Abstract
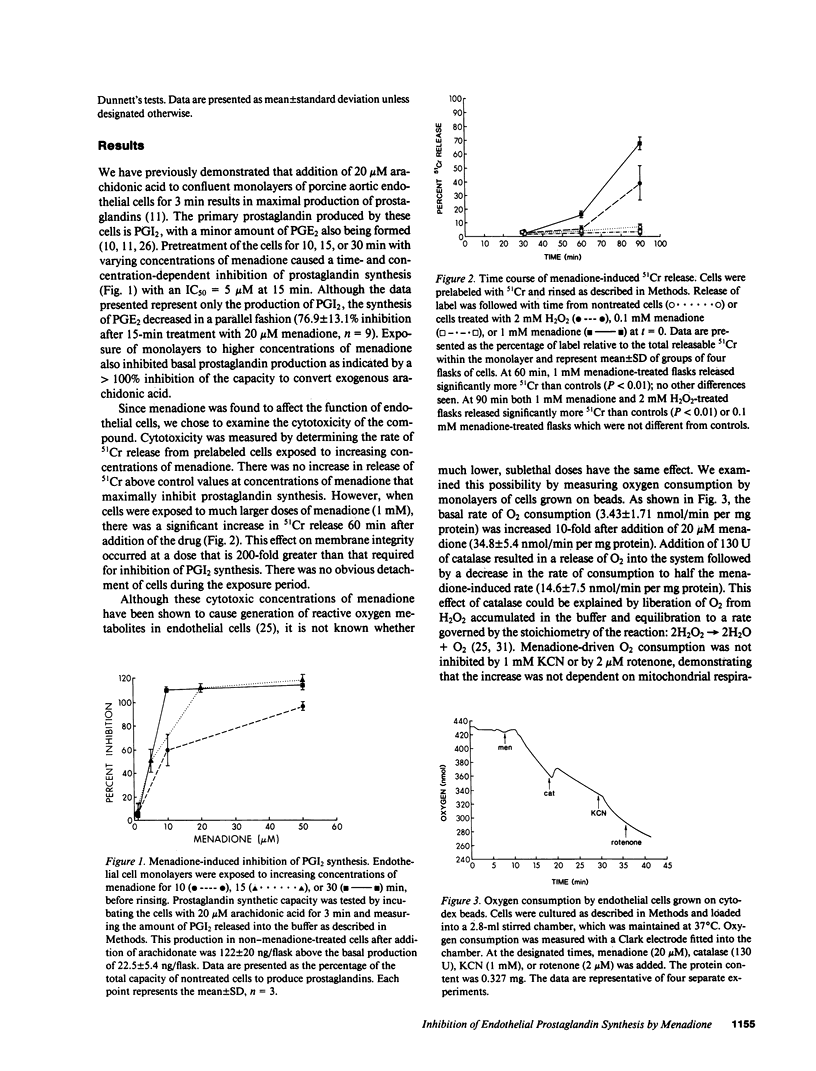
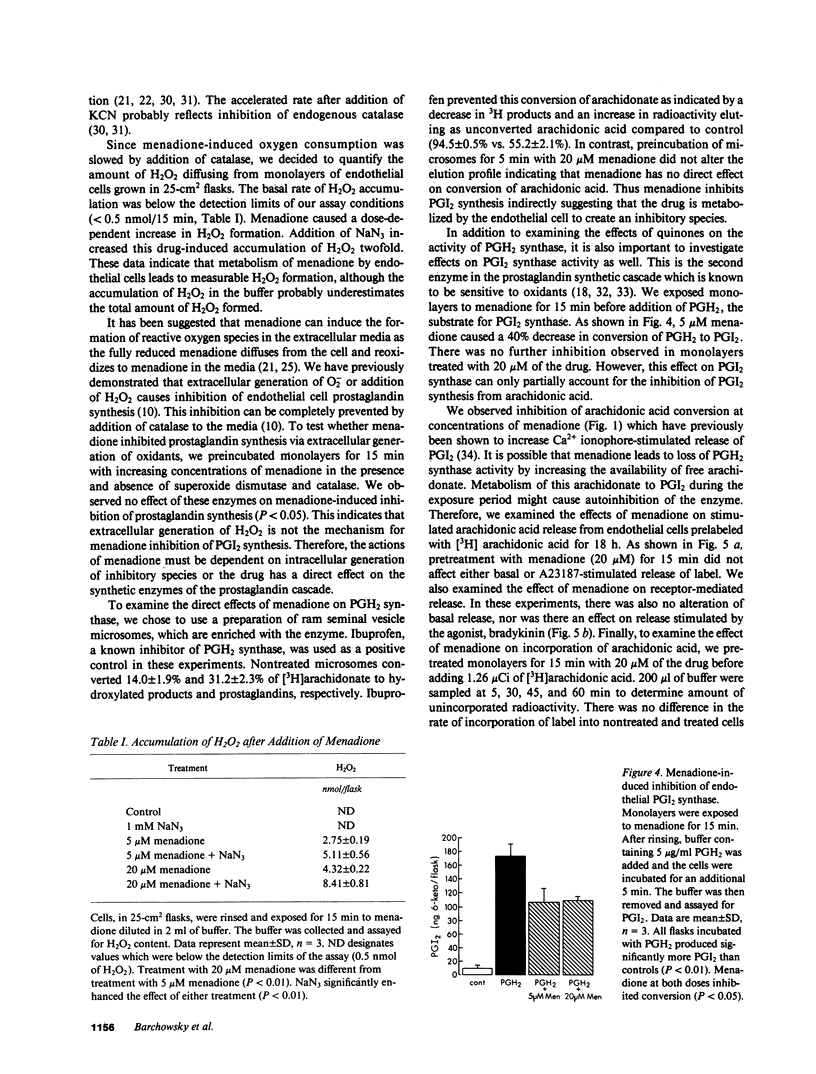
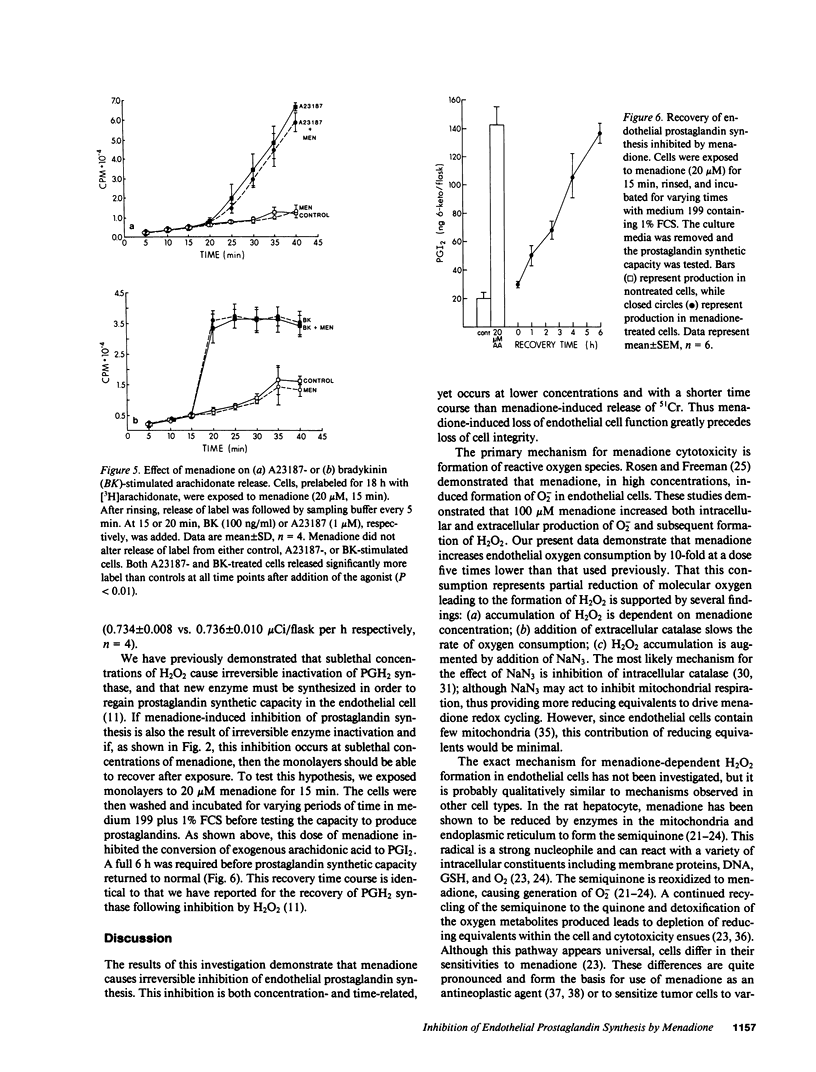
We have examined the effects of menadione on porcine aortic endothelial cell prostaglandin synthesis. Addition of 1-20 microM menadione caused a dose- and time-dependent inhibition of stimulated prostaglandin synthesis with an IC50 of 5 microM at 15 min. Concentrations greater than 100 microM menadione were necessary to increase 51Cr release from prelabeled cells. Recovery of enzyme inactivated by menadione required a 6-h incubation in 1% serum. In a microsomal preparation, menadione was shown to have no direct effect on conversion of arachidonic acid to prostaglandins. In intact cells menadione caused only a 40% inhibition of the conversion of PGH2 to prostacyclin. Enzymes involved in the incorporation and the release of arachidonic acid were not affected by menadione (20 microM, 15 min). Menadione undergoes oxidation/reduction reactions in intact cells leading to partial reduction of oxygen-forming, reactive oxygen species. In our cells menadione was found to increase KCN-resistant oxygen consumption. Further, an increased accumulation of H2O2 was observed with a time course consistent with menadione-induced inhibition of prostaglandin synthesis. We conclude that menadione at sublethal doses caused inhibition of prostaglandin synthesis. The mechanism involves inactivation of PGH2 synthase by a reactive species resulting from metabolism of menadione by endothelial cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y. Drug-induced pulmonary fibrosis. Environ Health Perspect. 1984 Apr;55:25–36. doi: 10.1289/ehp.845525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autor A. P., Bonham A. C., Thies R. L. Toxicity of oxygen radicals in cultured pulmonary endothelial cells. J Toxicol Environ Health. 1984;13(2-3):387–395. doi: 10.1080/15287398409530505. [DOI] [PubMed] [Google Scholar]
- BERGMEYER H. U. Zur Messung von Katalase-Aktivitäten. Biochem Z. 1955;327(4):255–258. [PubMed] [Google Scholar]
- Barchowsky A., Kent R. S., Whorton A. R. Recovery of porcine aortic endothelial cell prostaglandin synthesis following inhibition by sublethal concentrations of hydrogen peroxide. Biochim Biophys Acta. 1987 Mar 11;927(3):372–381. doi: 10.1016/0167-4889(87)90102-9. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Mirabelli F., DiMonte D., Richelmi P., Thor H., Orrenius C., Orrenius S. Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. A study with isolated hepatocytes and menadione (2-methyl-1,4-naphthoquinone). Biochem Pharmacol. 1987 Apr 15;36(8):1313–1320. doi: 10.1016/0006-2952(87)90087-6. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986 May;133(5):913–927. [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Prostacyclin biosynthesis in cultured vascular endothelium is limited by deactivation of cyclooxygenase. J Clin Invest. 1983 Oct;72(4):1255–1261. doi: 10.1172/JCI111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski R. T., Akman S. A., Block J. B. Vitamin K in the treatment of cancer. Cancer Treat Rev. 1985 Mar;12(1):49–63. doi: 10.1016/0305-7372(85)90012-x. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Fry G. L., Hoak J. C., Haycraft D. L. Inhibition of prostacyclin by treatment of endothelium with aspirin. Correlation with platelet adherence. J Clin Invest. 1979 May;63(5):1089–1092. doi: 10.1172/JCI109379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984 Dec;235(2):343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Dobrina A., Rossi F. Metabolic properties of freshly isolated bovine endothelial cells. Biochim Biophys Acta. 1983 Apr 5;762(2):295–301. doi: 10.1016/0167-4889(83)90084-8. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Kinnes D. A. Prostaglandin E1 and prostaglandin I2 modulation of superoxide production by human neutrophils. Biochem Biophys Res Commun. 1983 Jun 15;113(2):506–512. doi: 10.1016/0006-291x(83)91754-0. [DOI] [PubMed] [Google Scholar]
- Frei B., Winterhalter K. H., Richter C. Menadione- (2-methyl-1,4-naphthoquinone-) dependent enzymatic redox cycling and calcium release by mitochondria. Biochemistry. 1986 Jul 29;25(15):4438–4443. doi: 10.1021/bi00363a040. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S. Secretory function of vascular endothelium. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:397–404. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Jones T. W., Thor H., Orrenius S. Cellular defense mechanisms against toxic substances. Arch Toxicol Suppl. 1986;9:259–271. doi: 10.1007/978-3-642-71248-7_41. [DOI] [PubMed] [Google Scholar]
- Kent R. S., Diedrich S. L., Whorton A. R. Regulation of vascular prostaglandin synthesis by metabolites of arachidonic acid in perfused rabbit aorta. J Clin Invest. 1983 Aug;72(2):455–465. doi: 10.1172/JCI110993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulmacz R. J. Prostaglandin H synthase and hydroperoxides: peroxidase reaction and inactivation kinetics. Arch Biochem Biophys. 1986 Sep;249(2):273–285. doi: 10.1016/0003-9861(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Lazo J. S. Endothelial injury caused by antineoplastic agents. Biochem Pharmacol. 1986 Jun 15;35(12):1919–1923. doi: 10.1016/0006-2952(86)90720-3. [DOI] [PubMed] [Google Scholar]
- Madden M. C., Eling T. E., Friedman M. Ozone inhibits endothelial cell cyclooxygenase activity through formation of hydrogen peroxide. Prostaglandins. 1987 Sep;34(3):445–463. doi: 10.1016/0090-6980(87)90089-x. [DOI] [PubMed] [Google Scholar]
- Marshall P. J., Kulmacz R. J., Lands W. E. Constraints on prostaglandin biosynthesis in tissues. J Biol Chem. 1987 Mar 15;262(8):3510–3517. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nolan R. D., Eling T. E. Inhibition of prostacyclin synthesis in cultured bovine aortic endothelial cells by vitamin K1. Biochem Pharmacol. 1986 Dec 1;35(23):4273–4281. doi: 10.1016/0006-2952(86)90706-9. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Warner D. J. Effects of neutrophil-mediated pulmonary endothelial injury on the localization and metastasis of circulating Walker carcinosarcoma cells. Invasion Metastasis. 1987;7(3):183–196. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial injury and regeneration. IV. Endotoxin: a nondenuding injury to aortic endothelium. Lab Invest. 1983 Jan;48(1):25–34. [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. A., Smith D. R., Flower R. J., Moncada S., Vane J. R. Further studies on the enzymatic conversion of prostaglandin endoperoxide into prostacyclin by porcine aorta microsomes. Biochim Biophys Acta. 1978 Mar 14;523(1):250–262. doi: 10.1016/0005-2744(78)90028-1. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Lucchesi B. R. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med. 1987 Jul;110(1):13–30. [PubMed] [Google Scholar]
- Sjostrom K., Crapo J. D. Structural and biochemical adaptive changes in rat lungs after exposure to hypoxia. Lab Invest. 1983 Jan;48(1):68–79. [PubMed] [Google Scholar]
- Su Y. Z., Duarte T. E., Dill P. L., Weisenthal L. M. Selective enhancement by menadiol of in vitro drug activity in human lymphatic neoplasms. Cancer Treat Rep. 1987 Jun;71(6):619–625. [PubMed] [Google Scholar]
- Suttorp N., Simon L. M. Lung cell oxidant injury. Enhancement of polymorphonuclear leukocyte-mediated cytotoxicity in lung cells exposed to sustained in vitro hyperoxia. J Clin Invest. 1982 Aug;70(2):342–350. doi: 10.1172/JCI110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott R. E., Smith M. T., Giannini D. D. Inhibition of microsomal lipid peroxidation by naphthoquinones: structure-activity relationships and possible mechanisms of action. Arch Biochem Biophys. 1985 Aug 15;241(1):88–94. doi: 10.1016/0003-9861(85)90365-0. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Thayer W. S. Adriamycin stimulated superoxide formation in submitochondrial particles. Chem Biol Interact. 1977 Dec;19(3):265–278. doi: 10.1016/0009-2797(77)90050-3. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Whorton A. R., Montgomery M. E., Kent R. S. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. J Clin Invest. 1985 Jul;76(1):295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton A. R., Young S. L., Data J. L., Barchowsky A., Kent R. S. Mechanism of bradykinin-stimulated prostacyclin synthesis in porcine aortic endothelial cells. Biochim Biophys Acta. 1982 Jul 20;712(1):79–87. doi: 10.1016/0005-2760(82)90087-x. [DOI] [PubMed] [Google Scholar]
- Younes M., Cornelius S., Siegers C. P. Fe2+-supported in vivo lipid peroxidation induced by compounds undergoing redox cycling. Chem Biol Interact. 1985 Jun;54(1):97–103. doi: 10.1016/s0009-2797(85)80155-1. [DOI] [PubMed] [Google Scholar]
- de Groot H., Noll T., Sies H. Oxygen dependence and subcellular partitioning of hepatic menadione-mediated oxygen uptake. Studies with isolated hepatocytes, mitochondria, and microsomes from rat liver in an oxystat system. Arch Biochem Biophys. 1985 Dec;243(2):556–562. doi: 10.1016/0003-9861(85)90532-6. [DOI] [PubMed] [Google Scholar]


