Abstract
Until recently, culturing human pluripotent stem cells was hampered by three prominent technical problems: a high degree of unwanted cellular stress when the cells are passaged, unacceptably low cloning efficiency and poor recovery of cryopreserved stocks. This review discusses recent developments that address these problems. A major focus of the review is the use of p160 Rho-associated coiled-coil kinase inhibitors for improving both the cloning efficiency and the recovery of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. An underlying theme of this review is that the three problems have a common cause: separation of human pluripotent stem cells from one another increases cellular stress, which greatly decreases their viability unless special steps are taken.
Keywords: Accutase®, apoptosis, cloning efficiency, cryopreservation, embryonic stem cell, fasudil, induced pluripotent stem cell, Rho-associated coiled-coil kinase, Y-27632
Human pluripotent stem cells: technological challenges
Initiated by the first isolation of human embryonic stem cells (hESCs) [1] and propelled more recently by the production of human induced pluripotent stem (iPS) cells [2–4], there is a growing expectation that progress in the field of regenerative medicine will accelerate rapidly. In fact, work with hESCs and human iPS cells has already provided new and exciting developments that may eventually lead to the creation of novel cell-based therapies for the treatment of many human diseases [5,6]. However, until the last few years, work with human pluripotent stem cells was hampered by three prominent technical problems: a high degree of unwanted cellular stress during subculture, unacceptably low cloning efficiency and poor recovery of cryopreserved stocks of human pluripotent stem cells. This review discusses recent developments that address each of these problems. In addition, this review argues that the three problems appear to be due largely to a common cause – induction of cellular stress when human pluripotent stem cells are separated from one another.
A major focus of this review is the improvements in working with human pluripotent stem cells afforded by the use of small-molecule inhibitors, such as those that block the activity of p160 Rho-associated coiled-coil kinases (ROCK). More specifically, this review focuses heavily on recent studies that have led to improvements in the cloning efficiency of hESCs and the recovery of cryopreserved hESCs and human iPS cells. In this context, the review also discusses different methods used to subculture human pluripotent stem cells. Finally, in the ‘Future perspective’ section, the review comments on the need for improving the in vitro cultivation of human pluripotent stem cells and discusses fertile areas of research for producing new leads to improve the cultivation of these cells. A topic not discussed in detail in this review is the stress response of human pluripotent stem cells when separated into single cells. Readers interested in this topic are referred to an excellent review by Krawetz et al. [7].
Advances in methodology used to passage human pluripotent stem cells
It is widely recognized that most methods that involve subculturing hESCs as single cells lead to drastic reductions in plating efficiency and cell viability. For this reason, many, if not most, laboratories working with hESC subculture them as small clusters rather than single cells. When hESCs were first described by Thomson and coworkers, the cells were either subcultured using collagenase (type IV) or by manually transferring individual colonies as small clumps of cells (e.g., 50–100 cells per cluster) [1]. Using either method, the cells were maintained as small clusters and not separated into individual cells. Consequently, in this seminal report of hESCs, the cells described were not necessarily clonally derived. Over the next several years, the virtues of subculturing hESCs as small clusters were noted by other investigators [8–11]. Furthermore, several reports have argued that mechanical methods of subculturing hESCs as cell clumps are superior to enzymatic subculture methods as assessed by the criterion of karyotype stability after multiple passages [9,10]. However, other factors, for example, subculturing cells at high cell densities, may also contribute to the karyotype instability [12]. As discussed below, the methods used for subculturing hESCs are continuing to evolve. It is imperative that rigorous criteria continue to be used to assess these methodologies in order to determine which methods provide high-quality hESCs.
A survey of published studies indicates that instead of mechanical subculture of small clumps of cells, which is experimentally cumbersome, many laboratories passage hESCs as small clusters using collagenase [1]. The protease Dispase® is also used [13], but trypsin is usually avoided because it can greatly decrease the viability of the cells, especially when trypsin is used on its own [14,15]. Under most circumstances, the plating efficiency of hESCs after trypsinization is less than 1%. However, it has been reported that one can isolate hESCs that are capable of being subcultured routinely with trypsin. In this latter report, the cells were selected for survival at clonal densities after repeated dissociation with trypsin and plating at low density [14]. The resulting cells, which exhibited plating efficiency of approximately 25% (but not significantly higher), continued to possess a normal karyotype and retained the capacity to differentiate both in vitro and in vivo into cells derived from each of the three embryonic germ layers. Nonetheless, given that the selected cells differ from their unselected counterparts, one needs to consider whether the properties of the selected cells influence hESCs in other, more subtle, yet important ways.
A major limitation of subculturing hESCs as clusters of cells is encountered when one needs to clone hESCs. There are several solutions to this problem. One solution is to employ methods that maintain the viability of hESCs when they are dissociated into single cells. A recent study has shown that Accutase® can be used to subculture the hESC line H9 as single cells, without significant losses in viability or changes in their self-renewal and pluripotency after multiple passages with Accutase [15]. Unlike Dispase, which is a single protease, Accutase is a mixture of enzymes that possess proteolytic, col-lagenolytic and DNase activities. In the study by Bajpai et al., hESCs subcultured with Accutase were compared directly to those subcultured with collagenase and trypsin. There was little or no difference between the growth curves of cells subcultured as single cells using Accutase and those subcultured with collagenase as clusters, whereas, as reported by others, little or no growth was observed with cells subcultured with trypsin. Importantly, hESCs subcultured with Accutase exhibited typical pluripotent properties, including expression of Sox2, Oct4, Nanog and the cell surface marker SSEA4. In fact, hESCs subcultured with Accutase exhibited a higher percentage of Oct4-positive colonies than those subcultured with collagenase. Furthermore, even hESCs subcultured with Accutase for at least 15 passages retained the capacity to differentiate into cells that are normally derived from each of the three embryonic germ layers. Although this study did not report whether the karyotype of the cells was altered, others who have used Accutase reported the absence of karyotypic instability [16].
Interestingly, a recent study cited unpublished findings that a small molecule, thiazovivin, dramatically increases the survival of hESCs subcultured with trypsin [17]. At the time this review was prepared, details regarding the use of thiazovivin when subculturing hESCs with trypsin were not available. Currently, what we do know is that thiazovivin improves the reprogramming of human somatic cells into iPS cells, when used in conjunction with two other small-molecule inhibitors that disrupt TGF-β and MEK signaling [17]. In the future, it will be interesting to learn how thiazovivin acts and whether cells repeatedly subcultured with trypsin, in conjunction with thiazovivin, develop any unwanted properties. Moreover, it will be interesting to determine whether the use of thiazovivin can improve the cloning efficiency of hESCs. As discussed in the next section, the cloning efficiency of hESCs can be improved significantly by using either small-molecule inhibitors of ROCK or neurotrophic factors.
Improvements in the cloning efficiency of hESCs
There are many circumstances for which it is essential to establish clonal hESC lines. First and foremost, clonal hESC lines are needed to establish that pluripotency is due to a single cell, rather than the combined properties of different cell types in the population. For essentially the same reason, clonal populations are needed when isolating genetically modified hESCs. However, as discussed in the previous section, separation of hESC colonies into single cells can significantly decrease cell viability. Thus, it is not surprising that problems were encountered during the first efforts to clone hESCs, since single-cell preparations had to be used.
The first cloning of hESCs was reported by Amit and coworkers [18]. However, cloning efficiency was very low (<1%), and substantially lower than the cloning efficiency of mouse ESCs. In medium containing 20% serum, cloning efficiency of the hESC line H9 was reported to be approximately 0.24% and this was improved approximately threefold (still <1%) by using medium containing Knockout™ Serum Replacement (a serum-free propriety formulation) and bFGF (FGF2). Characterization of the two hESC clones (H9.1 and H9.2) described in this study demonstrated that they possessed a normal karyotype, expressed appropriate cell surface markers (Tra-1-160) and were capable of forming teratomas consisting of cells derived from each of the three embryonic germ layers. Although this study demonstrated that one could isolate clonal hESC lines with the desired phenotype, it was evident that improving the cloning efficiency of hESCs would have significant advantages (e.g., when isolating genetically modified hESCs or when undertaking high-throughput screening studies). Equally important, improving the culture conditions used during cloning could significantly reduce the high degree of stress imposed on the cells during their initial period of isolation from one another. This, in turn, could improve the quality of the clonally derived hESC lines.
A significant improvement in the cloning efficiency of hESCs was first reported by Pyle et al. [19]. In a search for factors that could improve the survival of hESCs, these investigators determined that hESCs (H1 and H9) express cell surface receptors belonging to the tropomyosin-related kinase (TRK) family, in particular TRKB and TRKC. This led to the finding that three neurotrophins, brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4, either as a group or individually, could improve the survival of hESCs at clonal cell densities. More specifically, they determined that a cocktail containing all three neurotrophins improved the cloning efficiency of hESCs (dissociated into single cells with trypsin) from less than 0.5% to nearly 15% (>30-fold improvement) (Table 1). Furthermore, it was determined that the addition of neurotrophins substantially reduces the apoptosis of hESCs, and that the effects of the neurotrophins can be blocked by inhibitors that target TRK receptors and PI-3K, but not by MEK or STAT inhibitors. Interestingly, this study provided new insights into the role of the growth-inactivated feeder layer, with the demonstration that neurotrophins produced by myocyte-enhancer factors (brain-derived neurotrophic factor, neurotrophin 3, neurotrophin 4 and NGF) improve the growth of hESCs at low density. Finally, and very importantly, it was determined that hESC clones isolated with the aid of neurotrophins maintained a normal karyotype, expressed key pluripotency markers (Oct4, SSEA3, SSEA4, Tra-1-60 and Tra-1-81) and maintained the ability to differentiate into cells that are normally derived from all three embryonic germ layers. Thus, treatment of hESCs with neurotrophins appears to reduce the stress response of the cells, especially after separation into single cells.
Table 1.
Improvements in the cloning and recovery of cryopreserved human pluripotent stem cells.
| Action taken | Results | Ref. |
|---|---|---|
| Neurotrophins added during the cloning of hESCs | Cloning efficiency increased >30-fold | [19] |
| Y-27632 added during clonal growth of hESCs | Cloning efficiency increased >25-fold | [20,21] |
| Y-27632 added before cryopreservation of hESCs | Colony number increased ~twofold | [21] |
| Y-27632 added after hESCs are thawed | Colony number and size increased ~eightfold | [25] |
| Fasudil added after hESCs are thawed | Colony number increased ~fourfold | [25] |
| Y-27632 added 5 days after hESCs are thawed | Colony number increased ~twofold in 24 h | [25] |
| Y-27632 added to hESCs after subculture | Colony number and size increased ~12-fold | [25] |
| Y-27632 added to hESCs 5 days after subculture | No increase in colony number | [25] |
| Y-27632 added to iPS cells after being thawed | Colony number increased ~sixfold | [25] |
| Y-27632 added to iPS cells after subculture | Colony number increased ~fourfold | [25] |
hESC: Human embryonic stem cell; iPS: Induced pluripotent stem.
Another improvement in the growth of hESCs at low density was reported by Watanabe and colleagues, who tested an unspecified list of kinase inhibitors, growth factors, trophic factors and caspase inhibitors [20]. Remarkably, they reported that addition of the kinase inhibitor Y-27632, which targets ROCK, improved the growth of hESCs more than 25-fold when the cells were plated at low density (500 cells/well in a 96-well plate). Based on alkaline phosphatase-positive clones (a low stringency test for self-renewal and pluripotency), cloning efficiency was estimated to rise from less than 1% to just over 25% in the presence of Y-27632 (Table 1). In this study, optimal conditions involved a brief exposure to Y-27632 prior to dissociation into single cells, and subsequent plating of the dissociated cells onto a growth-inactivated feeder layer in medium supplemented with Knockout Serum Replacement, bFGF and Y-27632. Importantly, cultivation of the cells for prolonged periods in the presence of Y-27632 did not alter their karyotype or their ability to form well-differentiated teratomas in the testes of severe combined immunodeficiency mice. Subsequently, others confirmed the improvement in cloning efficiency when Y-27632 was used during the cloning of other hESCs [21]. However, improvements in cloning efficiency in the latter study were less dramatic, reaching 6–7% in the presence of Y-27632. The mechanism by which Y-27632 acts on hESCs is discussed later in this review. As a technical note, Y-27632 is light sensitive. Hence, it is best to work with it in subdued light. In the author’s laboratory, its exposure to light is kept to a minimum and where needed it is handled in the presence of subdued yellow lighting. In addition, it is stored as 100X stock solution in culture media for up to 2 months at −20°C.
Improving the recovery of cryopreserved hESCs
Another significant technical problem encountered when working with hESCs is the poor recovery of cyropreserved stocks [16,21,22]. Until recently, recovery of hESCs was highest when the cells were preserved in liquid nitrogen and frozen as clumps by vitrification using the ‘open pulled straw’ method [10,22]. Vitrification involves placing the cells in a freezing solution containing a high concentration of cryopreservant and rapid cooling in liquid nitrogen. This method achieves a glass-like state in the cells that is believed to improve cell survival by preventing the formation of ice crystals. However, freezing of hESCs by vitrification, especially as clumps of cells, has several drawbacks. For example, vitrification can give variable results since it is important to pull the ‘straw’ properly in order to maximize the surface to volume ratio. It is also cumbersome and tedious to freeze hESCs in bulk quantities by vitrification. This is true even with the improvements reported for vitrification methods [23,24]. The more commonly used method of freezing cells in large numbers, including mouse ESCs, is the slow-freezing/rapid-thawing method. Using a controlled-rate freezing device, which achieves a freeze rate between 0.3 and 1.8°C per minute, it is possible to achieve survival levels of hESCs in the range of 20 to 80% [13]. However, the recovery of hESCs cryopreserved using the slow-freezing/rapid-thawing method, at least as employed in most laboratories, has generally been far lower.
During the past 2 years, three different studies have addressed the problem of low recovery of cryopreserved hESCs [16,21,25]. All three studies demonstrated that the addition of the ROCK inhibitor Y-27632 significantly improves the recovery of cryopreserved hESCs that had been frozen by the slow-freezing/rapid-thawing method. In these studies, the effects of Y-27632 were estimated under several conditions (Table 1). For example, addition of Y-27632 to the medium into which the cells was thawed and its addition to the culture medium daily when the medium was changed, increased the number of colonies recovered more than fourfold and increased the size of the recovered colonies more than twofold, for an overall effect that was more than eightfold [25]. In addition, there was a further increase (~twofold) in the recovery of the cryopreserved cells when hESCs were pretreated with Y-27632 before the cells were dissociated from the culture substratum and prepared for cryopreservation [21]. In each of these studies, Y-27632 was used at 10 μM. At higher concentrations, there were slight improvements in the size of the colonies [25], but Y-27632 is known to inhibit other kinases besides ROCK 1 and 2 at concentrations above 10 μM. Importantly, the effects of Y-27632 are not ESC line dependent [16,21,25]. Moreover, ROCK inhibitors have also been shown to improve the recovery of cryopreserved cynomolgus monkey ESCs [26].
The effects of Y-27632 appear to be mediated primarily via its effects on ROCK, because comparable effects were observed on the recovery of cryopreserved hESCs when a different ROCK inhibitor, fasudil, was used (Table 1). However, the possibility remains that Y-27632 and fasudil exert some of their effects via other kinases (e.g., PKC-related kinase), which are known to be inhibited at only slightly higher concentrations of ROCK inhibitors than those used in the recovery of cryopreserved hESCs. In addition, further study will be needed to determine whether Y-27632 and fasudil are acting by inhibiting one or both forms of ROCK, ROCK 1 and 2. This question can be addressed by knocking down ROCK 1 and 2 and/or with the use of commercially available inhibitors that target ROCK 2 more specifically.
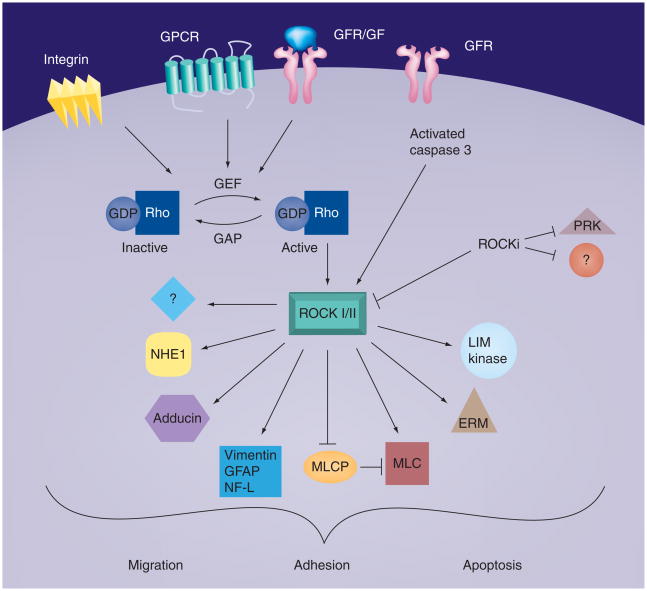
Both ROCK 1 and 2 are believed to be activated by several different pathways. ROCKs are known to be activated after conversion of Rho-GDP to Rho-GTP, in response to upregulation of several different upstream signaling pathways (Figure 1). However, a major effect of ROCK inhibitors on the recovery of cryopre-served hESCs may be due to their ability to block the stimulation of ROCK activity when caspase 3, a proximal mediator of apoptosis, is activated. In this regard, ROCK inhibitors have been shown to strongly block apoptosis when hESCs are placed in suspension. This is true for hESCs that are placed in suspension immediately after being thawed [16], as well as for hESCs actively growing in culture before being placed in suspension [20]. What is less clear are the downstream targets of activated ROCK that influence the recovery of cryopreserved hESCs. Given that activated ROCK has a large number downstream targets (Figure 1), this may be a difficult question to answer. Readers interested in the roles of ROCK in ESCs are directed to an excellent study by Harb et al. [27].
Figure 1. Rho-associated coiled-coil kinase signaling pathway.
The p160 ROCK is activated by several upstream pathways, including activated Rho-GTP and activated caspase 3. ROCK has a wide range of targets that are regulated when phosphorylated by ROCK. Downstream signaling of ROCK can influence many different biological responses, including cell migration, cell adhesion and apoptosis. ROCKi are believed to primarily inhibit the activity of ROCK 1 and 2, but may influence the activity of other kinases.
ERM: Ezrin, radixin and moesin; GF: Growth factor; GFAP: Glial fibrillary acidic protein; GFR: Growth factor receptor; GPCR: G-protein-coupled receptor; MLC: Myosin light chain; MLCP: Myosin light chain phosphatase; NF-L: Neurofilament protein; NHE: Na+/H+ exchanger; PRK: Protein kinase C-related kinase; ROCK: Rho-associated coiled-coil kinase; ROCKi: ROCK inhibitor.
Although a major effect of Y-27632 on hESCs is believed to be its prevention of apoptosis, this does not appear to be its only effect. To obtain the maximal benefit of Y-27632, it should be added to the medium in which the cells are thawed and cultured for the next 4–5 days. Nonetheless, Y-27632 need not be added immediately to the cells to improve the recovery of cryopreserved stocks. Surprisingly, one still observes significant improvements in recovery of the cells if one waits 5 days or more before adding Y-27632 (Table 1). In fact, in one instance, thawed cells, which exhibited virtually no growth 7 days after being placed in culture, quickly started to grow just 1 day after the addition of Y-27632 [Desler & Rizzino, Unpublished Data]. Equally interesting, once the colonies reach a critical size (~30–50 cells), the addition of Y-27632 exerts little or no effect on the growth of the cells [25]. Thus, in addition to blocking apoptosis, Y-27632 appears to kick-start the growth of hESCs when colonies are first beginning to form.
An important consideration when using ROCK inhibitors is whether they produce undesirable changes in hESCs. Published reports indicate that culturing hESCs in the presence of Y-27632 for multiple passages does not reduce their ability to express pluripotency markers or differentiate into multiple cells normally derived from each of the three embryonic germ layers [16,21,25]. Moreover, the cells retain a normal karyotype after long-term growth in medium supplemented with Y-27632 [16,21]. Although Y-27632 alters the morphology of hESCs, the cells revert to their original morphology soon after Y-27632 is removed from the culture medium [Cox & Rizzino, Unpublished Data]. However, the possibility remains that prolonged treatment of hESCs with Y-27632 alters the cells in some manner. Therefore, until further study is undertaken to probe for subtle changes in the cells, it would be prudent to limit the use of ROCK inhibitors to conditions where they would be most beneficial (e.g., during the initial recovery phase after cryopreserved hESCs are thawed or during the early stages of cloning). Addition of Y-27632 to actively growing cultures of hESCs after subculture improves the size and number of colonies during the first 2–3 days, but provides limited benefit with longer exposure (Table 1).
In the studies discussed above, where Y-27632 was used to improve the recovery of hESCs, the cells were thawed onto a growth-inactivated feeder layer in medium containing knockout serum replacement and bFGF [16,21,25]. However, the ROCK inhibitor Y-27632 also improved the recovery of cryopreserved hESCs when they were plated onto Matrigel™-coated dishes in mTeSR1 medium without a growth-inactivated feeder layer [25]. mTeSR1 medium is a serum-free, defined formulation that eliminates the need for a growth-inactivated feeder layer [28]. When cryopreserved hESCs were thawed and plated in mTeSR1 medium on Matrigel-coated tissue culture plates, Y-27632 improved the number of colonies more than twofold and the size of the colonies more than sevenfold. Thus, Y-27632 improves the recovery of cryopreserved hESCs under more than one culture condition.
Improving the recovery of cryopreserved human iPS cells
The problems encountered during the cloning, subculturing and recovery of frozen stocks of hESCs are also encountered when working with human iPS cells. This is not surprising given the similarities between hESCs and human iPS cells. This led Claassen et al. to test whether the ROCK inhibitor Y-27632 would improve the recovery of human iPS cells from frozen stocks [25]. When human iPS cells were thawed onto a growth-inactivated feeder layer in medium containing Knockout Serum Replacement and bFGF, the number of colonies formed by 48 h was more than fivefold higher in cultures supplemented with Y-27632. Moreover, the sizes of the colonies were much larger. In addition, Y-27632 was found to enhance the growth of human iPS cells approximately fourfold when they were subcultured using Accutase and plated onto a growth-inactivated feeder layer in medium containing Knockout Serum Replacement [25]. Although it has not yet been determined whether Y-27632 improves the cloning efficiency of human iPS cells, it is reasonable to predict that it will.
Future perspective
During the past several years, there have been substantial improvements in the methods used to clone, recover cryopreserved stocks and maintain actively growing cultures of human pluripotent stem cells. These improvements include the use of new cell culture media, such as mTeSR1 medium, use of Accutase or possibly trypsin in conjunction with small molecules (e.g., thiazovivin) for subculturing the cells, and the use of ROCK inhibitors for improving both the cloning and recovery of frozen stocks of human pluripotent stem cells. In addition, ROCK inhibitors have been used in the cultivation of hESCs in bioreactors [29,30] and in the derivation of hESCs [31,32]. Building on this base, optimal methods for working with human pluripotent stem cell needs to improve further over the next several years, especially as our understanding of human pluripotent cells improves. It is evident that the culture conditions used to handle human pluripotent stem cells can significantly influence their genetic stability and their propensity to differentiate. Moreover, there is growing awareness that the culture conditions used to establish hESCs and generate iPS cells substantially influence the properties of the cell lines produced.
Improvements in the in vitro handling of human pluripotent stem cells are likely to come from many areas of research. One area that is expected to be particularly fertile is the study of parameters that improve the reprogramming of somatic cells to iPS cells. For example, reducing O2 from 20 to 5% not only improves the efficiency of reprogramming [33], but also helps maintain hESCs in an undifferentiated state [34,35]. Another potential lead is the recent finding that addition of vitamin C improves the efficiency of producing human iPS cells [36]. It will be interesting to determine whether vitamin C can improve the cloning efficiency of human pluripotent stem cells and their recovery from cryopreserved stocks. Similarly, high-throughput screening studies that seek to identify small molecules (e.g., thiazovivin) that improve the reprogramming of somatic cells to human iPS cells [17] can be expected to identify small molecules that improve many aspects of in vitro cultivation of human pluripotent stem cells. Conversely, small molecules, such as Y-27632, which improve the in vitro growth of hESCs and human iPS cells, have been added to the culture medium during reprogramming of somatic cells to human iPS cells [37].
Identification of small molecules that improve the in vitro handling of human pluripotent stem cells may also have clinical applications during transplantation. Recent studies have shown that dissociation of ESC-derived neural precursors induces a high degree of anoikis (apoptosis induced by detachment from a suitable matrix), which can be suppressed by the use of the ROCK inhibitor Y-27632 [38]. Moreover, treatment of these cells with Y-27632 reduced apoptosis after transplantation into an animal model. Thus, ROCK inhibitors and other small molecules that are found to improve the in vitro growth of hESCs and their differentiated cells may also improve cell survival and engraftment of ESC-derived differentiated cells during transplantation.
Acknowledgments
The author wishes to thank J Cox for reading this review, and H Rizzino and M Desler for editorial assistance.
Footnotes
Financial & competing interests disclosure
Work in the Rizzino laboratory is supported by grants from the NIH (GM 080751) and Nebraska Department of Health (Stem Cell 2009–01). Additional support is provided by an NCI Training Grant (CA 36727) and an NCI Cancer Center Support Grant (CA 009476) for the support of core facilities of the UNMC Eppley Cancer Center. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1■.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5395):1145–1147. doi: 10.1126/science.282.5391.1145. First report demonstrating the isolation of human embryonic stem cells (hESCs) [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5.Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest. 2010;120(1):51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronaghi M, Erceg S, Moreno-Manzano V, Stojkovic M. Challenges of stem cell therapy for spinal cord injury: human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells. 2010;28(1):93–99. doi: 10.1002/stem.253. [DOI] [PubMed] [Google Scholar]
- 7.Krawetz RJ, Li X, Rancourt DE. Human embryonic stem cells: caught between a ROCK inhibitor and a hard place. Bioessays. 2009;31(3):336–343. doi: 10.1002/bies.200800157. [DOI] [PubMed] [Google Scholar]
- 8.Reubinoff BE, Pera MF, Fong C, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 9.Buzzard JJ, Gough NM, Crook JM, Colman A. Karyotype of human ES cells during extended culture. Nat Biotechnol. 2004;22(4):381–382. doi: 10.1038/nbt0404-381. [DOI] [PubMed] [Google Scholar]
- 10.Mitalipova MM, Rao RR, Hoyer DM, et al. Preserving the genetic integrity of human embryonic stem cells. Nat Biotechnol. 2005;23(1):19–20. doi: 10.1038/nbt0105-19. [DOI] [PubMed] [Google Scholar]
- 11.Trish E, Dimos J, Eggan K. Passaging HuES human embryonic stem cell-lines with trypsin. J Vis Exp. 2006;12(1):49. doi: 10.3791/49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews PW. Response letter. Nat Biotechnol. 2004;22(4):382. [Google Scholar]
- 13.Ware CB, Nelson AM, Blau CA. Controlled-rate freezing of hESC. Biotechniques. 2005;38(6):879–880. 882–883. doi: 10.2144/05386ST01. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa K, Fujioka T, Nakamura Y, Nakatsuji N, Suemori H. A method for the selection of human embryonic stem cell sublines with high replating efficiency after single-cell dissociation. Stem Cells. 2006;24(12):2649–2660. doi: 10.1634/stemcells.2005-0657. [DOI] [PubMed] [Google Scholar]
- 15■.Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75(5):818–827. doi: 10.1002/mrd.20809. First report describing the use of Accutase® with hESCs. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24(3):580–589. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- 17.Lin T, Ambasudhan R, Yuan X, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6(11):805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227(2):271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 19■■.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24(3):344–350. doi: 10.1038/nbt1189. First report describing factors that improve the cloning efficiency of hESCs. [DOI] [PubMed] [Google Scholar]
- 20■■.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681–686. doi: 10.1038/nbt1310. First report describing use of Rho-associated coiled-coil kinase (ROCK) inhibitors with hESCs. [DOI] [PubMed] [Google Scholar]
- 21■■.Martin-Ibanez R, Unger C, Stromberg A, Baker D, Canals J, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23(12):2744–2754. doi: 10.1093/humrep/den316. First report showing that ROCK inhibitors improve recovery of cryopreserved hESCs. [DOI] [PubMed] [Google Scholar]
- 22.Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16(10):2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- 23.Fujioka T, Yasuchika K, Nakamura Y, Naktsuju N, Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2009;48(10):1149–1154. doi: 10.1387/ijdb.041852tf. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Zhou C, Liu C, Mai Q, Zhuang G. Bulk vitrification of human embryonic stem cells. Human Reprod. 2008;23(2):358–364. doi: 10.1093/humrep/dem386. [DOI] [PubMed] [Google Scholar]
- 25■■.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76(8):722–732. doi: 10.1002/mrd.21021. First report showing that ROCK inhibitors improve recovery of cryopreserved human induced pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takehara T, Teramura T, Onodera Y, et al. Rho-associated kinase inhibitor Y-27632 promotes survival of cynomolgus monkey embryonic stem cells. Mol Human Reprod. 2008;14(11):627–634. doi: 10.1093/molehr/gan061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harb N, Archer TK, Sato N. The Rho–ROCK–myosin signaling axis determines cell–cell integrity of self-renewing pluripotent stem cells. PLoS ONE. 2008;3(8):e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3(8):637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 29.Kehoe DE, Jing D, Lock LT, Tzanakakis EM. Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng Part A. 2010;16(2):405–421. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krawetz R, Taiani JT, Liu S, et al. Large-scale expansion of pluripotent human embryonic stem cells in stirred suspension bioreactors. Tissue Eng Part C. 2010;16(4):573–582. doi: 10.1089/ten.TEC.2009.0228. [DOI] [PubMed] [Google Scholar]
- 31.Cortes JL, Sanchez L, Ligero G, et al. Mesenchymal stem cells facilitate the derivation of human embryonic stem cells from cryopreserved poor-quality embryos. Hum Reprod. 2009;24(8):1844–1851. doi: 10.1093/humrep/dep107. [DOI] [PubMed] [Google Scholar]
- 32.Taei A, Gourabi H, Seifinejad A, et al. Derivation of new human embryonic stem cell lines from preimplantation genetic screening and diagnosis-analyzed embryos. In vitro Cell Dev Biol Anim. 2010;46(3–4):395–402. doi: 10.1007/s11626-010-9293-3. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5(3):237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Westfall SD, Sachdev S, Das P, et al. Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells Dev. 2008;17(5):869–881. doi: 10.1089/scd.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139(1):85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esteban MA, Wang T, Qin B, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 38.Koyanagi M, Takahashi J, Arakawa Y, et al. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res. 2008;86(2):270–280. doi: 10.1002/jnr.21502. [DOI] [PubMed] [Google Scholar]