Abstract
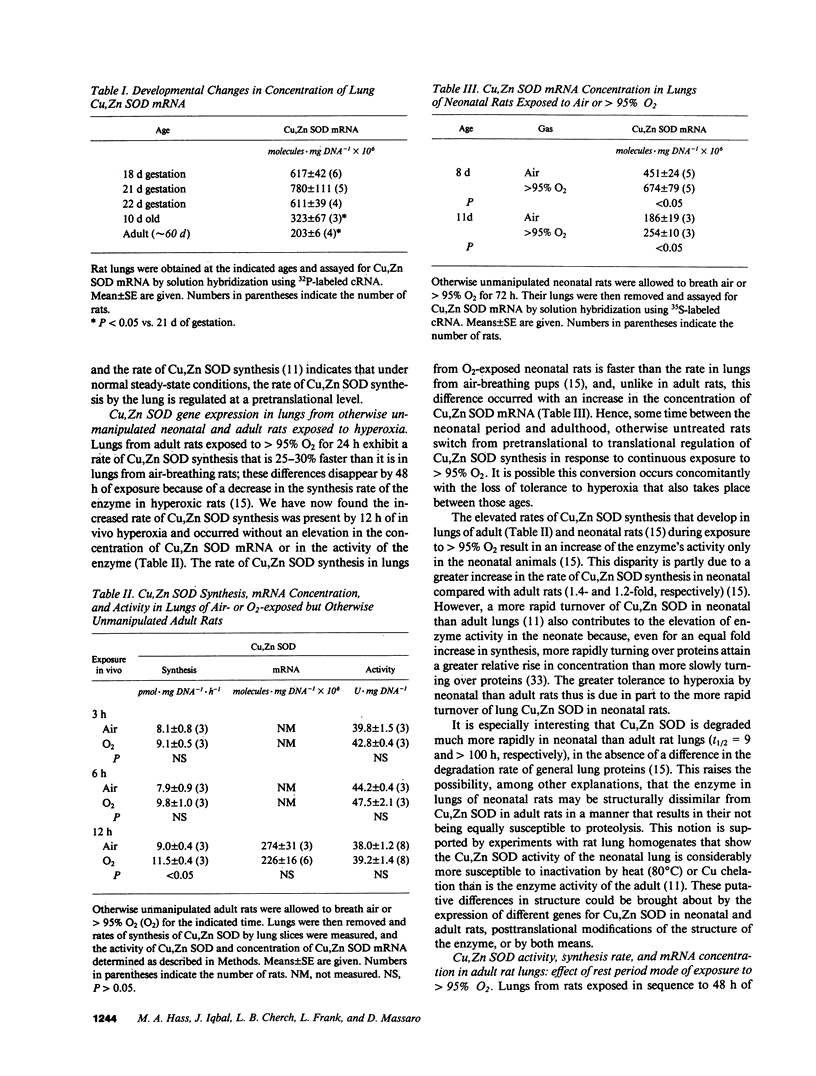
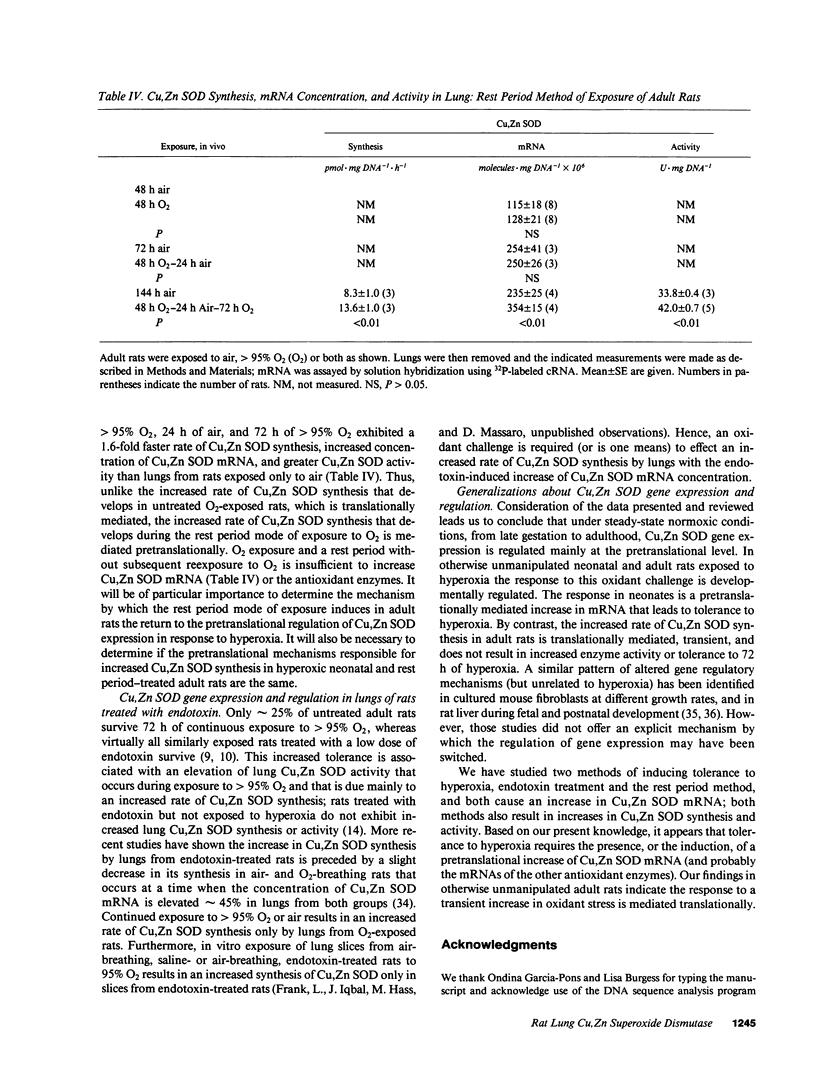
The synthesis of Cu,Zn SOD by rat lung increases spontaneously in the fetus in late gestation and during exposure of neonatal and adult rats to greater than 95% O2. To explore the regulation of these increases, we measured rat lung Cu,Zn SOD synthesis and activity. We also cloned and sequenced a rat lung Cu,Zn SOD cDNA that was used to measure Cu,Zn SOD mRNA concentration. We found that (a) under normal gestational and postgestational conditions the synthesis of this enzyme was regulated pretranslationally; (b) the increased synthesis that occurs under hyperoxia (greater than 95% O2), was pretranslationally mediated in otherwise unmanipulated neonatal rats but translationally controlled in hyperoxic adult rats; and (c) in lungs of rats made tolerant to greater than 95% O2 by allowing 24 h rest in air after an initial 48 h in greater than 95% O2, the increased Cu,Zn SOD synthesis that occurred during the second period of hyperoxia was regulated pretranslationally. We conclude Cu,Zn SOD gene expression in the lung is developmentally regulated under normal conditions and in response to an oxidant challenge. Tolerance, whether endogenous or induced, appears to require the accumulation of increased amounts of Cu,Zn SOD mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clerch L. B., Whitney P. L., Massaro D. Rat lung lectin synthesis, degradation and activation. Developmental regulation and modulation by dexamethasone. Biochem J. 1987 Aug 1;245(3):683–690. doi: 10.1042/bj2450683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerch L. B., Whitney P., Hass M., Brew K., Miller T., Werner R., Massaro D. Sequence of a full-length cDNA for rat lung beta-galactoside-binding protein: primary and secondary structure of the lectin. Biochemistry. 1988 Jan 26;27(2):692–699. doi: 10.1021/bi00402a030. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Delabar J. M., Nicole A., D'Auriol L., Jacob Y., Meunier-Rotival M., Galibert F., Sinet P. M., Jérôme H. Cloning and sequencing of a rat CuZn superoxide dismutase cDNA. Correlation between CuZn superoxide dismutase mRNA level and enzyme activity in rat and mouse tissues. Eur J Biochem. 1987 Jul 1;166(1):181–187. doi: 10.1111/j.1432-1033.1987.tb13500.x. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Frank L., Summerville J., Massaro D. Potection from oxygen toxicity with endotoxin. Role of the endogenous antioxidant enzymes of the lung. J Clin Invest. 1980 May;65(5):1104–1110. doi: 10.1172/JCI109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L., Yam J., Roberts R. J. The role of endotoxin in protection of adult rats from oxygen-induced lung toxicity. J Clin Invest. 1978 Feb;61(2):269–275. doi: 10.1172/JCI108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Fridovich I. Oxygen: boon and bane. Am Sci. 1975 Jan-Feb;63(1):54–59. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases: regularities and irregularities. Harvey Lect. 1983 1984;79:51–75. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Hass M. A., Frank L., Massaro D. The effect of bacterial endotoxin on synthesis of (Cu,Zn)superoxide dismutase in lungs of oxygen-exposed rats. J Biol Chem. 1982 Aug 25;257(16):9379–9383. [PubMed] [Google Scholar]
- Hass M. A., Massaro D. Developmental regulation of rat lung Cu,Zn-superoxide dismutase. Biochem J. 1987 Sep 15;246(3):697–703. doi: 10.1042/bj2460697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass M. A., Massaro D. Differences in CuZn superoxide dismutase induction in lungs of neonatal and adult rats. Am J Physiol. 1987 Jul;253(1 Pt 1):C66–C70. doi: 10.1152/ajpcell.1987.253.1.C66. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. cDNA and deduced amino acid sequence of rat copper-zinc-containing superoxide dismutase. Nucleic Acids Res. 1987 Aug 25;15(16):6746–6746. doi: 10.1093/nar/15.16.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. F., Williams J. G., Abelson H. T., Green H., Penman S. Changes in RNA in relation to growth of the fibroblast. III. Posttranscriptional regulation of mRNA formation in resting and growing cells. Cell. 1975 Jan;4(1):69–75. doi: 10.1016/0092-8674(75)90135-x. [DOI] [PubMed] [Google Scholar]
- Levanon D., Lieman-Hurwitz J., Dafni N., Wigderson M., Sherman L., Bernstein Y., Laver-Rudich Z., Danciger E., Stein O., Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985 Jan;4(1):77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panduro A., Shalaby F., Shafritz D. A. Changing patterns of transcriptional and post-transcriptional control of liver-specific gene expression during rat development. Genes Dev. 1987 Dec;1(10):1172–1182. doi: 10.1101/gad.1.10.1172. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. J., Michelson A. M., Puget K., Flohé L. The amino-acid sequence of rat Cu-Zn superoxide dismutase. Biol Chem Hoppe Seyler. 1986 Oct;367(10):1017–1024. doi: 10.1515/bchm3.1986.367.2.1017. [DOI] [PubMed] [Google Scholar]
- Yam J., Frank L., Roberts R. J. Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. Pediatr Res. 1978 Feb;12(2):115–119. doi: 10.1203/00006450-197802000-00010. [DOI] [PubMed] [Google Scholar]