC grew more rapidly in response to 10% serum with an increase in [3H]thymidine incorporation of 439% compared with 191% in WKY controls. Basal intracellular pH (pHi) values determined by fluorescent pH measurements were 7.37 +/- 0.04 and 7.27 +/- 0.03 (P less than 0.05) in early passage SHR and WKY, respectively. Acid recovery (initial pHi = 6.8) by SHR VSMC was faster than by WKY VSMC as measured by alkalinization (1.8 +/- 0.6 vs. 0.8 +/- 0.2 mmol H+/liter.min, P less than 0.05) or by amiloride-sensitive 22Na+ influx (14.5 +/- 1.2 vs. 4.0 +/- 0.5 nmol Na+/mg protein.min, P less than 0.05). In comparison to WKY cells early passage SHR VSMC exhibited 2.5-fold greater alkalinization and amiloride-sensitive 22Na+ influx in response to 100 nM angiotensin II. During serial passage, WKY cells acquired enhanced Na+/H+ exchange and growth rates so that by passage 6, these differences were no longer present. These findings in early cultures of SHR VSMC, removed from the in vivo neurohumoral milieu, suggest that increased Na+/H+ exchange in SHR may reflect alterations in Na+ homeostasis that might contribute to altered SHR VSMC function such as enhanced growth and vasoreactivity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida T., Yazaki Y., Ohuchi Y., Tanaka T., Ikeda M. Pressor responsiveness to vasopressin and angiotensin II in spontaneously hypertensive rats: effects of dietary sodium. Tohoku J Exp Med. 1983 Jan;139(1):51–59. doi: 10.1620/tjem.139.51. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Aronow M. S., Brock T. A., Cragoe E., Jr, Gimbrone M. A., Jr, Alexander R. W. Angiotensin II-stimulated Na+/H+ exchange in cultured vascular smooth muscle cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1987 Apr 15;262(11):5057–5064. [PubMed] [Google Scholar]
- Berk B. C., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Early agonist-mediated ionic events in cultured vascular smooth muscle cells. Calcium mobilization is associated with intracellular acidification. J Biol Chem. 1987 Apr 15;262(11):5065–5072. [PubMed] [Google Scholar]
- Blaustein M. P. Sodium transport and hypertension. Where are we going? Hypertension. 1984 Jul-Aug;6(4):445–453. doi: 10.1161/01.hyp.6.4.445. [DOI] [PubMed] [Google Scholar]
- Clubb F. J., Jr, Bell P. D., Kriseman J. D., Bishop S. P. Myocardial cell growth and blood pressure development in neonatal spontaneously hypertensive rats. Lab Invest. 1987 Feb;56(2):189–197. [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Phorbol ester-induced contraction of arterial smooth muscle and inhibition of alpha-adrenergic response. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1103–1109. doi: 10.1016/0006-291x(84)91397-4. [DOI] [PubMed] [Google Scholar]
- Feig P. U., D'Occhio M. A., Boylan J. W. Lymphocyte membrane sodium-proton exchange in spontaneously hypertensive rats. Hypertension. 1987 Mar;9(3):282–288. doi: 10.1161/01.hyp.9.3.282. [DOI] [PubMed] [Google Scholar]
- Folkow B., Hallbäck M., Lundgren Y., Weiss L. Structurally based increase of flow resistance in spontaneously hypertensive rats. Acta Physiol Scand. 1970 Jul;79(3):373–378. doi: 10.1111/j.1748-1716.1970.tb04737.x. [DOI] [PubMed] [Google Scholar]
- Friedman S. M. Evidence for enhanced sodium transport in the tail artery of the spontaneously hypertensive rat. Hypertension. 1979 Nov-Dec;1(6):572–582. doi: 10.1161/01.hyp.1.6.572. [DOI] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Berk B. C., Alexander R. W. Evidence that Na+/H+ exchange regulates angiotensin II-stimulated diacylglycerol accumulation in vascular smooth muscle cells. J Biol Chem. 1988 Aug 5;263(22):10620–10624. [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Rothstein A. Cytoplasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport. J Gen Physiol. 1984 Mar;83(3):341–369. doi: 10.1085/jgp.83.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther S., Gimbrone M. A., Jr, Alexander R. W. Identification and characterization of the high affinity vascular angiotensin II receptor in rat mesenteric artery. Circ Res. 1980 Aug;47(2):278–286. doi: 10.1161/01.res.47.2.278. [DOI] [PubMed] [Google Scholar]
- Hatori N., Fine B. P., Nakamura A., Cragoe E., Jr, Aviv A. Angiotensin II effect on cytosolic pH in cultured rat vascular smooth muscle cells. J Biol Chem. 1987 Apr 15;262(11):5073–5078. [PubMed] [Google Scholar]
- Hermsmeyer K. Cellular basis for increased sensitivity of vascular smooth muscle in spontaneously hypertensive rats. Circ Res. 1976 Jun;38(6 Suppl 2):53–57. doi: 10.1161/01.res.38.6.53. [DOI] [PubMed] [Google Scholar]
- Kanbe T., Nara Y., Tagami M., Yamori Y. Studies of hypertension-induced vascular hypertrophy in cultured smooth muscle cells from spontaneously hypertensive rats. Hypertension. 1983 Nov-Dec;5(6):887–892. doi: 10.1161/01.hyp.5.6.887. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lais L. T., Brody M. J. Vasoconstrictor hyperresponsiveness: an early pathogenic mechanism in the spontaneously hypertensive rat. Eur J Pharmacol. 1978 Jan 15;47(2):177–189. doi: 10.1016/0014-2999(78)90389-8. [DOI] [PubMed] [Google Scholar]
- Little P. J., Cragoe E. J., Jr, Bobik A. Na-H exchange is a major pathway for Na influx in rat vascular smooth muscle. Am J Physiol. 1986 Nov;251(5 Pt 1):C707–C712. doi: 10.1152/ajpcell.1986.251.5.C707. [DOI] [PubMed] [Google Scholar]
- Livne A., Balfe J. W., Veitch R., Marquez-Julio A., Grinstein S., Rothstein A. Increased platelet Na+-H+ exchange rates in essential hypertension: application of a novel test. Lancet. 1987 Mar 7;1(8532):533–536. doi: 10.1016/s0140-6736(87)90176-0. [DOI] [PubMed] [Google Scholar]
- Mahnensmith R. L., Aronson P. S. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985 Jun;56(6):773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- Matlib M. A., Schwartz A., Yamori Y. A Na+-Ca2+ exchange process in isolated sarcolemmal membranes of mesenteric arteries from WKY and SHR rats. Am J Physiol. 1985 Jul;249(1 Pt 1):C166–C172. doi: 10.1152/ajpcell.1985.249.1.C166. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Hansen O. K., Aalkjaer C. Direct evidence that the greater contractility of resistance vessels in spontaneously hypertensive rats is associated with a narrowed lumen, a thickened media, and an increased number of smooth muscle cell layers. Circ Res. 1978 Dec;43(6):854–864. doi: 10.1161/01.res.43.6.854. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Berk B. C., Brock T. A., Smith T. W. Na+-Ca2+ exchange in cultured vascular smooth muscle cells. Circ Res. 1988 Mar;62(3):486–493. doi: 10.1161/01.res.62.3.486. [DOI] [PubMed] [Google Scholar]
- Nabika T., Velletri P. A., Beaven M. A., Endo J., Lovenberg W. Vasopressin-induced calcium increases in smooth muscle cells from spontaneously hypertensive rats. Life Sci. 1985 Aug 12;37(6):579–584. doi: 10.1016/0024-3205(85)90472-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Ober S. S., Pardee A. B. Intracellular pH is increased after transformation of Chinese hamster embryo fibroblasts. Proc Natl Acad Sci U S A. 1987 May;84(9):2766–2770. doi: 10.1073/pnas.84.9.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N. E. Platelet-derived growth factor stimulates Na+ influx in vascular smooth muscle cells. Am J Physiol. 1984 Nov;247(5 Pt 1):C501–C505. doi: 10.1152/ajpcell.1984.247.5.C501. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Schwartz S. M. Alterations in vascular smooth muscle mass in the spontaneously hypertensive rat. Role of cellular hypertrophy, hyperploidy, and hyperplasia. Circ Res. 1982 Sep;51(3):280–289. doi: 10.1161/01.res.51.3.280. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Schwartz S. M., McCanna M. Evaluation of medial hypertrophy in resistance vessels of spontaneously hypertensive rats. Hypertension. 1988 Feb;11(2):198–207. doi: 10.1161/01.hyp.11.2.198. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss H. G., Goldin H. Serum renotropic activity and renal growth in spontaneously hypertensive rats. Kidney Int. 1983 Apr;23(4):635–642. doi: 10.1038/ki.1983.70. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L., Thomé F. S., Genest J. Vascular angiotensin II receptors in SHR. Hypertension. 1984 Sep-Oct;6(5):682–688. doi: 10.1161/01.hyp.6.5.682. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Brock T. A. Analysis of angiotensin-stimulated sodium transport in cultured smooth muscle cells from rat aorta. J Cell Physiol. 1983 Mar;114(3):284–290. doi: 10.1002/jcp.1041140306. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Smith L. Extracellular Na+ dependence of changes in free Ca2+, 45Ca2+ efflux, and total cell Ca2+ produced by angiotensin II in cultured arterial muscle cells. J Biol Chem. 1987 Dec 25;262(36):17455–17460. [PubMed] [Google Scholar]
- Tamura H., Hopp L., Kino M., Tokushige A., Searle B. M., Khalil F., Aviv A. Na+-K+ regulation in cultured vascular smooth muscle cell of the spontaneously hypertensive rat. Am J Physiol. 1986 Jun;250(6 Pt 1):C939–C947. doi: 10.1152/ajpcell.1986.250.6.C939. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Ishii M., Ishimitsu T., Sugimoto T. Enhanced phospholipase C activity in the vascular wall of spontaneously hypertensive rats. Hypertension. 1988 Jan;11(1):28–33. doi: 10.1161/01.hyp.11.1.28. [DOI] [PubMed] [Google Scholar]
- Vallega G. A., Canessa M. L., Berk B. C., Brock T. A., Alexander R. W. Vascular smooth muscle Na+-H+ exchanger kinetics and its activation by angiotensin II. Am J Physiol. 1988 Jun;254(6 Pt 1):C751–C758. doi: 10.1152/ajpcell.1988.254.6.C751. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Vanhoutte P. M. Sensitivity to noradrenaline in isolated tail arteries from spontaneously hypertensive rats. Clin Sci (Lond) 1979 Dec;57 (Suppl 5):31s–33s. doi: 10.1042/cs057031s. [DOI] [PubMed] [Google Scholar]
- Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol. 1988 Feb;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]
- Yamabe H., Lovenberg W. Increased incorporation of 14C-lysine into vascular proteins of the spontaneously hypertensive rat. Eur J Pharmacol. 1974 Nov;29(1):109–116. doi: 10.1016/0014-2999(74)90177-0. [DOI] [PubMed] [Google Scholar]


