Abstract
Fission yeast Schizosaccharomyces pombe is an important genetic model organism for studying the mechanisms of endocytosis and cytokinesis. However, most work on the biochemical properties of fission yeast actin-binding proteins has been done with skeletal muscle actin for matters of convenience. When simulations of mathematical models of the mechanism of endocytosis were compared with events in live cells, some of the reactions appeared to be much faster than observed in biochemical experiments with muscle actin. Here, we used gelsolin affinity chromatography to purify actin from fission yeast. S. pombe actin shares many properties with skeletal muscle actin but has higher intrinsic nucleotide exchange rate, faster trimer nucleus formation, faster phosphate dissociation rate from polymerized actin, and faster nucleation of actin filaments with Arp2/3 complex. These properties close the gap between the biochemistry and predictions made by mathematical models of endocytosis in S. pombe cells.
Keywords: Actin, ADP, ATP, Protein Assembly, Yeast, Arp2/3 Complex, Cofilin, Profilin
Introduction
Actin plays pivotal roles in endocytosis, cell migration, vesicle trafficking, and cytokinesis. The sequences of actin genes have been highly conserved during evolution, and cells use a variety of actin-binding proteins to regulate actin polymerization and depolymerization spatially and temporally (1). Biochemical analysis of the interactions of actin with actin-binding proteins is essential for understanding the mechanisms of physiological processes in cells other than muscle, but most biochemical and biophysical experiments are done with skeletal muscle actin for practical reasons.
Fission yeast Schizosaccharomyces pombe is a genetic model organism used to study actin dynamics during endocytosis and cytokinesis (2–4). Comparisons of mathematical models with quantitative measurements on live cells are powerful tests of our understanding of both endocytosis and cytokinesis (5, 6). However, computer simulations of these models have depended on quantitative parameters from experiments with muscle actin due to the lack of the information about S. pombe actin. To obtain good fits of simulations to measurements in cells, it was necessary to assume that muscle and S. pombe actin differ in some ways. For example, to account for the time course of endocytic actin patch assembly, Berro et al. (5) had to assume that the reactions leading to actin filament branch formation are much faster in cells than in biochemical experiments with muscle actin and fission yeast Arp2/3 complex and nucleation-promoting factors. Similarly Berro et al. (5) had to assume that γ-phosphate dissociates much more rapidly from polymerized S. pombe ADP·Pi·actin than muscle actin to account for the time course of actin patch disassembly.
Budding yeast actin has been characterized in considerable detail (7–9), but budding and fission yeast diverged during evolution more than 400 million years ago, so the properties of their actins likely differ. Takaine and Mabuchi described a method to purify S. pombe actin (10), but we could not obtain good yields of active actin by their method. Ohki et al. (11) purified human cytoplasmic actin from HeLa cells by affinity chromatography with the C-terminal half of mouse gelsolin. We found that a modification of their method yields highly purified, active fission yeast actin. We confirm that S. pombe actin shares many properties with muscle actin but differs quantitatively in several features, including faster nucleotide exchange, no effect of its own profilin on nucleotide exchange, more efficient nucleation of actin filaments with its own Arp2/3 complex, and much faster dissociation of the γ-phosphate from ADP·Pi·actin subunits in filaments. All of these differences are important for mathematical modeling of the actin system in fission yeast.
EXPERIMENTAL PROCEDURES
Purification of C-terminal Half of Mouse Gelsolin
We purified the C-terminal half of His6-tagged mouse gelsolin (G4-6) from Rosetta (DE3) pLysS-competent Escherichia coli cells (Novagen) (11) with the following modifications. Cells were grown in LB medium with 100 μg/ml ampicillin and 34 μg/ml chloramphenicol, and expression was induced at A595 = 1.0–1.2 with 0.4 mm isopropyl-1-thio-β-d-galactopyranoside at 15 °C for 16 h. We lysed cells from a 1-liter culture (∼15 g of cells) by sonication (Branson Sonifier 450) in 35 ml of lysis buffer (50 mm KCl, 10 mm Tris, pH 8.0, 5 mm CaCl2, 1 mm ATP, 7 mm β-mercaptoethanol, and one Roche Applied Science protease inhibiter tablet 11873580001) and centrifuged the homogenate at 4 °C at 38,000 rpm in a Ti45 rotor (Beckman Coulter) for 30 min. The supernatant was mixed with 7 ml of nickel-Sepharose 6 Fast Flow (GE Healthcare, 17-5318-01) at 4 °C for 1.5 h, loaded onto a column using gravity flow, and washed with 100 ml of lysis buffer without protease inhibitor. G4-6 was eluted with lysis buffer containing 200 mm imidazole (pH 8.0) and dialyzed against 1 liter of lysis buffer containing 1 mm NaN3. The yield was ∼350 mg from 6 liters of cell culture. Gelsolin G4-6 purification was finished 1 day before fission yeast actin purification.
Purification of Actin from S. pombe
Six flasks containing 1 liter of YE5S medium were inoculated with 10 ml of an overnight culture of S. pombe strain TP150 in YE5S medium and grown in a shaking incubator at 28 °C for 15 h. When the A595 was ∼1.5–2.5, each liter of culture was fed with another 70 g of YE5S powder and incubated at 32 °C until the A595 = 5.5–6.5. Six 1-liter cultures yielded ∼120 g of cells, which were suspended in 100 ml of ice-cold lysis buffer (10 mm Tris, pH 8.0, 10 mm CaCl2, 1 mm ATP, 7 mm β-mercaptoethanol, 1 mm NaN3, eight tablets of Roche Applied Science protease inhibitor 11873580001) and lysed with a Microfluidizer (Microfluidics, M110-EH) using a pressure of 25,000 p.s.i. All subsequent steps were at 0–4 °C. The cell lysate was mixed with ∼350 mg of dialyzed G4-6, and the mixture was stirred on ice for 3 h. After centrifugation at 20,000 rpm for 20 min and at 45,000 rpm for 1 h in a Ti45 rotor, the supernatant was mixed with 7 ml of nickel-Sepharose 6 Fast Flow (GE Healthcare, 17-5318-01) on ice for 1.5 h. The resin was loaded onto the column using gravity flow and washed with 100 ml of washing buffer (10 mm Tris, pH 8.0, 5 mm CaCl2, 1 mm ATP, 7 mm β-mercaptoethanol, 1 mm NaN3). Then the resin was transferred to 50-ml centrifuge tubes. After the beads settled and the supernatant was removed, actin was eluted by suspending the beads in 100 ml of elution buffer (10 mm Tris, 5 mm EGTA, 1 mm MgCl2, 1 mm ATP, 7 mm β-mercaptoethanol, 1 mm NaN3, pH 8.0) for 1 min and pelleting the beads. The eluted protein was dialyzed overnight against 1 liter of dialysis buffer (10 mm Tris, pH 8.0, 5 mm MgCl2, 1 mm ATP, 7 mm β-mercaptoethanol, 1 mm NaN3) followed the next day by 1 liter of dialysis buffer for 2 h. The protein solution was transferred into two 50-ml centrifuge tubes with 200 μl of nickel-Sepharose, and the mixture was incubated for 15 min. After spinning at 3,500 rpm for 10 min, actin was polymerized in 100 mm KCl and 10 mm MgCl2 for 1 h at 4 °C. Actin filaments were pelleted at 38,000 rpm in a Ti45 rotor for 2 h. The supernatant was discarded, and the surface of actin filament pellet was washed with 5 ml of G-buffer (2 mm Tris, 0.1 mm CaCl2, 0.2 mm ATP, 0.5 mm DTT, 1 mm NaN3, pH 8.0). The pellet was resuspended in 10 ml of G-buffer with a Dounce homogenizer (Wheaton 432-1272). The actin solution was dialyzed against four changes of 1 liter of G-buffer over 2 days to depolymerize the filaments. Depolymerized actin was centrifuged for 2 h at 38,000 rpm in Ti70.1 rotor to remove any insoluble materials. The top two-thirds of the supernatant was loaded onto a 2.6 × 69-cm Sephacryl S-300 gel filtration column equilibrated with G-buffer. Fractions of 110 drops were collected, and the actin concentration was measured by absorption at 290 nm (ϵ = 26,000 m−1 cm−1). We stored purified S. pombe actin by continuous dialysis against 1 liter of G-buffer changed every 12 h and used fractions containing >5 μm actin within 5 days. To generate ADP or ADP·Pi·actin filaments, actin monomers were polymerized in MEI (1 mm MgCl2, 1 mm EGTA, 10 mm imidazole, 0.1 mm ATP, 1 mm DTT, pH 7.0) with 25 mm potassium phosphate, pH 7.0, or 25 mm K2SO4 for 1 h on ice. Cys-374 of S. pombe actin was labeled with N-(1-pyrene)iodoacetamide (P29, Invitrogen) (12) or Oregon green 488 iodoacetamide (O-6010, Invitrogen) (13, 14).
Purification of Other Proteins
We purified Arp2/3 complex from fission yeast2 and recombinant fission yeast cofilin (15) and fission yeast and human profilin (16) from E. coli BL21(DE3) pLysS cells.
Actin Monomer Nucleotide Exchange
A slurry containing 90 μl of Dowex AG 1-X4 resin (Bio-Rad 143-1345) in 100 mm Tris, pH 8.0, was washed twice with 600 μl of nucleotide free G-buffer using a Cortex 0.22-μm spin column. Free ATP was removed from 400 μl of actin by incubation with the washed Dowex for 3 min and pelleting the resin. The 400 μl of actin were mixed with 50 μl of 5 mm ϵ-ATP, 2.5 μl of 10 mm MgCl2, and 2 μl of 50 mm EGTA for 30 min at 4 °C. Free ϵ-ATP and ATP were removed by incubation with 50 μl of Dowex for 2.5 min, and the ϵ-ATP·actin was used within about 1 h. Equal volumes of 0.2 μm ϵ-ATP·actin monomers in KMEI (MEI buffer with potassium) and KMEI with 0.2 mm ATP and 0.1 mm DTT containing a range of profilin concentrations were mixed with a KinTek stopped-flow mixer with a dead time of ∼7 ms. The fluorescence intensity was followed over time at five points per second with excitation at 345 nm and emission at 410 nm.
Release of Phosphate during Actin Polymerization
ATP was removed from actin monomers in a Cortex 0.22-μm spin column with 600 μl of Sephadex G-25 resin (Sigma-Aldrich G2550) previously washed twice with nucleotide-free G-buffer (1 mm NaN3, 0.1 mm CaCl2, and 2 mm Tris, pH 8.0). Centrifugation at 1,400 × g for 2 min removed the buffer outside the beads (17). A solution of 400 μl of actin monomers was added on top of each of three washed G-25 columns, which were spun at 1,400 × g for 2 min, yielding 1 ml of actin monomer in nucleotide-free buffer. The specific activity of our 10 mCi/ml stock of [γ-32P]ATP was 6,000 Ci/mmol. We mixed 981 μl of G-25 filtered actin monomer with 6.4 μl of 1.67 μm [γ-32P]ATP, 5 μl of 10 mm MgCl2, and 4 μl of 50 mm EGTA for 30 min at 4 °C. Free [γ-32P]ATP was removed by adding 200-μl aliquots of radioactive actin monomer to the top of 600 μl of washed G-25 and centrifuging at 1,400 × g for 2 min. The concentration of radioactive actin monomer was estimated by Bradford assay. Samples of 1.5 ml of 3 μm actin were polymerized in 1× KMEI buffer at 25 °C. At each time point, a 100-μl aliquot was added to a 0.5-ml 30-kDa molecular mass cut-off Amicon ultracentrifuge tube (Millipore UFC503096) and spun at 23,000 × g for 1 min to separate a small fraction of the buffer containing 32P from the retained protein. The radioactivity of the filtrate was measured with a liquid scintillation analyzer (Packard, TRI-CARB 2900TR).
RESULTS
Purification of S. pombe Actin
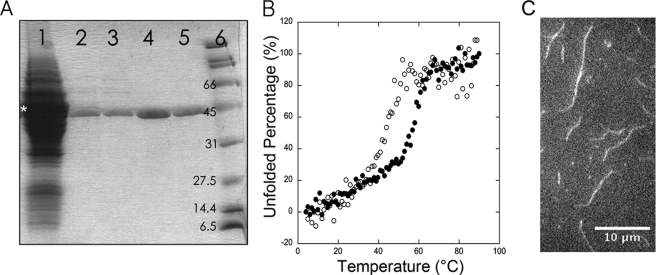
After homogenization of fission yeast, a substantial fraction of the actin pelleted with cell debris (data not shown) as expected because ∼13% of cellular actin is polymerized and associated with endocytic actin patches (2). We purified actin from the supernatant by affinity chromatography with the C-terminal half (domains 4–6) of gelsolin (11). When added to a cell lysate in Ca2+ buffer, His-tagged gelsolin G4-6 bound actin monomers and capped the barbed end of actin filaments (18, 19). After binding His-tagged gelsolin G4-6 with associated actin to nickel-Sepharose, actin was eluted with Mg2+ buffer containing EGTA. After adding another 200 μl of nickel-Sepharose to the eluted actin solution to bind any free G4-6, actin was polymerized, pelleted, depolymerized, and gel-filtered. Affinity chromatography produced the largest gain in purity and the cycle of polymerization, and gel filtration removed minor contaminants as shown by gel electrophoresis with Coomassie Blue staining (Fig. 1A). Quantitative Western blots showed that the yield of actin from 120 g of cells was ∼40 mg from the nickel-gelsolin column, 10 mg in the actin pellet, and 5 mg from the peak fractions after gel filtration (Fig. 1A). Given ∼0.5 mg of actin/g of wet fission yeast cells (2), the overall yield was 10% of the total actin from 120 g of cells.
FIGURE 1.
Purification, thermal stability, and filaments of S. pombe actin. A, SDS-PAGE stained with Coomassie Brilliant Blue of samples from the steps in the purification of fission yeast actin. Lane 1, total cell lysate plus purified C terminus of gelsolin (G4-6). The asterisk shows the band of purified gelsolin on 10–20% SDS-PAGE. Lane 2, protein eluted by EGTA from the nickel-gelsolin column. Lane 3, supernatant after the protein shown in lane 2 was treated with nickel-Sepharose. Lane 4, resuspended actin filament pellet. Lane 5, purified actin monomers eluted from the S-300 gel filtration column. Lane 6, protein standards with molecular masses. Fission yeast actin has the same molecular mass as His6-G4-6 (41.7 kDa), but the actin has higher electrophoresis mobility. Actin bands were confirmed by Western blot with primary anti-actin antibody (SC-47778, Santa Cruz Biotechnology). B, thermal denaturation measured by CD at 222 nm. Conditions were as follows: 2.5 ml of 2 μm actin in 2 mm Tris, 0.1 mm CaCl2, 1 mm NaN3, 0.05 mm ATP, 0.125 mm DTT, pH 8.0. ○, S. pombe actin; ●, chicken skeletal muscle actin. The temperature was increased at 5 °C/min in 1 °C-steps from 4 to 95 °C, and the solution was equilibrated at each temperature for 1 min before measuring CD with an Aviv CD spectrometer. The Tm values were 55 °C for muscle actin and 45 °C for S. pombe actin. C, fluorescence micrograph made by total internal reflection excitation of a field of 2 μm S. pombe actin filaments stained with 66 μm Alexa Fluor 488 phalloidin. 2 μm S. pombe actin was polymerized in the chamber for 10 min before staining for 5 min with phalloidin. Bar is 10 μm.
We used circular dichroism (CD) to assess the thermal stability of S. pombe actin. S. pombe and chicken muscle actin have similar CD spectra between 190 and 350 nm (supplemental Fig. S1). We monitored the change of CD at 222 nm when the protein was heated from 4 to 95 °C. The melting temperature (Tm) of fission yeast actin monomers was ∼45 °C in G-buffer, 10 °C lower than chicken skeletal muscle actin in our control experiments (Fig. 1B) and as measured previously by DNase I inhibition (20) and differential scanning calorimetry (21).
Polymerization of S. pombe Actin
In polymerization buffer containing KCl, EGTA, and Mg2+, S. pombe actin spontaneously formed filaments that bound Alexa Fluor 488 phalloidin. Fluorescence microscopy showed that they ranged in length from 0.5 to 10 μm (Fig. 1C).
To study the kinetics of polymerization, we labeled fission yeast actin Cys-374 with pyrene as widely applied for muscle actin (22, 23). When mixed with KMEI polymerization buffer, 5% pyrene-labeled S. pombe actin polymerized spontaneously (Fig. 2A) with a shorter initial lag than muscle actin (supplemental Fig. S2).
FIGURE 2.
Kinetics of S. pombe actin polymerization measured by the fluorescence of pyrene-labeled actin. Conditions were as follows: KMEI buffer containing 50 mm KCl, 1 mm MgCl2, 1 mm EGTA, 10 mm imidazole, 0.1 mm ATP, and 1 mm DTT, pH 7.0. A, spontaneous polymerization of 5% pyrene-labeled S. pombe actin monomers. Actin monomer concentrations were as follows: ●, 4 μm; ■, 3 μm; △, 2 μm; ○, 1 μm; □, 0.5 μm; and ▴, 0.3 μm. The solid lines are simulations of the reaction with trimer nuclei forming at the apparent rate constant of 0.1125 μm−1 s−1 for 4 μm (orange), 3 μm (blue), and 2 μm (red) actin monomer. B, elongation of 5% pyrene-labeled S. pombe actin monomers from 0.2 μm actin filament seeds. Actin monomer concentrations were as follows: X, 2 μm; ♢, 1.5 μm; △, 1 μm; □, 0.8 μm; ○, 0.6 μm; ▴, 0.4 μm; ■, 0.2 μm; and ●, 0 μm. C, dependence of the initial elongation rate on the concentration of actin monomers. The initial actin elongation rate was the slope of the first 100 s of each elongation reaction from panel B. A line with a slope of 0.17 fit the data with R2 = 0.99 and an X-intercept equal to the critical concentration of 0.07 μm. A.U., arbitrary units. D, simulations were performed using Virtual Cell, version 4.7. The model of actin assembly and phosphate release is as follows: the green circles are molecular species, and the yellow circles are reactions. Reaction 1, formation of actin dimer from two Mg2+-ATP·actin monomers with k+ = 35.7 μm−1 s−1 and k− = 1.63 × 108 s−1 (24). Reaction 2, irreversible reaction of a MgATP·actin dimer with a MgATP-actin monomer to form an actin trimer nucleus for elongation of a filament. The apparent rate constants were acquired by fitting the time course of pyrene actin polymerization (A). Reaction 3, elongation of both ends of filaments by the addition of MgATP·actin monomers. Self-assembled actin nuclei function as a catalyst (dashed line) in this reaction, and the rate constants (sum of two ends) were k+ = 12.9 μm−1 s−1 and k− = 2.2 s−1 (52). Reaction 4, the irreversible hydrolysis of ATP by polymerized MgATP·actin subunits yielding MgADP·Pi·actin subunits with a rate constant of 0.3 s−1 (25). Reaction 5, phosphate dissociation from MgADP·Pi·actin subunits (Fig. 3). Phosphate binds MgADP·actin subunits with a rate constant of 10−5 μm−1 s−1 (28), and the phosphate dissociation rate constant was estimated by fitting simulations of the model the to experimental data.
We analyzed the underlying reactions with a simple nucleation model with irreversible formation of actin trimer nuclei (Fig. 2D). Simulations with dimerization parameters from previous work (24) and a trimer formation rate constant of ∼0.11 μm−1 s−1 fit the time courses of polymerization over a range of S. pombe actin concentrations (Fig. 2A, supplemental Fig. S2, supplemental Fig. S3). Using the trimer formation rate constant (0.11 μm−1 s−1) that best fit the time course of 2 μm S. pombe actin polymerization gave simulated time courses for 4 and 3 μm S. pombe actin with only slightly longer lag phases than the experimental data (Fig. 2A). The best fits over a range of actin concentrations show that S. pombe actin forms trimers at least 5-fold faster than muscle actin (supplemental Fig. S3). The higher nucleation rate explains why S. pombe actin polymerizes faster than muscle actin (Fig. 2A, supplemental Fig. S2).
Elongation assays with 0.5 μm unlabeled S. pombe actin filament nuclei (Fig. 2B) gave initial polymerization rates proportional to the concentration of 5% pyrene-labeled S. pombe actin monomers. The x axis intercept gave the critical concentration of 0.11 ± 0.04 μm (Fig. 2C, six experiments), which is similar to muscle actin (0.052 ± 0.011 μm in two experiments; supplemental Fig. S2).
Rapid Phosphate Release from S. pombe Actin Filaments
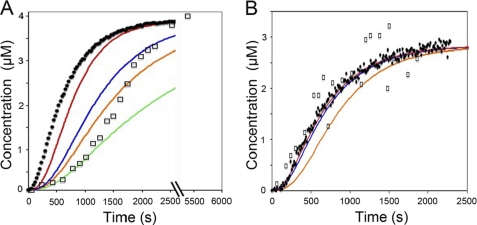
We followed the time course of phosphate release from ADP·Pi·actin filaments during the polymerization of [γ-32P]ATP·actin monomers. Incorporation of ATP·actin monomers into muscle actin filaments increases the rate of ATP hydrolysis by 40,000-fold (25, 26) followed by the dissociation of the γ-phosphate from each subunit. We separated [32P]phosphate that dissociated from polymerized actin from 32P bound to actin monomers and filaments by centrifugation through a 30-kDa molecular mass cut-off membrane. We monitored the polymerization of parallel samples of pyrene-labeled actin.
Time courses of S. pombe actin polymerization and [γ-32P]phosphate release overlapped (Fig. 3B), whereas muscle actin filaments released phosphate after a lag of nearly 10 min (Fig. 3A). We used two methods to estimate the rate of phosphate release. For muscle actin, we compared the concentrations of ADP·Pi·actin filaments and rates of [γ-32P]phosphate accumulation during the progress of actin polymerization and calculated a [γ-32P]phosphate release rate constant of ∼0.0015 s−1 (Fig. 3A), similar to published values (27, 28). For both types of actin, we also used a simple nucleation model (Fig. 2D) to estimate the phosphate release rate required to fit the observations.
FIGURE 3.
Time course of phosphate release during polymerization of muscle and S. pombe actin monomers. Conditions were as follows: KMEI buffer at 25 °C. □, [32P]phosphate released from polymerized [γ-32P]ATP·actin. ●, pyrene signal from polymerization of 5% pyrene-labeled actin. A, polymerization and phosphate release of 4 μm chicken muscle actin monomers. Simulations with different phosphate dissociation rate constants was as follows: red, 0.005 s−1; blue, 0.0015 s−1; orange, 0.001 s−1; green, 0.0005 s−1. B, polymerization and phosphate release of 3 μm fission yeast actin monomers. The data points are the average of three individual experiments. Simulations with different phosphate dissociation rate constants was as follows: red, 0.1 s−1; blue, 0.025 s−1; orange, 0.005 s−1.
For S. pombe actin simulations with phosphate, dissociation rate constants >0.025 s−1 fit the experimental time course of phosphate dissociation (Fig. 3B), but given the limited temporal resolution of the filtration assay, phosphate release could be much faster. A rate constant of 0.025 s−1 gives a 1-min lag of phosphate release behind actin polymerization, and the rate constant of 0.1 s−1 gives a lag of ∼15 s. Thus MgADP·Pi subunits in S. pombe actin filaments release hydrolyzed phosphate at least 10-fold faster and possibly 100-fold faster than subunits in muscle actin filaments.
For muscle actin, the simulated time courses do not fit the sigmoidal time course of phosphate release (Fig. 3A). Early in the reaction, phosphate dissociates from MgADP·Pi subunits at 0.0005 s−1, but the reaction is ∼10-fold faster later in the reaction. This change suggests that the rate of phosphate release depends on the nucleotide composition of muscle actin filaments.
S. pombe Actin Monomers Exchange Nucleotide Faster than Muscle Actin
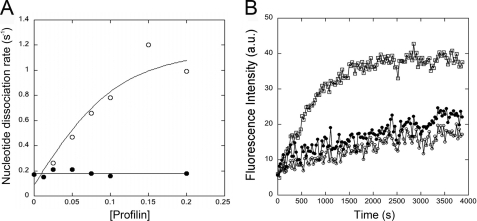
We measured dissociation of ϵ-ATP from actin monomers under polymerizing conditions in KMEI buffer by mixing 0.1 mm ATP with 0.1 μm Mg-ϵ-ATP·actin monomers and monitoring the fluorescence decrease as ϵ-ATP dissociated and was replaced by the excess of ATP. ϵ-ATP dissociated from S. pombe actin monomers at 0.17 s−1 (Fig. 4A), 40-fold faster than the rate of 0.004 s−1 for muscle actin (not shown).
FIGURE 4.
Effects of profilin on nucleotide exchange and polymerization of fission yeast actin monomers. A, dependence of the rate of ϵ-ATP dissociation from S. pombe Mg2+-actin monomers on profilin concentration. Conditions were as follows: Mg2+-ϵ-ATP·actin was mixed with 0.2 mm ATP and a range of profilin concentrations at 25 °C in KMEI buffer, and the dissociation of ϵ-ATP was measured by the decrease in fluorescence. ϵ-ATP dissociates from S. pombe actin at ∼0.17 s−1. 0.1 μm S. pombe actin titrated with human profilin (○) or S. pombe profilin (●). In experiments not shown, the nucleotide dissociation rates of muscle actin were 0.004 s−1 for muscle actin alone, 0.12 s−1 when saturated with human profilin, and 0.02 s−1 when saturated with S. pombe profilin. B, effect of profilin on the spontaneous polymerization of S. pombe actin monomers measured by the fluorescence of pyrene-labeled actin. Conditions were as follows: polymerization of 5% pyrene-labeled 3 μm S. pombe actin at 25 °C in KMEI buffer was monitored at 407 nm. □, S. pombe actin alone; ○, S. pombe actin with 2 μm human profilin; and ●, S. pombe actin with 2 μm S. pombe profilin. A.U., absorbance units.
Human profilin stimulated the rate of ϵ-ATP dissociation from fission yeast actin monomers from 0.17 to 1 s−1, but S. pombe profilin had no effect on ϵ-ATP dissociation from S. pombe actin (Fig. 4A), although low concentrations of S. pombe profilin stimulate ϵ-ATP dissociation from muscle actin 2.3-fold (29). Although S. pombe profilin did not stimulate nucleotide exchange of S. pombe actin monomers, it binds to these actin monomers because both 2 μm S. pombe and human profilin inhibited spontaneous polymerization of 3 μm S. pombe actin (Fig. 4B).
Phosphate Inhibits Binding of Cofilin to S. pombe ADP·Actin Filaments
We used the reduction in fluorescence of pyrene-labeled actin filaments (30) to measure binding of S. pombe cofilin to S. pombe actin filaments. As in the case of muscle actin filaments (15), fission yeast cofilin reduced the pyrene fluorescence of S. pombe ADP·actin filament faster than S. pombe ADP·Pi·actin filaments (Fig. 5A). The observed rate constants were close to those with muscle actin filaments3 (15). Because of cooperative binding of human (31) and fission yeast (32) cofilin to actin filaments, these time courses were nearly exponential even without pseudo-first order conditions (15). The observed rate constants were proportional to the S. pombe cofilin concentration, and the slopes of these plots were greater with S. pombe ADP·actin filaments than with S. pombe ADP·Pi·actin filaments (Fig. 5B).
FIGURE 5.
Binding of fission yeast cofilin to ADP and S. pombe ADP·Pi·actin filaments. Conditions were as follows: 2 μm 20% pyrene-labeled S. pombe actin filaments were mixed with an equal volume of S. pombe cofilin using a KinTek stopped-flow mixer with a dead time of ∼7 ms, and cofilin binding was measured by the decrease of pyrene fluorescence at 390 nm over time. □, ADP·actin filaments; ○, ADP·Pi·actin filaments. A, time course of the fluorescence changes in the presence of 2 μm fission yeast cofilin. Single exponential fits to the time courses gave kobs for cofilin binding to actin filaments, 17 s−1 for ADP·actin filaments and 3.1 s−1 for ADP·Pi·actin filaments. A.U., absorbance units. B, dependence of kobs on the cofilin concentration.
Nucleation of S. pombe Actin Filaments by S. pombe Arp2/3 Complex
We used fission yeast Arp2/3 complex labeled with pyrene on A317C of ARPC2 to measure binding to actin filaments (Fig. 6E). The fluorescence increase in the presence of actin filaments is interpreted as Arp2/3 complex binding to the side of actin filaments (33)2. As in the case of muscle actin filaments, the fluorescence changed very slowly after mixing 0.2 μm pyrene-labeled Arp2/3 complex with 6 μm S. pombe ADP or ADP·Pi·actin filaments. One micromolar fission yeast Wsp1p·CA4 blocked 0.2 μm S. pombe Arp2/3 complex from binding 15 μm muscle actin filaments2, but 10 times more Wsp1p·CA was required to inhibit binding to 6 μm S. pombe actin filaments.
FIGURE 6.
Interactions of S. pombe Arp2/3 complex with S. pombe and muscle actins. A–D, actin polymerization monitored over time at 25 °C by the increase of pyrene fluorescence intensity at 407 nm. A and B, dependence of the time course of the polymerization on the concentration of S. pombe Arp2/3 complex in the presence of a nucleation-promoting factor in KMEI buffer. A, 4 μm pyrene-labeled S. pombe actin. B, 4 μm pyrene-labeled chicken muscle actin. Conditions were as follows: 1 μm S. pombe Wsp1p-VCA with the following concentrations of S. pombe Arp2/3 complex: ○, 0 nm; ♢, 2 nm; □, 5 nm; △, 10 nm; ●, 20 nm; ■, 100 nm; or Δ, 100 nm GST N-WASp-VVCA with 5 nm Arp2/3 complex. C and D, dependence of the time course of the polymerization on the concentration of S. pombe Arp2/3 complex without a nucleation-promoting factor in KMEI buffer. C, 3 μm pyrene-labeled S. pombe actin, D, 3 μm pyrene-labeled chicken muscle actin with the following concentrations of Arp2/3 complex: □, 0 nm; ○, 100 nm; ♢, 500 nm. E, time course of 0.2 μm pyrene-labeled S. pombe Arp2/3 complex binding to 6 μm (○) ADP or (□) ADP·Pi·actin filaments measured by fluorescence intensity at 390 nm at 25 °C. Conditions were as follows: MEI buffer (1 mm MgCl2, 1 mm EGTA, 10 mm imidazole, 0.1 mm ATP, 1 mm DTT, pH 7.0) containing 25 mm K2SO4 or 25 mm potassium phosphate, pH 7.0, was mixed with actin filaments to generate either ADP or ADP·Pi·actin filaments. A.U., absorbance units.
Reaction of fission yeast Arp2/3 complex and a nucleation-promoting factor produced more rapid polymerization with S. pombe actin than with muscle actin (Fig. 6 and Table 1). We calculated the concentrations of actin filament barbed ends from the polymerization rates when half of the actin was polymerized. The two actins produced about the same concentrations (0.3 nm) of barbed ends in the absence Arp2/3 complex (Table 1), but over a range of concentrations of S. pombe Arp2/3 complex with 1 μm Wsp1p-VCA S. pombe actin generated 3-fold more barbed ends than muscle actin. As observed previously with muscle actin, VCA dimers had much higher nucleation activity than VCA monomers (Table 1). In the absence of nucleation-promoting factors, S. pombe Arp2/3 complex generated similar numbers of barbed ends with S. pombe or muscle actin monomers (Fig. 6, C and D, Table 2).
TABLE 1.
Production of actin filament barbed ends by fission yeast and muscle actin with Arp2/3 complex and VCA
Wsp1p-VCA is a protein construct consisting of the VCA domains of fission yeast WASp. N-WASP-VVCA is a protein construct consisting of the VVCA domains of N-WASP. Concentrations of ends were calculated from the time course of polymerization of 4 μm actin when half of the actin monomers were polymerized.
| Arp2/3 complex concentration | Nucleation-promoting factor | Barbed end concentration at t½ |
|
|---|---|---|---|
| S. pombe actin | Muscle actin | ||
| nm | |||
| 0 | 1 μm Wsp1p·VCA | 0.19 ± 0.07 | 0.25 ± 0.1 |
| 2 nm | 1 μm Wsp1p·VCA | 0.49 ± 0.8 | 0.36 ± 0.06 |
| 5 nm | 1 μm Wsp1p·VCA | 1.6 ± 0.7 | 0.64 |
| 10 nm | 1 μm Wsp1p·VCA | 2.6 ± 0.3 | 1.4 ± 0.1 |
| 20 nm | 1 μm Wsp1p·VCA | 4.4 ± 1 | 2.1 ± 0.5 |
| 100 nm | 1 μm Wsp1p·VCA | 12.9 ± 2.6 | 4.4 ± 0.6 |
| 5 nm | 0.1 μm GST N-WASP·VVCA | 2.9 ± 1.2 | 0.58 ± 0.09 |
TABLE 2.
Production of actin filament barbed ends by 3 μm fission yeast and muscle actin monomers with Arp2/3 complex without a nucleation-promoting factor
Concentrations of ends were calculated from the time course of polymerization when half of the actin monomers were polymerized.
| Reaction | Concentration of barbed ends at t½ |
|
|---|---|---|
| S. pombe actin | Muscle actin | |
| nm | ||
| Actin only | 0.15 ± 0.01 | 0.15 ± 0.02 |
| 100 nm Arp2/3 | 0.39 ± 0.01 | 0.27 ± 0.05 |
| 500 nm Arp2/3 | 1 ± 0.12 | 0.6 ± 0.04 |
DISCUSSION
We purified fission yeast actin and characterized its most relevant biochemical properties because one cannot rely on the properties of muscle or budding yeast actin for quantitative analysis of the actin system in fission yeast. Since fungi diverged from animals more than 1 billion years ago, and Saccharomyces cerevisiae and S. pombe diverged ∼400 million years ago (34, 35), about 10% of the residues in these actins changed (supplemental Fig. S4). These widely dispersed substitutions have resulted in functionally important differences in biochemical properties.
Comparisons of S. pombe Actin with Muscle and Budding Yeast Actins
Nucleotide binding to apo-actin is fast (k+ = 5 μm−1 s−1, (36)), so dissociation is rate-limiting for nucleotide exchange when ADP·actin monomers that dissociate from filaments need to be activated by binding ATP. The bound divalent cation strongly influences the dissociation rate, and Mg2+ is the physiologically relevant divalent cation. Our finding that the rate constant for Mg-ϵ-ATP dissociation is about 40-fold faster for S. pombe actin than muscle actin means that nucleotide dissociation occurs spontaneously with a half-time of only 4 s. Takaine and Mabuchi (10) found that Ca-ϵ-ATP dissociates only three times faster from S. pombe actin (0.013 s−1) than muscle actin (0.004 s−1) in low salt buffer.
Most profilins (37–39), including both S. cerevisiae and S. pombe profilin (29, 40), stimulate nucleotide exchange of muscle and cytoplasmic actin monomers, but plant profilins (40–42) do not. Remarkably, S. pombe profilin does not stimulate nucleotide dissociation of S. pombe Mg-ϵ-ATP·actin (Fig. 4A) or S. pombe Ca-ϵ-ATP·actin (10), but S. pombe profilin stimulates dissociation of both Mg-ϵ-ATP and Ca-ϵ-ATP from muscle actin despite having a 10-fold higher affinity for S. pombe CaATP·actin monomers (Kd = 0.15 μm) than for muscle actin monomers (10) in a low salt buffer. The inability of S. pombe profilin to stimulate nucleotide exchange of its own actin is not due simply to the intrinsically high rate of ATP dissociation because human profilin stimulated the rate of ϵ-ATP dissociation from fission yeast actin monomers 6-fold. A possible explanation for the lower nucleotide exchange activity of S. pombe profilin is shorter strands β4, β5, and β6 that flank both sides of the actin-profilin interface at the barbed end of the actin monomer (43). Because the nucleotide binding cleft and the barbed end groove of the actin monomers open and close in a reciprocal fashion, human profilin may favor the open nucleotide binding cleft because its longer strands provide additional interactions at the barbed end of actin monomers.
The inability of S. pombe profilin to stimulate nucleotide exchange of fission yeast actin is surprising given evidence that nucleotide exchange activity is required to complement the defects in profilin-null and temperature-sensitive mutations in fission yeast (29). It is thus likely that defects in affinity for actin explain the lack of function of these mutant profilins. The rapid, spontaneous dissociation of nucleotide accounts for the ability of Arabidopsis profilin (lacking nucleotide exchange activity (42)) to complement the phenotype of S. pombe profilin temperature-sensitive mutations (43, 44).
The critical concentration for polymerization of our preparation of S. pombe MgATP·actin was similar to muscle actin. Because the critical concentration is the ratio of the rate constants for elongation (k−/k+) and because the barbed end dominates measurements of elongation, we are confident that the rate constants for barbed end elongation of S. pombe actin are similar to those of muscle actin (and other actins such as Acanthamoeba actin) despite the fact that we were unable to measure the rate constants directly by fluorescence microscopy because of limited materials for labeling with Oregon green. The fact that both actins depolymerize at about the same rate in latrunculin A (10) reinforces this conclusion. Takaine and Mabuchi (10) measured critical concentrations of 0.5 μm for both S. pombe and muscle actins in MgCl2 and KCl by steady state light scattering. The difference from our observations is likely the presence of EGTA in our physiological buffer, which binds the Ca2+ in the actin monomer buffer and lowers the critical concentration. Electron microscopy and optical diffraction established that the structure of S. pombe actin filaments is indistinguishable from muscle actin filaments (10). Both types of filaments bind and activate the ATPase of muscle myosin heavy meromyosin (10).
Like the S. pombe actin prepared by Takaine and Mabuchi (10), the spontaneous polymerization of our preparation of S. pombe actin was faster than muscle actin (Fig. 2, supplemental Fig. S1), and our computer simulations indicate that the rate of nucleation was about five times faster. Spontaneous polymerization of budding yeast actin is also faster than muscle actin (45, 46).
We used a new filtration assay with small samples and cleaner background signal than previous methods (27, 47) to show that S. pombe actin filaments release phosphate at least 10 times faster than muscle actin filaments. Phosphate release keeps up with polymerization of S. pombe actin, so we can put a lower limit on the rate constant for phosphate dissociation of ∼0.025 s−1, but rate constants as high as 0.1 s−1 are also consistent with the data. Phosphate release from budding yeast actin also overlaps the time course of polymerization (48). The phosphate dissociation rate constant from budding yeast actin, calculated without consideration of the concentration of polymerized actin, is ∼0.012 s−1 (49), so this value is smaller than our rate constant taking the concentrations of ADP·Pi·actin subunits into account. We did not measure the rate at which S. pombe actin filaments hydrolyze ATP, but note that the active sites of muscle and S. pombe actin are identical and that the measured phosphate dissociation rate is close to the rate of ATP hydrolysis by muscle actin (0.3 s−1) (25, 26), so we expect that the hydrolysis rate for S. pombe actin is similar to muscle actin.
As in previous work (27, 47), we observed that phosphate release lags far behind the polymerization of muscle actin filaments, but our simulations of a simple model of actin polymerization fit the experimental time course of phosphate release poorly (Fig. 3). Thus rate of phosphate release appears to increase during the time course of polymerization and phosphate dissociation. Perhaps the conformational changes within the filament that are coupled to phosphate release might increase the rate of phosphate release from adjacent ADP·Pi·actin subunits as the filament ages.
Like all other profilins tested, S. pombe profilin strongly inhibits spontaneous polymerization of S. pombe MgATP·actin monomers (Fig. 4B) (10). Contrary to most other profilins tested, Takaine and Mabuchi (10) presented evidence that S. pombe profilin also reduces the extent of polymerization of S. pombe actin and the length of the filaments at steady state. The absence of EGTA in their buffer is one plausible explanation for this difference.
S. pombe cofilin binds faster to S. pombe actin filaments with bound ADP than bound ADP·Pi. The rates and cooperativity are similar for S. pombe cofilin and muscle actin filaments (15).
In the presence of the fission yeast nucleation-promoting factor Wsp1p-VCA, S. pombe Arp2/3 complex generated 3-fold more barbed ends with S. pombe actin than with muscle actin (Table 1). This may be due to the higher affinity of S. pombe Arp2/3 complex for S. pombe actin filaments because a higher concentration of Wsp1p-CA was required to inhibit the interaction between S. pombe Arp2/3 complex and S. pombe actin filament, but limited amounts of S. pombe actin precluded detailed characterization of these reactions.
Budding yeast Arp2/3 complex does not require a nucleation-promoting factor to stimulate polymerization of its own actin (7). In the absence of a nucleation-promoting factor, fission yeast Arp2/3 complex also stimulates the polymerization of its own actin slightly better than muscle actin (Fig. 6, C and D, Table 2). The conditions differed, but S. pombe Arp2/3 complex appears to be less active on its own than budding yeast Arp2/3 complex (7).
Functional Implications of the New Information about Fission Yeast Actin
The purification and characterization of the basic properties of S. pombe actin in this study and by Takaine and Mabuchi (10) provide information essential for studying endocytosis and cytokinesis using S. pombe as the model organism. S. pombe actin is more dynamic than muscle actin in terms of faster nucleotide exchange by monomers, faster actin filament nucleation, faster dissociation of phosphate from ADP·Pi·actin filaments, and faster actin filament formation with S. pombe Arp2/3 complex. These properties help to account for the rapid assembly and turnover of actin filaments in fission yeast and assist us in understanding the mechanisms of endocytosis and cytokinesis.
Arp2/3 complex and its activators WASp (Wsp1p) and myosin-I (Myo1p) are required to assemble actin filaments at sites of endocytosis in budding and fission yeast (50, 51). Simulations of a mathematical model that accounts for the assembly and disappearance of actin filaments at the site of endocytosis in fission yeast indicated that the formation of actin filament branches is several hundred times faster than observed with purified Arp2/3 complex, WASp-VCA, and muscle actin monomers (3, 5). The F-BAR protein Bzz1p can increase the rate of nucleation by about 4-fold,5 and our finding that S. pombe Arp2/3 complex forms new filaments faster with S. pombe actin than muscle actin further narrows the gap between measurements with purified proteins and in cells.
Because of rapid ATP hydrolysis and phosphate release after polymerization of S. pombe actin, filaments will have a high proportion of ADP·actin subunits, favoring S. pombe cofilin binding (Fig. 5) and severing of the filaments. This new information about rapid phosphate release supports the mathematical model, which showed that the rapid phosphate release rate of S. pombe actin filaments is required for the rapid loss of actin filaments from patches in 10 s (5). Once filaments depolymerized in cells, fast spontaneous nucleotide exchange with a half-time of 4 s should be fast enough to recharge ADP·actin for another round of polymerization despite the fact that S. pombe profilin does not stimulate nucleotide exchange.
Supplementary Material
Acknowledgments
We thank Aaron Downs for helpful suggestions. We also thank Ronald Breaker for assisting us with the [γ-32P]ATP experiments in the Breaker laboratory.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Research Grant GM-026338 (to T. D. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
S. C. Ti, C. Jurgenson, B. J. Nolen, and T. D. Pollard, manuscript submitted for publication.
Q. Chen and T. D. Pollard, manuscript in revision.
Wsp1p·CA is a protein construct consisting of the C and A domains of fission yeast WASp.
R. Arasada and T. D. Pollard, manuscript in revision.
REFERENCES
- 1. Pollard T. D., Cooper J. A. (2009) Science 326, 1208–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu J. Q., Pollard T. D. (2005) Science 310, 310–314 [DOI] [PubMed] [Google Scholar]
- 3. Sirotkin V., Berro J., Macmillan K., Zhao L., Pollard T. D. (2010) Mol. Biol. Cell 21, 2894–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pollard T. D., Wu J. Q. (2010) Nat. Rev. Mol. Cell Biol. 11, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berro J., Sirotkin V., Pollard T. D. (2010) Mol. Biol. Cell 21, 2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vavylonis D., Wu J. Q., Hao S., O'Shaughnessy B., Pollard T. D. (2008) Science 319, 97–100 [DOI] [PubMed] [Google Scholar]
- 7. Wen K. K., Rubenstein P. A. (2005) J. Biol. Chem. 280, 24168–24174 [DOI] [PubMed] [Google Scholar]
- 8. Wen K. K., McKane M., Houtman J. C., Rubenstein P. A. (2008) J. Biol. Chem. 283, 9444–9453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wen K. K., Rubenstein P. A. (2009) J. Biol. Chem. 284, 16776–16783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takaine M., Mabuchi I. (2007) J. Biol. Chem. 282, 21683–21694 [DOI] [PubMed] [Google Scholar]
- 11. Ohki T., Ohno C., Oyama K., Mikhailenko S. V., Ishiwata S. (2009) Biochem. Biophys. Res. Commun. 383, 146–150 [DOI] [PubMed] [Google Scholar]
- 12. Pollard T. D. (1984) J. Cell Biol. 99, 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuhn J. R., Pollard T. D. (2005) Biophys. J. 88, 1387–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovar D. R., Kuhn J. R., Tichy A. L., Pollard T. D. (2003) J. Cell Biol. 161, 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan C., Beltzner C. C., Pollard T. D. (2009) Curr. Biol. 19, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaiser D. A., Goldschmidt-Clermont P. J., Levine B. A., Pollard T. D. (1989) Cell Motil. Cytoskeleton 14, 251–262 [DOI] [PubMed] [Google Scholar]
- 17. Helmerhorst E., Stokes G. B. (1980) Anal. Biochem. 104, 130–135 [DOI] [PubMed] [Google Scholar]
- 18. Haddad J. G., Harper K. D., Guoth M., Pietra G. G., Sanger J. W. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 1381–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun H. Q., Yamamoto M., Mejillano M., Yin H. L. (1999) J. Biol. Chem. 274, 33179–33182 [DOI] [PubMed] [Google Scholar]
- 20. Schüler H., Lindberg U., Schutt C. E., Karlsson R. (2000) Eur. J. Biochem. 267, 476–486 [DOI] [PubMed] [Google Scholar]
- 21. Bertazzon A., Tian G. H., Lamblin A., Tsong T. Y. (1990) Biochemistry 29, 291–298 [DOI] [PubMed] [Google Scholar]
- 22. Kouyama T., Mihashi K. (1981) Eur. J. Biochem. 114, 33–38 [PubMed] [Google Scholar]
- 23. Cooper J. A., Walker S. B., Pollard T. D. (1983) J. Muscle Res. Cell Motil. 4, 253–262 [DOI] [PubMed] [Google Scholar]
- 24. Sept D., McCammon J. A. (2001) Biophys. J. 81, 667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blanchoin L., Pollard T. D. (2002) Biochemistry 41, 597–602 [DOI] [PubMed] [Google Scholar]
- 26. Rould M. A., Wan Q., Joel P. B., Lowey S., Trybus K. M. (2006) J. Biol. Chem. 281, 31909–31919 [DOI] [PubMed] [Google Scholar]
- 27. Carlier M. F., Pantaloni D. (1986) Biochemistry 25, 7789–7792 [DOI] [PubMed] [Google Scholar]
- 28. Carlier M. F., Pantaloni D. (1988) J. Biol. Chem. 263, 817–825 [PubMed] [Google Scholar]
- 29. Lu J., Pollard T. D. (2001) Mol. Biol. Cell 12, 1161–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carlier M. F., Laurent V., Santolini J., Melki R., Didry D., Xia G. X., Hong Y., Chua N. H., Pantaloni D. (1997) J. Cell Biol. 136, 1307–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao W., Goodarzi J. P., De La Cruz E. M. (2006) J. Mol. Biol. 361, 257–267 [DOI] [PubMed] [Google Scholar]
- 32. Andrianantoandro E., Pollard T. D. (2006) Mol. Cell 24, 13–23 [DOI] [PubMed] [Google Scholar]
- 33. Beltzner C. C., Pollard T. D. (2008) J. Biol. Chem. 283, 7135–7144 [DOI] [PubMed] [Google Scholar]
- 34. Sipiczki M. (1995) Antonie. Van. Leeuwenhoek 68, 119–149 [DOI] [PubMed] [Google Scholar]
- 35. Sipiczki M. (2000) Genome. Biol. 1, REVIEWS1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De La Cruz E. M., Pollard T. D. (1995) Biochemistry 34, 5452–5461 [DOI] [PubMed] [Google Scholar]
- 37. Mockrin S. C., Korn E. D. (1980) Biochemistry 19, 5359–5362 [DOI] [PubMed] [Google Scholar]
- 38. Nishida E. (1985) Biochemistry 24, 1160–1164 [DOI] [PubMed] [Google Scholar]
- 39. Goldschmidt-Clermont P. J., Machesky L. M., Doberstein S. K., Pollard T. D. (1991) J. Cell Biol. 113, 1081–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eads J. C., Mahoney N. M., Vorobiev S., Bresnick A. R., Wen K. K., Rubenstein P. A., Haarer B. K., Almo S. C. (1998) Biochemistry 37, 11171–11181 [DOI] [PubMed] [Google Scholar]
- 41. Kovar D. R., Drøbak B. K., Staiger C. J. (2000) Plant Cell 12, 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perelroizen I., Didry D., Christensen H., Chua N. H., Carlier M. F. (1996) J. Biol. Chem. 271, 12302–12309 [DOI] [PubMed] [Google Scholar]
- 43. Ezezika O. C., Younger N. S., Lu J., Kaiser D. A., Corbin Z. A., Nolen B. J., Kovar D. R., Pollard T. D. (2009) J. Biol. Chem. 284, 2088–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christensen H. E., Ramachandran S., Tan C. T., Surana U., Dong C. H., Chua N. H. (1996) Plant J. 10, 269–279 [DOI] [PubMed] [Google Scholar]
- 45. Buzan J. M., Frieden C. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim E., Miller C. J., Reisler E. (1996) Biochemistry 35, 16566–16572 [DOI] [PubMed] [Google Scholar]
- 47. Carlier M. F. (1987) Biochem. Biophys. Res. Commun. 143, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 48. Yao X., Rubenstein P. A. (2001) J. Biol. Chem. 276, 25598–25604 [DOI] [PubMed] [Google Scholar]
- 49. Bryan K. E., Rubenstein P. A. (2005) J. Biol. Chem. 280, 1696–1703 [DOI] [PubMed] [Google Scholar]
- 50. Kaksonen M., Toret C. P., Drubin D. G. (2006) Nat. Rev. Mol. Cell Biol. 7, 404–414 [DOI] [PubMed] [Google Scholar]
- 51. Galletta B. J., Cooper J. A. (2009) Curr. Opin. Cell Biol. 21, 20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pollard T. D. (1986) J. Cell Biol. 103, 2747–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.