Short abstract

The accumulation of harmful and persistent organic molecules in soils and sediment is a major environmental concern. Removal by physical means such as riverine, lacustrine, or marine dredging can be prohibitively difficult, expensive, and may not ultimately prove effective. An alternative is to locally change the geochemistry to stabilize and sequester the contaminants and render them biologically unavailable. Ghosh et al. report on pilot projects to determine whether activated carbon would be so useful. Their Feature concludes with what more needs to be done to minimize anthropogenic chemical blights in soil and sediments.
Aquatic sediments form the ultimate repositories of past and ongoing discharges of hydrophobic organic compounds (HOCs) such as polychlorinated biphenyls (PCBs), many pesticides, and dioxins, as well as mercury (Hg) and methylmercury (MeHg). These sediment-bound pollutants serve as long-term exposure sources to aquatic ecosystems. Approximately 10% of the sediment underlying the United States' surface water is sufficiently contaminated with toxic pollutants to pose potential risks to fish and fish-eating wildlife and humans.(1) Remediation of contaminated sediments remains a technological challenge. Traditional approaches do not always achieve risk reduction goals for human health and ecosystem protection and can even be destructive for natural resources. Though removal of contaminated sediment by dredging and disposal in a secure landfill can be effective under certain conditions, a recent study by the National Research Council found a wide range of outcomes.(2) Among the problems with dredging are unfavorable site conditions, resuspension of contaminated sediment into the water column, and contaminated sediment residuals. While capping contaminated sediment with clean sand may be a viable remedial option at some sites, often the alteration of sediment bathymetry may not be acceptable and the control of contaminant transport through the cap can be a challenge. In addition, both dredging and conventional capping result in the destruction of existing benthic ecosystems. Therefore, development of new techniques offering greater flexibility in contaminated sediment management and avoiding some of the problems with conventional dredging and capping is highly desirable.
This feature article summarizes research by several groups in the U.S. and Europe to develop a novel approach for in situ sediment remediation that minimizes or eliminates some of the problems with traditional technologies. The efforts involve introducing sorbent amendments into contaminated sediments that alter sediment geochemistry, increase contaminant binding, and reduce contaminant exposure risks to people and the environment. We present here a description of recently concluded laboratory studies and a brief outline of ongoing pilot-scale trials, field challenges, regulatory issues, and further research needs.
Bioavailability of Sediment-Bound Legacy Contaminants
Sediment HOCs can be taken up by aquatic or benthic organisms through ingestion and dermal absorption, and subsequently passed on to higher organisms and humans. For both of these pathways, the uptake depends on the bioavailability of contaminants in sediment, which is determined by how strongly the contaminants are bound to the sediment particles.3,4 Strong binding in the sediment matrix reduces contaminant bioavailability to organisms. Work in the last two decades has improved our understanding of how sediment geochemistry controls contaminant bioavailability. For example, black carbonaceous particles in sediments such as soot, coal, and charcoal very strongly bind HOCs, and their presence in sediments (both natural and anthropogenic) reduces exposure and risk,5,6 often by one order of magnitude or more compared to natural organic matter.
Contaminant Sequestration by Active Amendments
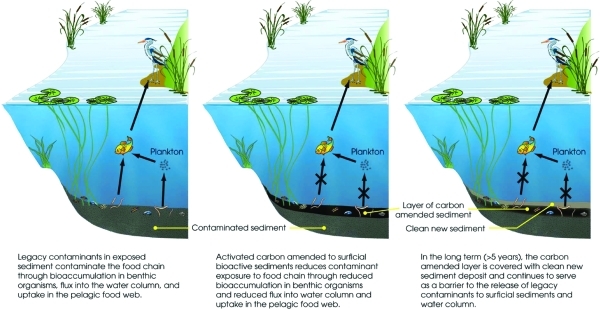
“Natural” contaminant sequestration in native carbonaceous particles can be greatly enhanced by the addition of clean, manufactured carbonaceous materials into sediments, such as activated carbon (AC). AC is produced from coal or biomass feedstock and treated at high temperature to produce a highly porous structure with great sorption capacity. Activated carbons have been used widely for drinking water purification and human poisoning abatement. McLeod et al (7) showed in clam particle feeding studies that the biouptake of a tetrachloro-PCB in the gut was only 1−2% for AC-sorbed PCBs, compared to 90% for diatom-sorbed ones. As illustrated in Figure 1, amending or thin-capping the bioactive surface layer of sediment with AC will transfer contaminants from the sediment to the strongly binding AC particles, reducing bioavailability to benthic organisms and contaminant flux into the water column, and thus accumulation in the aquatic food-chain. Sediment turnover by benthic organisms and other natural mixing processes can further incorporate the added AC into deeper or newly depositing sediment layers.(11) In depositional sediment environments, where legacy contaminants are often found, over time new clean sediment can cover the AC-treated sediment layer (Figure 1).
Figure 1.
Conceptual model of how sorbent amendment of sediment reduces contaminant exposure pathways of benthic organism accumulation and flux from the sediment bed.
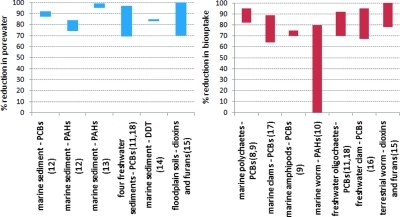
Laboratory tests with contaminated sediment show proof-of-concept through reductions in HOC bioavailability (Figure 2). These studies evaluated HOC bioavailability through measurement of equilibrium aqueous concentration and biouptake in a range of benthic organisms. The study sediments were all field-collected and had aged for decades in freshwater or marine environments. HOC concentrations in sediment porewater provide a useful assessment of the potential sediment-to-water flux, especially when legacy contaminated sediments are the primary pollution source. Sediment porewater concentration is also predictive of HOC biouptake in benthic organisms.(19) Tests with a range of field sediments showed that AC amendment in the range of 1−5% reduces equilibrium porewater concentration of PCBs, PAHs, DDT, dioxins, and furans in the range of 70−99%, thus reducing the driving force for the diffusive flux of HOCs into the water column and transfer into organisms. Most of the studies using benthic organisms show a reduction of biouptake of HOCs in the range of 70−90% compared to untreated control sediment (Figure 2).
Figure 2.
Percent reduction ranges of aqueous equilibrium concentration and contaminant biouptake in different laboratory studies of activated carbon amendment to sediments and soils from the field. These studies range from freshwater to marine sediments and cover a wide range of benthic organisms. The dose of activated carbon used in these laboratory experiments typically ranged from 1 to 5% by dry sediment weight (29).
Recent work on metal-contaminated sediments demonstrated reduced biouptake of cadmium (Cd) (20) and Hg/MeHg (21) after amendment of AC and thiol-functionalized silica into sediments. Significant reductions in Hg from water may be feasible with polysulfide-rubber polymer-coated AC.(22) AC mixed into sediment showed about one order of magnitude weaker sorption than pure AC for HOCs,13,23 probably attributable to sorptive competition with native HOCs and/or biomolecules or pore clogging.(24) In total, the varied laboratory results demonstrate that the effectiveness of sorbent amendment on lowering contaminant bioavailability increases with decreasing AC particle size, increasing dose of AC, greater mixing, and contact time. Biodynamic modeling with species-specific physiological parameters was able to describe invertebrate tissue concentrations and response to reduced uptake efficiency and pore water concentrations for strongly bound contaminants.8,23,25 There are many specialty carbons available in the market, but those most suitable for use in sediment remediation will have good sorption properties for the target contaminant (PCBs or Hg for example), will need to have no inherent toxicity, and will need to be low-cost. While some studies14,22 have compared different types of AC for use in sediment remediation, there is potential for more research in this area.
Current Status of Technology Development: Ongoing Pilot-Scale Demonstrations
Motivated by encouraging bench-scale results, pilot-scale field trials were recently conducted at five sites in the U.S. and Norway as shown in Figure S1 and Table S1 in the Supporting Information. These field experiments are evaluating different methods of applying AC to sediments to reduce the bioavailability of hydrophobic contaminants. The field sites span a range of contaminated aquatic environments: (1) tidal mudflat, (2) freshwater river, (3) marine harbor, (4) deep-water fjord, and (5) tidal creek and marsh. Each site poses varied engineering challenges in the application of AC and monitoring of the effectiveness. The key objectives of the pilot-scale experiments are to study the feasibility of application of AC using large-scale equipment in contaminated field sites, persistence of the AC and its binding potential after application to sediment in the natural environment, effectiveness of the AC in reducing contaminant bioavailability, reductions of sediment porewater contaminant concentrations and sediment-to-water fluxes, and effects of AC addition on the existing benthic community.
A major challenge in pilot evaluations is accounting for transient and/or long-term changes that take place naturally in the open environment. Pilot-studies by design occupy a relatively small footprint in a large contaminated sediment area that typically is overlain by contaminated water mass. Thus, in situ measurements of pore water concentrations at the sediment surface or bioaccumulation assessments using benthic organisms exposed to contaminants in the water phase (e.g., filter-feeding bivalves) can be impacted by the contaminated water above the treatment zone. Finally, over time the small pilot-treatment areas may become covered with newly deposited, contaminated sediment from the surrounding area or upstream locations. Some of the challenges in field assessments can be addressed through appropriate study designs:
-
(1)
Observations of changes in bioaccumulation at treatment sites need to be contrasted to ongoing changes at properly selected background control sites.
-
(2)
Using deposit-feeding organisms for biomonitoring is preferable to using filter feeders for assessing pilot-scale remediation.
-
(3)
In situ assessments should preferably have an ex situ laboratory component to delineate overlying water and depositional impacts.
-
(4)
The number of replicate samplings should be large enough to account for spatial variability at the site.
-
(5)
Multiple lines of evidence for exposure reduction, including physical, chemical, and biological, need to be pursued to obtain confidence in the observations.
Findings from Hunters Point and Big Picture
Results from the first pilot study at Hunters Point in San Francisco Bay were recently published.26,27 The Hunters Point study found that AC can be placed in sediment in a large scale, is physically stable in the environment, and remains effective at binding contaminants in sediments several years after application.(27) The AC applied at Hunters Point did not show a significant impact on benthic community as judged by the diversity of species and their overall abundance. This community-level observation from the field is in contrast to a laboratory study where potential toxic effects of AC on benthic organisms were indicated.(28)
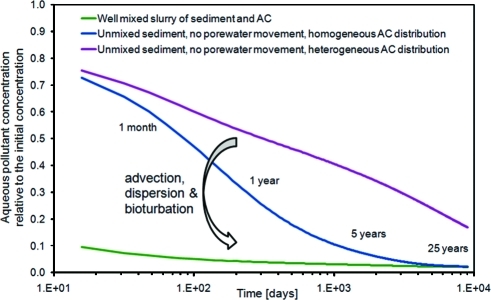
Typical AC dosing at the various test sites was 2−5% by weight of dry sediment (matching the native organic carbon content of sediment) in the top 10−30 cm of sediment. Even under poor mixing conditions, mass transfer of PCBs to a passive sampler in sediment was greatly reduced in the presence of AC.(29) Homogeniety of AC distribution and mixing regime will influence the time required to observe full treatment benefits under field conditions (Figure 3). Small-scale heterogeneity of sorbent distribution at the scale of 1 cm will extend the time required, whereas porewater movement by advection or mechanical dispersion and/or bioturbation will enhance contact between sediment and the added sorbents.
Figure 3.
Simulated decrease in the average aqueous PCB-101 concentration for Hunters Point sediment amended with 3.4% by weight activated carbon with a mean particle size of 150 μm. The simulation of the heterogeneous distribution assumes 1 cm spherical volumes of activated carbon free sediment surrounded by activated carbon rich sediment.
The amount of AC required to remediate a site with 5% in the top 10 cm of bioactive sediment is 35,000 kg/ha which amounts to about $75,000/ha at a bulk cost of AC of about $2.2/kg. Cost of AC application will depend on several factors including the need for mixing into sediment, and whether the application and mixing can be accomplished in an exposed sediment surface or needs to be performed underwater. The full cost of AC application is being evaluated through the ongoing pilot studies. By comparison, dredging and disposal cost for the Hudson River cleanup has been projected at $2.5M/ha (30) and reported actual for phase I at $15M/ha.(31) Thus, the material cost of AC required for treatment is at least an order of magnitude lower than typical full cost of remediation by dredging and disposal.
The technology is especially attractive at locations where dredging is not feasible or appropriate, such as (i) under piers and around pilings, (ii) in sediment full of debris, (iii) in areas where overdredging is not possible, and (iv) in ecologically sensitive sites such as wetlands. In situ amendments can also be used in combination with other remedies. For example, sorbent amendments can be applied during and immediately after a dredging process to minimize aqueous contaminant release from resuspended sediments and residuals, or as an amendment to sand caps to enhance retardation capacity.
Potential Use of Biochars and Carbon Sequestration
Charcoals, especially anthropogenic ones created under high-temperature conditions (“biochar”), are known to persist for thousands of years in soils and sediments, indicating carbon storage opportunities for greenhouse gas abatement.32,33 AC manufactured from biomass waste products such as pine chips, corn stalk, and poultry litter thus offer an exciting opportunity for efficient resource utilization and carbon sequestration along with sediment remediation.(34) New types of ACs made from renewable resources are being developed and are claimed to have superior metal sorption characteristics.(35) In addition, the U.S. Environmental Protection Agency’s new Green Remediation strategy aims to minimize the environmental footprints of a cleanup.(36) Therefore, technologies that can diminish or reverse the carbon footprint while reducing risks will likely be favored in the future. Major unknowns are currently whether a technology can be developed to place (activated) biochars on a sediment bed, and to what extent these materials can be effective in reducing organic and metal contaminant bioavailability in sediments.
Potential Barriers to Using in Situ Amendments and Future Research Needs
Sorbent amendment does not decrease total sediment concentrations of contaminants. Rather, it decreases contaminants available for biouptake and transport to surface- and groundwater. Sediment risk management is often based on bulk total concentrations and chemical mass with these measures being considered indicative of exposure.5,37 Although regulatory confidence and comfort are building for the explicit consideration of bioavailability in assessments and remedial decisions, there is still a bias against remedies other than removal. There are also natural perceptions and regulatory precedents to “get it out”. This surgical view of sediment remediation is appropriate in many cases but there are numerous situations where removal is not warranted and can be destructive or potentially ineffective for risk reduction. A more balanced evaluation of less invasive remedial measures such as in situ remedies can be achieved by broadening the decision context to include all relevant factors, such as short- and long-term ecological impacts and benefits, residual impacts, and performance. Comparisons of alternatives could involve comparative life cycle assessments.
The pilot studies are starting to provide valuable information to address concerns about long-term effectiveness both in terms of physical stability of the AC and chemical permanence of the remedy. To gain acceptance and advance the technology, it is likely that pilot-scale studies will have to lead to full-scale experimental remedies at a few sites with long-term monitoring to evaluate effectiveness not only near the base of the food chain, but also into evaluating recovery of fish and higher animals that are often the drivers for risk management.
To that end, further research is needed in the following areas:
-
(1)
development of novel amendments that can actively bind contaminants of concern other than HOCs;
-
(2)
improved fundamental understanding of mechanisms of HOC binding to AC, especially in the sediment matrix where fouling can be a concern;
-
(3)
development of efficient, low-impact delivery methods for amendments into sediments;
-
(4)
pilot-scale studies at various hydrodynamic and ecological environments to understand where the technology is best suited;
-
(5)
assessment of ecosystem recovery;
-
(6)
potential for microbial processes to degrade sorbed contaminants
-
(7)
full-scale demonstration to go beyond what can be learned through small-scale pilot studies;
-
(8)
development of modeling tools to interpret field results, understand food web transfer, predict long-term performance, and optimize AC dose and engineering methods of application;
-
(9)
life-cycle analyses including carbon footprints of different sediment remediation technologies.
Acknowledgments
We acknowledge numerous researchers and graduate students listed as authors in the cited papers (7−27) who have made major contributions in advancing the understanding of in situ amendments. We also thank the various sponsors of this research, notably, the U.S. Department of Defense through the SERDP/ESTCP programs, the National Institutes of Health through the Superfund Research Program, USEPA SBIR and GLNPO, Alcoa, Schlumberger, The Dow Chemical Company, DuPont, the Leverhulme Trust (UK), and the Norwegian Research Council.
Biography
Upal Ghosh is an associate professor of Civil and Environmental Engineering at the University of Maryland Baltimore County. Richard G. Luthy is Silas H. Palmer Professor in the Department Civil and Environmental Engineering, and Senior Fellow, Woods Institute for the Environment at Stanford University. U.G. and R.G.L. performed initial research on in situ sediment amendments at Stanford University and led the pilot-scale demonstrations at Hunters Point and Grasse River. Gerard Cornelissen is an environmental chemist at the Norwegian Geotechnical Institute, Professor at the University of Life Sciences, Norway, and also attached to Stockholm University, Sweden. He is leading the two AC amendment field studies in Norway along with Dr. Espen Eek and NGI-colleagues. Dr. David Werner is a Senior Lecturer in Environmental Engineering in the School of Civil Engineering and Geosciences at Newcastle University and has been closely involved in AC amendment studies especially with respect to contaminant mass transfer and biouptake modeling in AC-amended sediments. Charles A. Menzie is the Director of EcoSciences Practice at Exponent and has expertise in Environmental Risk Assessment. U.G. and C.A.M. codeveloped SediMite through a USEPA SBIR program and are leading the pilot-scale demonstrations in two wetland systems.
Supporting Information Available
A summary of ongoing pilot demonstration projects. This information is available free of charge via the Internet at http://pubs.acs.org/.
Features in Environmental Science & Technology can no longer include Supporting Information. This manuscript was received prior to January 1, 2011 and is therefore exempt. See the Instructions to Authors for more information on acceptable manuscript formats.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- U.S. Environmental Protection Agency. EPA’s Contaminated Sediment Management Strategy; EPA-823-R-98-001; Office of Water, April 1998. [Google Scholar]
- National Research Council. Sediment Dredging at Superfund Megasites: Assessing the Effectiveness; The National Academies Press: Washington, DC, 2007. [Google Scholar]
- Luthy R. G.; Aiken G. R.; Brusseau M. L.; Cunningham S. D.; Gschwend P. M.; Pignatello J. J.; Reinhard M.; Traina S. J.; Weber W. J. Jr.; Westall J. C. Sequestration of hydrophobic organic contaminants by geosorbents. Environ. Sci. Technol. 1997, 31, 3341–3347. [Google Scholar]
- The National Research Council. Bioavailability of Contaminants in Soils and Sediments; National Academies Press: Washington, DC, 2003. [Google Scholar]
- Cornelissen G.; Gustafsson O.; Bucheli T. D.; Jonker M. T. O.; Koelmans A. A.; van Noort P. C. M. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Critical Review. Environ. Sci. Technol. 2005, 39, 6881–6895. [DOI] [PubMed] [Google Scholar]
- Ghosh U.; Luthy R. G.; Gillette J. S.; Zare R. N. Microscale location, characterization, and association of polycyclic aromatic hydrocarbons on harbor sediment particles. Environ. Sci. Technol. 2000, 34, 1729–1736. [Google Scholar]
- McLeod P. B.; Van Den Heuvel-Greve M. J.; Allen-King R. M.; Luoma S. N.; Luthy R. G. Effects of particulate carbonaceous matter on the bioavailability of benzo[a]pyrene and 2,2′,5,5′-tetrachlorobiphenyl to the clam, Macoma balthica. Environ. Sci. Technol. 2004, 38, 4549–4556. [DOI] [PubMed] [Google Scholar]
- Janssen E. M. L.; Croteau M. N.; Luoma S. N.; Luthy R. G. Measurement and modeling of polychlorinated biphenyl bioaccumulation from sediment for Neanthes arenaceodentata and response to sorbent amendment. Environ. Sci. Technol. 2010, 44, 2857–2863. [DOI] [PubMed] [Google Scholar]
- Millward R. N.; Bridges T. S.; Ghosh U; Luthy R. G.; Zimmerman J. R. Addition of activated carbon to sediments to reduce PCB bioaccumulation by the polychaete, Neanthes arenaceodentata, and the amphipod, Leptocheirus plumulosus. Environ. Sci. Technol. 2005, 39, 2880–2887. [DOI] [PubMed] [Google Scholar]
- Cornelissen G.; Breedveld G. D.; Oen A. M. P.; Næs K.; Ruus A. Bioaccumulation of native PAHs from sediment by a polychaete and a gastropod: Freely dissolved concentrations and activated carbon amendment. Environ. Toxicol. Chem. 2006, 25, 2349–2355. [DOI] [PubMed] [Google Scholar]
- Sun X.; Ghosh U. PCB bioavailability control in Lumbriculus variegatus through different modes of activated carbon addition to sediments. Environ. Sci. Technol. 2007, 41, 4774–4780. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. R.; Werner D; Ghosh U.; Millward R. N.; Bridges T. S.; Luthy R. G. The effects of dose and particle size on activated carbon treatment to sequester PCBs and PAHs in marine sediments. Environ. Toxicol. Chem. 2004, 24, 1594–1601. [DOI] [PubMed] [Google Scholar]
- Cornelissen G.; Breedveld G. D.; Christanis K.; Kalaitzidis S.; Kibsgaard A.; Oen A. M. P. Strong sorption of native PAHs to pyrogenic and unburned carbonaceous geosorbents in sediments. Environ. Sci. Technol. 2006, 40, 1197–1203. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J. T.; Werner D.; Luthy R. G. Activated carbon amendment as a treatment for residual DDT in sediment from a superfund site in San Francisco Bay, Richmond, California, USA. Environ. Toxicol. Chem. 2007, 26, 2143–2150. [DOI] [PubMed] [Google Scholar]
- Fagervold S. K.; Chai Y.; Davis J. W.; Wilken M.; Ghosh U. Bioaccumulation of polychlorinated dibenzo-p-dioxins/dibenzofurans in E. fetida from floodplain soils and the effect of activated carbon amendment. Environ. Sci. Technol. 2010, 44, 5546–5552. [DOI] [PubMed] [Google Scholar]
- McLeod P. B.; Luoma S. N.; Luthy R. G. Biodynamic modeling of PCB uptake by Macoma balthica and Corbicula fluminea from sediment amended with activated carbon. Environ. Sci. Technol. 2008, 42, 484–490. [DOI] [PubMed] [Google Scholar]
- McLeod P. B.; van den Heuvel-Greve M. J.; Luoma S. N.; Luthy R. G. Biological uptake of polychlorinated biphenyls by Macoma balthica from sediment amended with activated carbon. Environ. Toxicol. Chem. 2007, 26, 980–987. [DOI] [PubMed] [Google Scholar]
- Sun X.; Ghosh. U. The effect of activated carbon on partitioning, desorption, and biouptake of native PCBs in four freshwater sediments. Environ. Toxicol. Chem. 2008, 27, 2287–2295. [DOI] [PubMed] [Google Scholar]
- Werner D; Hale S. E.; Kwon S.; Ghosh U.; Luthy R. G. Polychlorinated biphenyl sorption and availability in field-contaminated sediments. Environ. Sci. Technol. 2010, 44, 2809–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S.; Thomas J. W.; Reed B. E.; Levine L.; Magar V. S.; Ghosh U. Evaluation of sorbent amendments for in situ remediation of metal contaminated sediments. Environ. Toxicol. Chem. 2010, 29, 1883–1892. [DOI] [PubMed] [Google Scholar]
- Ghosh U.; et al. Rational selection of tailored amendment mixtures and composites for In Situ Remediation of Contaminated Sediments (SERDP Project # ER 1491), Final Report submitted to Strategic Environmental Research and Development Program, U.S. Department of Defense, 2008. http://docs.serdp-estcp.org/index.cfm.
- Kim E.; Seyfferth A. L.; Fendorf S.; Luthy R. G. Immobilization of Hg(II) in water with polysulfide-rubber (PSR) polymer-coated activated carbon. Water Res. 2011, 45, 453–460. [DOI] [PubMed] [Google Scholar]
- Werner D.; Ghosh U.; Luthy R. G. Modeling polychlorinated biphenyl mass transfer after amendment of contaminated sediment with activated carbon. Environ. Sci. Technol. 2006, 40, 4211–4218. [DOI] [PubMed] [Google Scholar]
- Hale S. E.; Tomaszewski J. E.; Luthy R. G.; Werner D. Sorption of dichlorodiphenyltrichloroethane (DDT) and its metabolites by activated carbon in clean water and sediment slurries. Water Res. 2009, 43, 4336–4346. [DOI] [PubMed] [Google Scholar]
- Sun X.; Werner D.; Ghosh U. Modeling PCB mass transfer and bioaccumulation in a freshwater oligochaete before and after amendment of sediment with activated carbon. Environ. Sci. Technol. 2009, 43, 1115–1121. [DOI] [PubMed] [Google Scholar]
- Cho Y.; Smithenry D. W.; Ghosh U.; Kennedy A. J.; Millward R. N.; Bridges T. S.; Luthy R. G. Field methods for amending marine sediment with activated carbon and assessing treatment effectiveness. Mar. Environ. Res. 2007, 64, 541–549. [DOI] [PubMed] [Google Scholar]
- Cho Y.; Ghosh U.; Kennedy A. J.; Grossman A.; Ray G.; Tomaszewski J. E.; Smithenry D.; Bridges T. S.; Luthy R. G. Field application of activated carbon amendment for in-situ stabilization of polychlorinated biphenyls in marine sediment. Environ. Sci. Technol. 2009, 43, 3815–3823. [DOI] [PubMed] [Google Scholar]
- Jonker M. T. O.; Suijkerbuijk M. P. W.; Schmitt H.; Sinnige T. L. Ecotoxicological effects of activated carbon addition to sediments. Environ. Sci. Technol. 2009, 43, 5959–5966. [DOI] [PubMed] [Google Scholar]
- Hale S. E.; Werner D. Modeling the mass transfer of hydrophobic organic pollutants in briefly and continuously mixed sediment after amendment with activated carbon. Environ. Sci. Technol. 2010, 44, 3381–3387. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. EPA proposes comprehensive plan to clean up Hudson River PCBs; USEPA, Dec 6, 2000. http://www.epa.gov/history/topics/pcbs/02.htm.
- Hill M. Hudson River PCB cleanup has cost $561M so far, GE says. Daily Freeman, April 30, 2010. http://www.dailyfreeman.com/.
- Marris E. Putting the carbon back: Black is the new green. Nature 2006, 442, 624–626. [DOI] [PubMed] [Google Scholar]
- Lehmann J. A handful of carbon. Nature 2007, 447, 143–144. [DOI] [PubMed] [Google Scholar]
- Beesley L.; Moreno-Jimenez E.; Gomez-Eyles J. L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [DOI] [PubMed] [Google Scholar]
- Fitzmorris K. B.; Lima I. M.; Marshall W. E.; Reimers R. S. Anion and cation leaching or desorption from activated carbons from municipal sludge and poultry manure as affected by pH. Water Environ. Res. 2006, 88, 2324–2329. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Superfund and Green Remediation. http://www.epa.gov/superfund/greenremediation/.
- Ehlers L. J.; Luthy R. G. Contaminant bioavailability in soil and sediment. Environ. Sci. Technol. 2003, 37, 295A–302A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.