Abstract
Cells sense the rigidity of their environment and respond to it. Most studies have been focused on the role of adhesion complexes in rigidity sensing. In particular, it has been clearly shown that proteins of the adhesion complexes were stretch-sensitive and could thus trigger mechano-chemical signaling in response to applied forces. In order to understand how this local mechano-sensitivity could be coordinated at the cell scale, we have recently carried out single cell traction force measurements on springs of varying stiffness. We found that contractility at the cell scale (force, speed of contraction, mechanical power) was indeed adapted to external stiffness and reflected ATPase activity of non-muscle myosin II and acto-myosin response to load. Here we suggest a scenario of rigidity sensing where local adhesions sensitivity to force could be coordinated by adaptation of the acto-myosin dependent cortical tension at the global cell scale. Such a scenario could explain how spreading and migration are oriented by the rigidity of the cell environment.
Key words: single cell, mechano-sensing, mechano-transduction, contractility, spreading, polarization
Introduction
Cells' life and fate are controlled by their mechano-chemical environment.1–3 In particular, rigidity (the ability of a material to resist deformation) influences many cellular processes such as spreading,4,5 migration6 or even differentiation.7,8 The challenge is thus to understand how cells sense rigidity and respond to it.
In principle, determining the rigidity of a given material implies to apply some force on it and then to measure the resulting deformation. Indeed, it was early demonstrated that cells do apply traction forces on their surroundings.9 These forces result from acto-myosin contraction and are transmitted to the cell substrate through complex molecular assemblies associated with integrin adhesion receptors.10 Since proteins involved in the adhesion complexes act as force transmitters, they were natural candidates for rigidity sensing through molecular deformation.
On stiff-weakly deformable-substrates, cell contractility could result in high traction forces, leading to substantial stretching of mechanosensory molecules of the adhesion complexes. Molecular stretching would then activate specific mechano-chemical signaling pathways and enhance, in turn, contractility (Fig. 1).6,11–14 Conversely, on soft highly-deformable substrates, cell contractility could only induce low generated forces. Adhesion complexes would then be weakly deformed, leading to a weak cell response. Many studies were thus devoted to the search of the mechanosensing element (reviewed in ref. 2). In particular, p130cas, a substrate of SRC kinases, was unambiguously shown to be stretch sensitive.15
Figure 1.
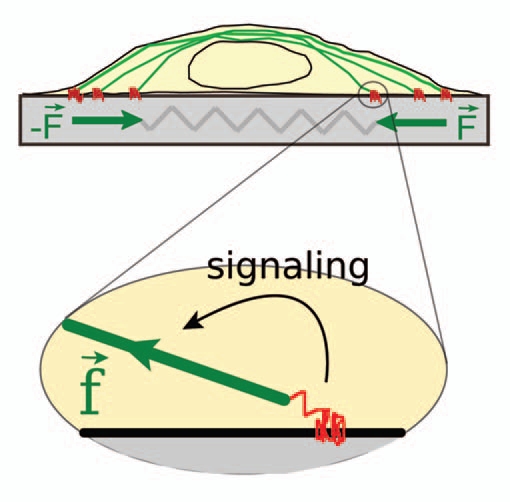
Acto-myosin contractility, adhesion complexes and rigidity. An adherent cell applies traction forces that are generated by the contractile acto-myosin machinery (green) and are transmitted to the substrate through integrin-based adhesion complexes (red). The resultant dipole forces, F, are resisted by the elasticity of the substrate which acts basically as a spring. Current models of rigidity sensing are mainly based on (local) stretching of sensory molecules of the adhesion complexes (zoom). On rigid-weakly deformable-substrates local force components, f, induce stretching and phosphorylation of specific molecules, thus triggering mechano-chemical signaling cascades that enhance in turn contractility.
In sum, the current model for cell rigidity sensing assumes that local (molecular level) deformations trigger specific mechano-chemical signaling pathways to enhance contractility at the cell scale. Although reasonable, this scenario implies the interplay of some centralized regulation process to integrate local events into a coordinated and coherent response at the cell level.16 This integration process could be achieved through appropriate, although quite complex, cell signaling. It could also be carried out through direct adaptation of the cell structure to mechanical cues of its environment.17 Following this idea, we suggest here a scenario of rigidity sensing where acto-myosin based build-up of tension at the cell scale could by itself be dependent on the rigidity of the cell substrate and may thus spatially coordinate adhesion protein recruitment to ensure an appropriate orientation of cell spreading and migration.
Acto-Myosin Dependent Force Build Up is Sensitive to Rigidity
Since applying a given amount of deformation to substrates of increasing stiffness requires an increasing amount of force, cell traction on substrates of increasing rigidity is equivalent to pulling on increasing loads. Like any force generator (an engine, a muscle), the cell contractile machinery should have a specific load dependent output, and contractility at the cell scale could thus have its own response to the rigidity of the environment. In order to test this assumption, we have recently carried out single cell traction force measurements with a custom-made parallel microplates setup.18
In this study, we let single cells spread between and pull on two parallel glass microplates coated with fibronectin. One plate was rigid, the other flexible and used as a nano-Newton force sensor (i.e., a spring) of calibrated stiffness (Fig. 2). A computer controlled detection of the flexible plate deflection allowed us to carry out real-time single cell traction force measurements.19 Using flexible plates of different stiffness, we could determine the effect of rigidity on contractility at the cell scale.
Figure 2.
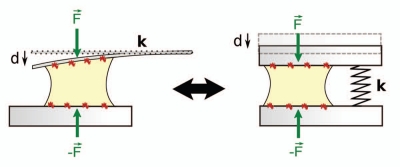
Principle of a single cell traction force assay. A single cell spreads between and pull on two parallel glass microplates coated with fibronectin. Cell traction force is measured through the deflection d of a flexible plate of calibrated stiffness k; F = kd. The setup is equivalent to one where the cell would be compressing a spring of stiffness k. By using plates of different stiffness, we have recently investigated the effect of rigidity on the contractile activity at the cell scale, i.e., on the overall force F.
The main observation was that force increased faster when cells were pulling on stiffer plates. Consequently, after a given time t, cells applied higher forces on stiffer plates (Fig. 3). This is an important observation since pulling harder when the environment is rigid is one strategy allowing cells to orient and migrate along the stiffest direction of their substrate (Sup. materials of ref. 18).
Figure 3.
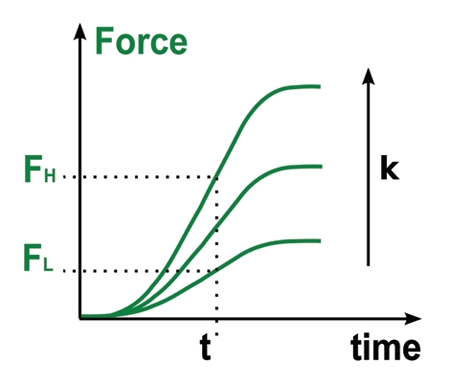
Force generation is dependent on stiffness. The rate of force build-up (dF/dt, slope of the force curves) increased with stiffness. This implies that, after a given time t, cells apply higher forces FH on stiff plates than on soft ones Fl. This phenomenon could explain why cells migrating on anisotropic substrates orient along the stiffest axis.
It is also remarkable that reaching higher forces after a given time t means that cells involve more energy in deforming stiffer substrates. This could be due to adaptation of the cell contractile machinery, generating more power for increasing stiffness. Measuring, in real time, the traction force F, as well as the speed of shorting V, we could determine the mechanical power p = FV (i.e., the amount of energy per unit time) that a single cell produce to bend flexible plates of different stiffness.
It turned out that the mechanical power increased with increasing rigidity in the low stiffness range where previous studies on 2D substrates have reported cell adaptation to rigidity. These earlier studies have shown that equilibrium traction forces were proportional to stiffness, leading to the idea that cell response could be controlled by deformation.20 Our findings suggested a different interpretation. Increasing forces with increasing stiffness could be the consequence of an adaptation of the mechanical output power of force generators within the cell [increasing rate of force generation, slope of the F(t) curve, Fig. 3].
To further test this idea, we investigated the role of acto-myosin activity in force generation. Traction force measurements were carried out with increasing doses of myosin inhibitor blebbistatin. We obtained a graded force inhibition similar to the one observed for mouse cardiac muscles on the one hand and resembling the inhibition of the ATPase activity of non-muscle myosin II measured in chemical assays on the other hand (similar inhibitory constant Ki). Thus, force generation in single cells reflected the ATPase activity of non-muscle myosin II.
We then compared the force-velocity (or equivalently the load-power) data measured in single-cell traction force experiments to the Hill F-V relationship observed for muscle contraction. Using dimensionless parameters v = V/Vmax and f = F/Fmax (Vmax and Fmax being the maximum speed of shortening and the maximum traction force of an isolated cell), we showed that single cell data fit the universal Hill dimensionless f-v equation accounting for muscle contraction and reflecting the force-dependent kinetics of myosin binding to actin. Thus acto-myosin contractile units could by themselves act as mechanosensors, adapting mechanical power to the stiffness of the cell substrate. In fact, in the low (physiological) stiffness range, increasing stiffness implies decrease of the cell speed of contraction and, hence, a decrease in the internal friction. This means less energy lost into heat and acto-myosin contraction could thus become more efficient on stiffer substrates, leading to higher rates of force increase on a rigid environment.
Mechanical Cell-Scale Response to Stiffness is Nearly Instantaneous
The results depicted above suggested that, beyond local adhesion complexes sensitivity to force, cell response to stiffness could also involve a cell-scale acto-myosin based adaptation of tension build-up. These two mechanisms are quite different in nature and should take place on different characteristic time scales. For instance, integrin-based sensing involves biochemical signaling cascades and structural reorganization at the cell scale that are characterized by time scales in the minute range.21,22 Conversely, mechanical adaptation of the rate of tension build-up (acto-myosin dependent sensing) should be instantaneous.
Thus, in order to reveal the kinetics of cell response to the rigidity of its environment, we have recently designed a method allowing us to change dynamically (t < 0.1 s) the effective stiffness felt by a single living cell, regardless of the level of cell-substrate forces.23 In these conditions, measuring the traction force generated by single cells, we found that the rate of force build-up was tuned to stiffness in less than 0.1 second and was not dependent on the level of the cell-substrate force, nor on the cell strain.24 It seems unlikely that this instantaneous cell response to the stiffness of its environment could be explained by the slow, force-dependent, mechano-chemical signaling processes described until now.
Global and Local Sensing: Towards a Two Steps Model?
We can now speculate on how local (integrin dependent) and global (acto-myosin) sensing could act together to ensure an appropriate cell response to the rigidity of its environment. For instance, let us consider an experiment where a cell is plated on a 2D substrate of anisotropic stiffness (Fig. 4). Before contacting the substrate, the cell is rounded which indicates that acto-myosin based tension is isotropic in the cell cortex. When the cell reaches the substrate and begins to spread, cortical tension is transferred to the substrate through newly established adhesions at the cell periphery. What our data imply is that tension applied on adhesions situated along the high stiffness axis will increase faster than that applied on complexes aligned with the soft axis. Thus, tension will become high along the stiff axis and low along the soft one. As a consequence, following the well established adhesion complexes reinforcement and growth under applied force, adhesions situated along the stiff axis will grow notably and stabilize, while those aligned with the soft axis won't and could even shrink if they could not reach some hypothetical threshold force to form stable adhesions. Such a scenario would lead to preferential cell spreading along the stiffer axis as observed experimentally.25
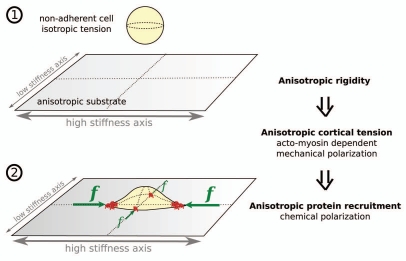
Figure 4.
A model of initial cell “polarization” on a substrate with anisotropic rigidity. (1) When cell is non-adherent, acto-myosin based cortical tension is isotropic and the cell is rounded. (2) When cell reaches the substrate and begins to spread, tension in the free part of the cortex is transferred to the substrate through adhesions situated at the cell periphery. Since the rate of force build-up increases with stiffness, cortical tension will become anisotropic. This will result in higher forces applied on the cell poles situated along the stiffest axis of the substrate. Following adhesions sensitivity to force, these poles will concentrate adhesion complexes and their related mechano-chemical signaling processes.
In summary, we suggest that the anisotropy in substrate rigidity could be first (∼instantaneously) translated into an anisotropy in acto-myosin dependent cortical tension at the cell scale (mechanical polarization). This anisotropic tension would then be translated locally (typical time scales of tens of seconds or minutes), at the cell periphery, into adhesion complexes of adapted size and stability (chemical polarization). Eventually, this inhomogeneous distribution of adhesion complexes would lead to concentration of adhesions-dependent chemical signaling at the cell poles situated along the stiffer axis, and could thus guide cell polarization (Fig. 4). It is indeed noteworthy that anisotropy of cortical forces was shown to orient Drosophila tissue morphogenesis.26 Note also that, at a single cell level, stress fibers were shown to develop in the apical cortex, constituting thus a tensile dome-like cap.27
In order to test the two-step scenario of rigidity sensing proposed here, we are now working on a new setup combining force measurements and TIRFM visualization of labeled proteins of the adhesion complexes. We could thus be able to monitor, simultaneously, within the same single cell, the kinetics of force adaptation as well as of adhesion complexes redistribution/reinforcement, after a switch in the effective stiffness.
Besides a focus on the kinetics of cell response to stiffness, we are also working on a better characterization of the relation between cell shape and traction force generation. In fact, cell spreading and force increase seemed clearly related in our experiments. In these conditions cell shortening can not be as simple as a muscle contraction. We need thus to understand how acto-myosin dependent cortical tension, when applied on an increasing cell-plate contact line (spreading cell), could lead to the force curves obtained experimentally. In particular, it should be informative to visualize the distribution and geometry of stress fibers.
Acknowledgements
This work was supported by grants from the Ministère de la Recherche (ACI Jeune chercheur), from the Centre National de la Recherche Scientifique (Physique et Chimie du Vivant), from the Paris-Diderot (Paris 7) University (Bonus Qualité Recherche) and from the Association pour la Recherche sur le Cancer (subvention libre # 3115). “Physique du Vivant” is member of the GDR 3070 CellTiss of the CNRS.
References
- 1.Discher D, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 3.Discher D, Mooney D, Zandstra P. Growth factors, matrices and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solon J, Levental I, Sengupta K, Georges P, Janmey P. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo C, Wang H, Dembo M, Wang Y. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engler A, Griffin M, Sen S, Bönnemann C, Sweeney H, Discher D. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engler A, Sen S, Sweeney H, Discher D. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Harris A, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 10.Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 11.Riveline D, Zamir E, Balaban N, Schwarz U, Ishizaki T, Narumiya S, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balaban N, Schwarz U, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 13.Tamada M, Sheetz M, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Kostic A, Sheetz M. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell. 2006;17:2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawada Y, Tamada M, Dubin-Thaler B, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingber D. Mechanosensation through integrins: Cells act locally but think globally. Proc Natl Acad Sci USA. 2003;100:1472–1474. doi: 10.1073/pnas.0530201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Butler J, Ingber D. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 18.Mitrossilis D, Fouchard J, Guiroy A, Desprat N, Rodriguez N, Fabry B, et al. Single-cell response to stiffness exhibits muscle-like behavior. Proc Natl Acad Sci USA. 2009;106:18243–18248. doi: 10.1073/pnas.0903994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desprat N, Guiroy A, Asnacios A. Microplates-based rheometer for a single living cell. Rev Sci Instrum. 2006;77:055111. [Google Scholar]
- 20.Saez A, Buguin A, Silberzan P, Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys J. 2005;89:52–54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshigi M, Hoffman L, Jensen C, Yost M, Beckerle M. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lele T, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber D. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 23.Mitrossilis D, Browaeys J, Asnacios A. European patent #EP09305991. Method for determining the response of a contractile or expansible biological material. 2009
- 24.Mitrossilis D, Fouchard J, Pereira D, Postic F, Richert A, Saint-Jean M, et al. Real-time single cell response to stiffness. Proc Natl Acad Sci USA. 2010;107:16518–16523. doi: 10.1073/pnas.1007940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc Natl Acad Sci USA. 2007;104:8281–8286. doi: 10.1073/pnas.0702259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauzi M, Verant P, Lecuit T, Lenne P. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- 27.Khatau S, Hale C, Stewart-Hutchinson P, Patel M, Stewart C, Searson P, et al. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]