Abstract
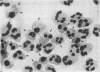
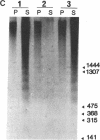
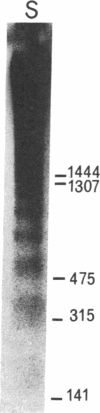
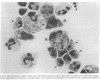
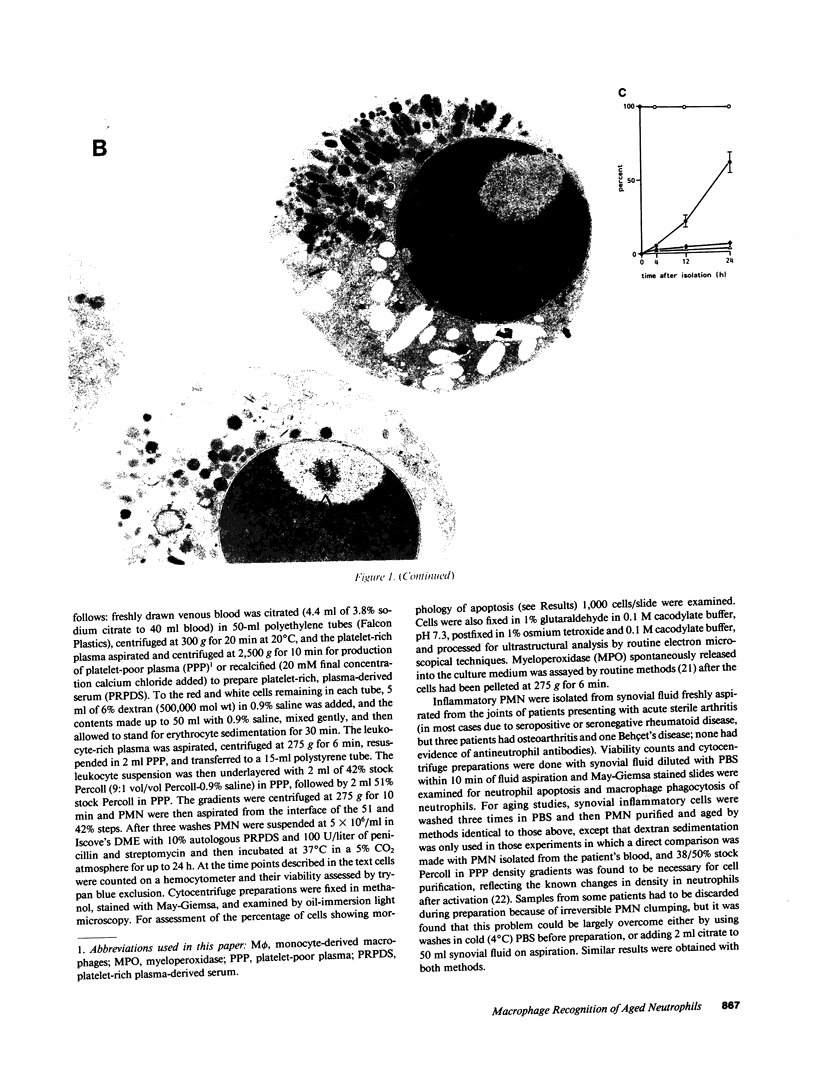
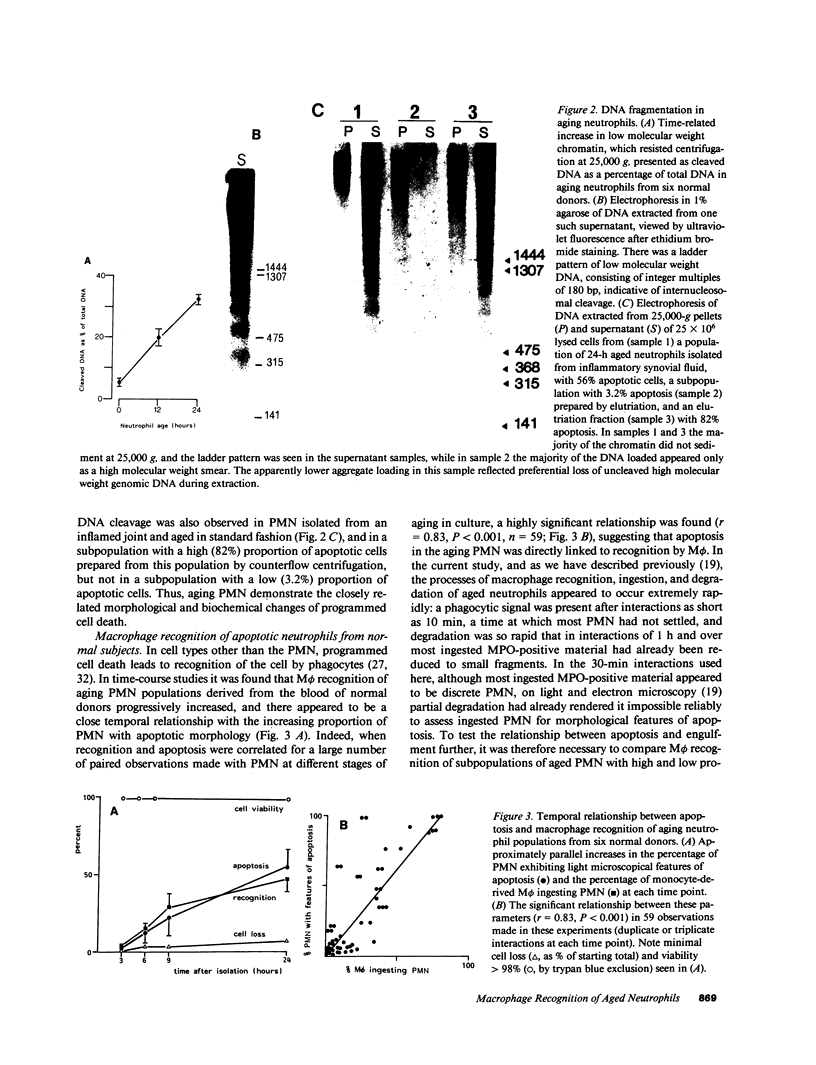
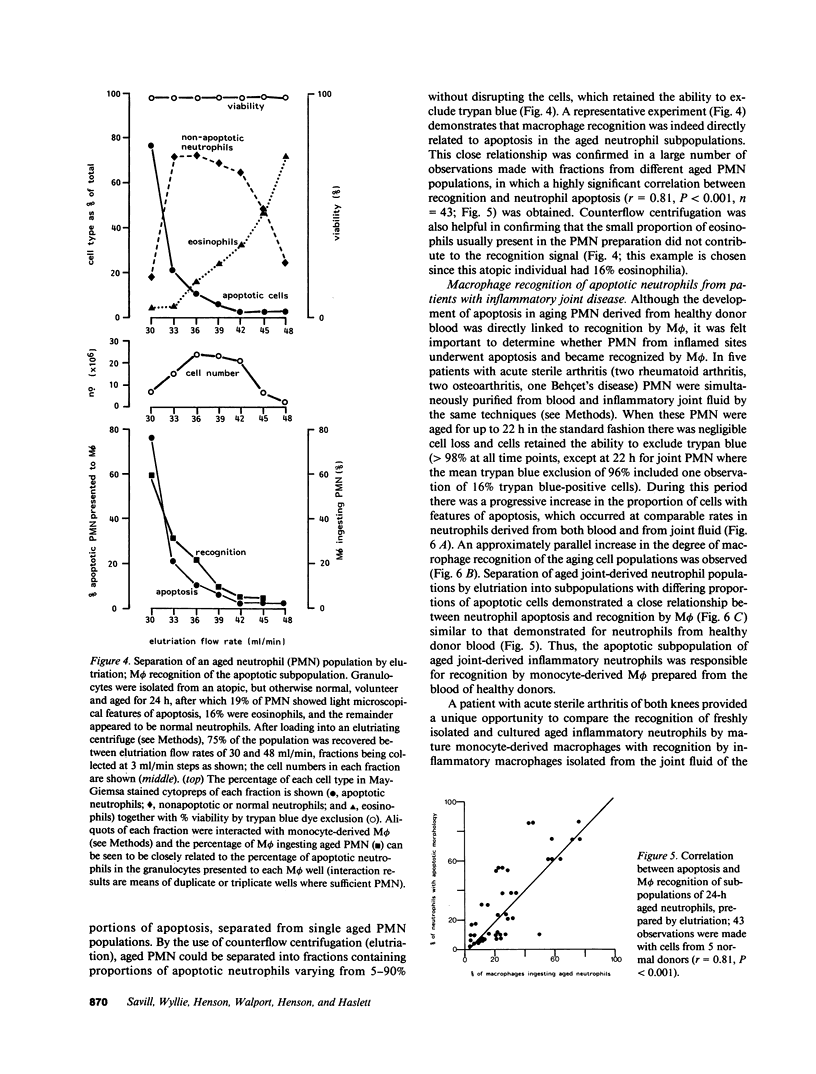
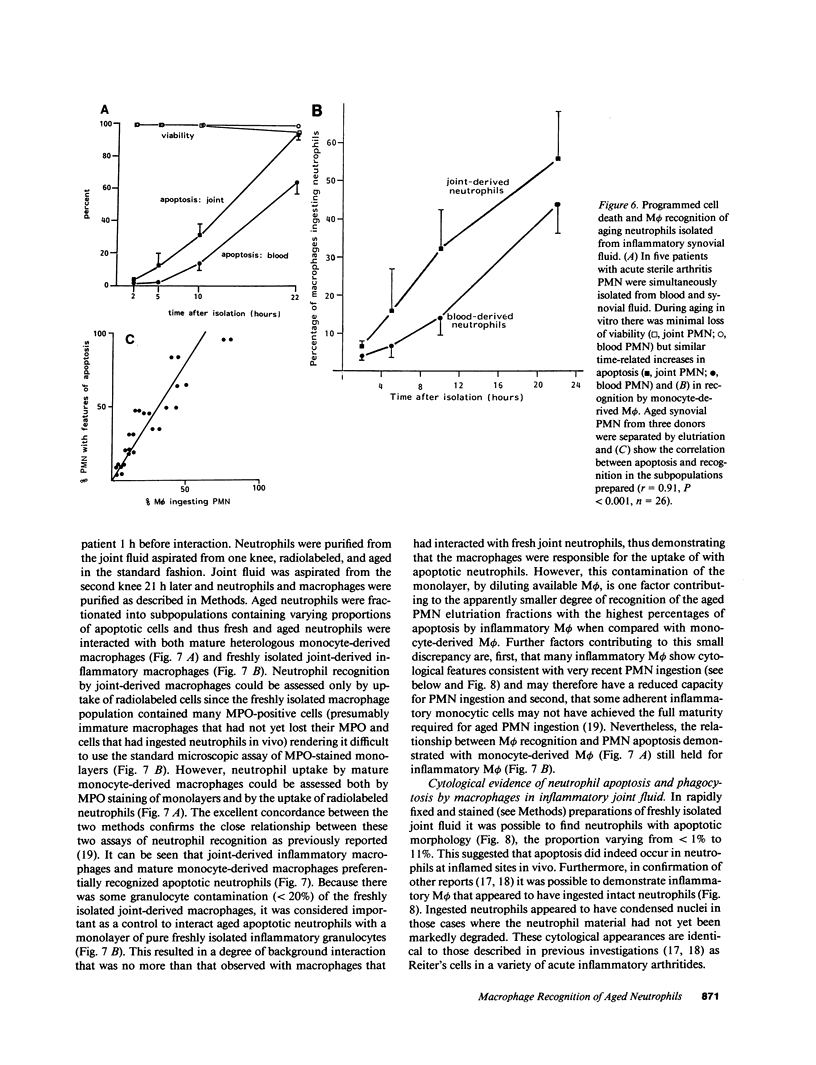
Mechanisms governing the normal resolution processes of inflammation are poorly understood, yet their elucidation may lead to a greater understanding of the pathogenesis of chronic inflammation. The removal of neutrophils and their potentially histotoxic contents is one prerequisite of resolution. Engulfment by macrophages is an important disposal route, and changes in the senescent neutrophil that are associated with their recognition by macrophages are the subject of this investigation. Over 24 h in culture an increasing proportion of human neutrophils from peripheral blood or acutely inflamed joints underwent morphological changes characteristic of programmed cell death or apoptosis. Time-related chromatin cleavage in an internucleosomal pattern indicative of the endogenous endonuclease activation associated with programmed cell death was also demonstrated. A close correlation was observed between the increasing properties of apoptosis in neutrophils and the degree of macrophage recognition of the aging neutrophil population, and a direct relationship between these parameters was confirmed within aged neutrophil populations separated by counterflow centrifugation into fractions with varying proportions of apoptosis. Macrophages from acutely inflamed joints preferentially ingested apoptotic neutrophils and histological evidence was presented for occurrence of the process in situ. Programmed cell death is a phenomenon of widespread biological importance and has not previously been described in a cell of the myeloid line. Because it leads to recognition of intact senescent neutrophils that have not necessarily disgorged their granule contents, these processes may represent a mechanism for the removal of neutrophils during inflammation that also serves to limit the degree of tissue injury.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREWER D. B. ELECTRON-MICROSCOPE OBSERVATIONS ON THE PHAGOCYTOSIS OF NEUTROPHIL POLYMORPHONUCLEAR LEUCOCYTES BY MACROPHAGES. J Pathol Bacteriol. 1964 Jul;88:307–309. doi: 10.1002/path.1700880139. [DOI] [PubMed] [Google Scholar]
- Ballard K. J., Holt S. J. Cytological and cytochemical studies on cell death and digestion in the foetal rat foot: the role of macrophages and hydrolytic enzymes. J Cell Sci. 1968 Jun;3(2):245–262. doi: 10.1242/jcs.3.2.245. [DOI] [PubMed] [Google Scholar]
- Chapes S. K., Haskill S. Evidence for granulocyte-mediated macrophage activation after C. parvum immunization. Cell Immunol. 1983 Feb 1;75(2):367–377. doi: 10.1016/0008-8749(83)90334-9. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Doherty D. E., Downey G. P., Worthen G. S., Haslett C., Henson P. M. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab Invest. 1988 Aug;59(2):200–213. [PubMed] [Google Scholar]
- Duvall E., Wyllie A. H., Morris R. G. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology. 1985 Oct;56(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- FLIEDNER T. M., CRONKITE E. P., ROBERTSON J. S. GRANULOCYTOPOIESIS. I. SENESCENCE AND RANDOM LOSS OF NEUTROPHILIC GRANULOCYTES IN HUMAN BEINGS. Blood. 1964 Oct;24:402–414. [PubMed] [Google Scholar]
- Hacbarth E., Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986 Nov;29(11):1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Worthen G. S., Giclas P. C., Morrison D. C., Henson J. E., Henson P. M. The pulmonary vascular sequestration of neutrophils in endotoxemia is initiated by an effect of endotoxin on the neutrophil in the rabbit. Am Rev Respir Dis. 1987 Jul;136(1):9–18. doi: 10.1164/ajrccm/136.1.9. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Kadri A., Noinuddin M., de Leeuw N. K. Phagocytosis of blood cells by splenic macrophages in thrombotic thrombocytopenic purpura. Ann Intern Med. 1975 Jun;82(6):799–802. doi: 10.7326/0003-4819-82-6-799. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Hargreaves A. D., Duvall E., Wyllie A. H. Hormone-induced cell death. 2. Surface changes in thymocytes undergoing apoptosis. Am J Pathol. 1984 Jun;115(3):426–436. [PMC free article] [PubMed] [Google Scholar]
- Musson R. A. Human serum induces maturation of human monocytes in vitro. Changes in cytolytic activity, intracellular lysosomal enzymes, and nonspecific esterase activity. Am J Pathol. 1983 Jun;111(3):331–340. [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley R. T., Crist W. M., Ragab A. H., Boxer L. A., Malluh A., Findley H. Phagocytosis of neutrophils by marrow macrophages in childhood chronic benign neutropenia. J Pediatr. 1981 Feb;98(2):207–212. doi: 10.1016/s0022-3476(81)80636-1. [DOI] [PubMed] [Google Scholar]
- Pekin T. J., Jr, Malinin T. I., Zvaifler N. J. Unusual synovial fluid findings in Reiter's syndrome. Ann Intern Med. 1967 Apr;66(4):677–684. doi: 10.7326/0003-4819-66-4-677. [DOI] [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Sanui H., Yoshida S., Nomoto K., Ohhara R., Adachi Y. Peritoneal macrophages which phagocytose autologous polymorphonuclear leucocytes in guinea-pigs. I: induction by irritants and microorgansisms and inhibition by colchicine. Br J Exp Pathol. 1982 Jun;63(3):278–284. [PMC free article] [PubMed] [Google Scholar]
- Spriggs A. I., Boddington M. M., Mowat A. G. Joint fluid cytology in Reiter's disease. Ann Rheum Dis. 1978 Dec;37(6):557–560. doi: 10.1136/ard.37.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S. Cytotoxic T lymphocytes and glucocorticoids activate an endogenous suicide process in target cells. Nature. 1987 May 7;327(6117):62–64. doi: 10.1038/327062a0. [DOI] [PubMed] [Google Scholar]
- Vartio T., Seppä H., Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981 Jan 10;256(1):471–477. [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G. Hormone-induced cell death. Purification ad properties of thymocytes undergoing apoptosis after glucocorticoid treatment. Am J Pathol. 1982 Oct;109(1):78–87. [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]