Abstract
Objective
To examine the impact of a recent surgery on development of endometriosis-related adhesions in a chimeric model and to determine the therapeutic efficacy of pioglitazone (PIO).
Design
Human endometrial biopsies were maintained in estradiol (E) with or without PIO for 24 hrs prior to intraperitoneal injection into immunocompromised mice at multiple timepoints following peritoneal surgery. The presence and extent of adhesions was examined in animals relative to the initial establishment of experimental endometriosis.
Setting
Medical School Research Center
Patients
Endometrial biopsies for experimental studies described herein were provided by normally cycling women without a medical history indicative of endometriosis or adhesions.
Interventions
None
Main Outcome Measure
Examination of the development of endometriosis-related adhesions in an experimental model.
Results
Without therapeutic intervention, injection of E-treated human endometrial tissue into mice near the time of peritoneal surgery resulted in multiple adhesions and extensive endometriotic-like disease. In contrast, PIO treatment reduced adhesive disease and experimental endometriosis related to surgical injury.
Conclusions
The presence of human endometrial tissue fragments in the peritoneal cavity of mice with a recent surgical injury promoted development of both adhesive disease and experimental endometriosis. Targeting inflammation and angiogenesis with PIO therapy limited the development of postsurgical adhesions associated with ectopic endometrial growth.
Keywords: Adhesions, Endometriosis, Surgery, Mice
Introduction
Endometriosis, the growth of endometrial glands and stroma outside the uterus, is diagnosed by surgical observation of ectopic tissue and subsequent histopathological confirmation. The disease is thought to occur most frequently following retrograde menstruation into the peritoneal cavity of reproductive-age women. However, it remains unclear whether or not endometriosis results as a consequence of an altered endometrial physiology (1-2) or an altered inflammatory-like peritoneal microenvironment (3). Regardless, the invasive establishment of endometriosis contributes to peritoneal inflammation and proinflammatory cytokine and chemokine production likely affects disease progression. The co-morbidity of adhesive disease often occurs in endometriosis patients, potentially leading to bowel obstruction, chronic abdominal or pelvic pain and infertility (4). Hospital charges related to adhesive disease alone may represent 3.5 to 5 billion dollars annually in the United States (5). Furthermore, complications of adhesive disease in endometriosis patients may require additional surgery, increasing the potential for further functional injury to organs within the peritoneal cavity. Therefore, therapeutic intervention to prevent adhesion formation in response to various stimuli, including surgical procedures related to the treatment of endometriosis, would represent a significant clinical advance.
The basic pathophysiology of postsurgical adhesion development and the initial establishment of endometriosis share biological commonality around the inflammatory processes that occur during normal wound healing. Thus, within the peritoneal cavity, immune cell responses to injury, similar to infection or radiation therapy, may promote formation of adhesions (6). Certainly, macrophages and neutrophils play key roles in the initiation of inflammation related to wound healing, by releasing both inflammatory cytokines and proangiogenic factors. Nevertheless, current adhesion-prevention strategies are largely limited to mechanical barriers, such as oxidized regenerated cellulose (Gynecare Interceed, Ethicon, Inc., Somerville, NJ) or sodium hyaluronate with carboxymethylcellulose (Saprafilm, Genzyme, Inc., Cambridge, MA). While the effectiveness of these barrier agents in clinical practice remains inconsistent (7-9) other studies indicate that limiting inflammatory processes and/or angiogenesis may provide efficacy in reducing adhesion formation (10-12).
In this report, we describe a novel human/murine experimental model that demonstrates a cooperative link between the presence of endometrial tissue fragments within the peritoneal cavity and the risk that postsurgical adhesions will develop. Our previous studies have indicated that inflammatory processes are involved in the establishment and progression of endometriosis (2); therefore, targeting inflammatory mediators represent a logical therapeutic approach for the treating endometriosis-related adhesive disease. Specifically, thiazolidinediones, a class of pharmacologic agents which activate the PPAR-γ receptor family, have potent anti-angiogenic and anti-inflammatory affects (13) and a member of this family (rosiglitazone) has been found to be effective in preventing adhesive disease in rats (14). Therefore, in the current study, we examined whether pioglitazone (PIO) treatment would reduce the development of surgical adhesions associated with experimental endometriosis. The choice of PIO for our studies was based on the improved pharmacologic profile of PIO compared to rosiglitazone (15). Our results demonstrated that targeted therapy with PIO in our chimeric model of endometriosis-related postsurgical adhesions resulted in a reduction in adhesive disease.
Materials and Methods
Acquisition of Human Endometrial Tissue
Approval for human tissue use was obtained from the Vanderbilt University Institutional Review Board and Committee for the Protection of Human Subjects. After written informed consent, endometrial samples (n=12) were obtained by Pipelle biopsy (Unimar, Inc, Wilton, CT) during the proliferative phase (days 9-12) from a donor population at Vanderbilt University Medical Center. Donors were normally cycling women with no prior medical or surgical history of adhesions or endometrial disease, including symptoms suggestive of endometriosis. A serum progesterone level of <1.5 ng/ml was required for inclusion. Individuals with a history (< 3 months) of hormonal therapy (i.e., oral contraceptives) or other medications that could impact study results were excluded.
Human Tissue Preparation and Culture Conditions
Endometrial specimens were minced into 1 × 1 mm3 cubes, then suspended in tissue culture inserts (Millipore Corp., Bedford, MA) as described previously.15 Endometrial fragments were maintained overnight under serum-free conditions in DME/F-12 media supplemented with 1nM estradiol (Sigma, St. Louis, MO), 1% insulin-transferrin-selenium (ITS +, Collaborative Biomedical, Bedford, MA) and 0.1% Excyte (Miles Scientific, Kankakee, IL) and incubated in a 5% CO2 humidified chamber at 37°C. Some tissues were additionally treated with 700 ng/mL pioglitazone (Takeda Pharmaceuticals, Deerfield, IL) in 1X phosphate buffered saline (PBS). Prior to injection into mice, endometrial fragments were washed in sterile, prewarmed PBS. Each biopsy was typically adequate for induction of experimental disease in 8-12 mice. Biopsies (N = 12) from different patients were not mixed and only a single biopsy was used for each individual experiment. Since endometrial biopsies were not typically adequate for 8 different groups of mice (3 control and 5 experimental groups), each of the 12 experiments included at least one control group (group 1: sham surgery plus endometrial tissues; group 2: surgery plus PBS only; group 3: surgery more than five days prior to tissue injection) and two or more experimental groups (tissue injected 0, 4, 16, 24-36 or 120 hrs after ovariectomy [experimental groups 1-5]). For each independent experiment, all groups within that experiment consisted of 2-3 mice per group and each group was replicated at least 3 times with 3 different human biopsies.
Ovariectomy and Sham Surgeries
Five-week-old athymic nude mice (Harlan Laboratories, Indianapolis, IN) were anesthetized with isoflurane and subjected to standard surgical ovariectomy via a single 5mm, dorsal/ventral incision between the rib cage and hind limb.7 The incision was held open with small rat tooth forceps and the ovary pulled out through the incision with blunt forceps by grasping the surrounding fat pad. Mosquito hemostats are placed at the boundary between the oviduct and uterus and left in place for several seconds. On each side, the ovary and oviduct are removed just below the hemostat using scissors; after hemostat release, the site was inspected for bleeding. During sham surgery, the ovary and oviduct were similarly surgically identified and the ovarian fat pad excised, but no clamping with hemostats occurred. At the time of surgery, all mice were implanted subcutaneously with a slow-release estradiol capsule made in our laboratory as previously described (16). Experiments described herein were approved by Vanderbilt University’s Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act.
Experimental Endometriosis/Adhesion Model
In contrast to our previous experimental endometriosis studies which allowed mice to fully recover from ovariectomy surgery for 5-10 days before intraperitoneal (ip) injection of human endometrial tissue (for example, (17)), ovariectomy in the current study was experimentally performed at times closer to human tissue injection. Specifically, mice were ovariectomized 0, 4, 16, 24-36 or 120 hrs prior to ip injection of tissues along the ventral midline (supplemental FIGURE 1). Three sets of control mice were utilized in this study: Control Group 1 was ovariectomized 5 days (120 hrs) prior to tissue injection, by which time the surgical injury had healed. Control Group 2 was ovariectomized 16 hrs prior to injection with PBS only (no tissue). Control Group 3 underwent sham surgery 16 hrs prior to injection of tissue. All animals were sacrificed 5 days after injection of tissues (or PBS only).
At necropsy, the presence or absence of peritoneal adhesions and the presence and extent of ectopic sites of endometrial growth was determined. Endometriotic-like lesions were further characterized by size, the larger denoted “a” and the smaller denoted “b” and total volume, calculated by standard methodology using the formula: V = a × b2 × 0.5 (18).
Pioglitazone Inhibition of Adhesion Formation
Since rosiglitazone has previously been found to prevent/reduce adhesions in a rat uterine horn adhesion model (14), we examined the therapeutic efficacy of a similarly acting thiazolidinedione (PIO) using our experimental model of endometriosis-related adhesion development. Within additional studies, mice were similarly subjected to ovariectomy or sham-surgery as described above. Mice undergoing ovariectomy 16 hrs prior to injection of endometrial tissue received fragments maintained overnight in 1 nM estradiol with or without 700 ng/mL PIO. Of the mice receiving estradiol only-treated tissues, half were also administered PIO by gavage once daily beginning 2 days prior to surgery and continuing until the end of the study (5 days after endometrial tissue injection). All mice receiving PIO-treated tissues were provided 1 μg/kg PIO by gavage for the same 7 days. At necropsy, the presence of ectopic endometrial disease and the presence or absence of adhesion formation was determined.
Immunohistochemistry and Assessment of Microvessel Density
Immunohistochemical staining of experimental lesions for von Willebrand factor (vWF) was performed by standard methodology for formalin-fixed, paraffin-embedded tissues using a rabbit anti-human von Willebrand factor (1-3:000 dilution, DakoCytomation, Carpinteria, CA) and visualized using the Dako Envision+ HRP/DAB System (DakoCytomation) following counterstaining with Mayer’s hematoxylin.
Microvessels were enumerated by standard methodology after scanning at low power (40x magnification) to identify areas of dense vascularization. Of these areas, five random fields were selected, then vWF-positive vessels counted at a magnification of 200x. The average number of microvessels obtained from 5 (or the maximum number of obtainable fields) and multiple specimens is shown (TABLE 1).
Table 1.
Impact of Timing of Tissue Injection on Experimental Endometriosis and Adhesion Development
| Surgical Timing Prior to Tissue Injection (hrs) |
Percent with Experimental Endometriosis |
Percent with Adhesions |
Lesion size (total avg. volume, mm3) |
Microvessel Density (Avg) |
|||
|---|---|---|---|---|---|---|---|
| N | Surgical Site |
Other Sites |
Surgical Site |
Other Sites |
|||
| Ovx (0 hrs) | 10 | 75 | 100 | 75 | 100 | 2.5 | 71.5 |
| Ovx (4-6 hrs) | 10 | 100 | 100 | 100 | 100 | 2.8 | 73.8 |
| Ovx (16 hrs) | 15 | 100 | 100 | 100 | 93 | 5.4 | 65.1 |
| Ovx (24-36 hrs) | 10 | 40 | 100 | 44 | 40 | 1.96 | 47.7 |
| Ovx (5 days) | 15 | 10 | 90 | 0 | 10 | 1.5 | 14.8 |
| Sham surgery (16 hrs) | 6 | 0 | 100 | 0 | 0 | 1.4 | 18.3 |
| Ovx (PBS at 16 hrs) | 3 | N/A | 0 | 0 | N/A | NA | |
OVX: Ovariectomy
N/A: Not applicable
Results
Adhesion Formation
Mice undergoing ovariectomy within 16 hrs of receiving human endometrial tissues developed extensive adhesive disease (TABLE 1 and FIGURE 1), including not only adhesions at the site of surgical injury, but also at peritoneal sites distal from the site of surgical injury (TABLE 1 and FIGURE 1). In contrast, adhesions did not develop in mice undergoing sham surgery or PBS injection in the absence of human tissues and adhesions were an infrequent finding in mice receiving human tissues more than 16 hrs postsurgery (TABLE 1).
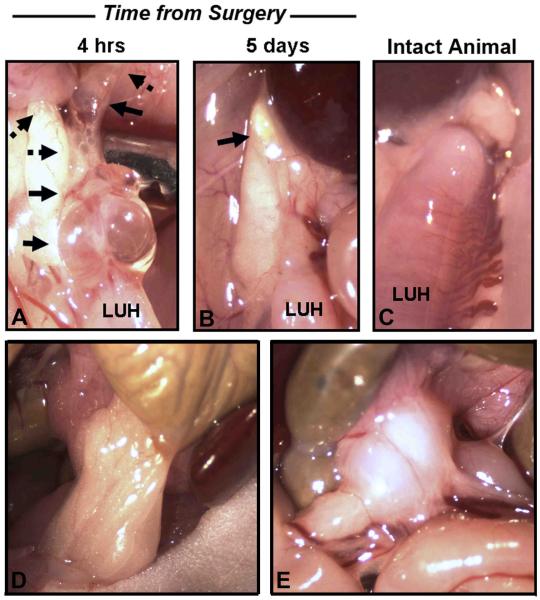
Figure 1. Gross Morphology of Experimental Lesions and Adhesions and Timing of Tissue Injection.
A-B. Gross photomicrographs of lesions (solid arrows) and adhesions (dotted arrows) identified in mice at the site of ovariectomy. Human endometrial tissues were injected 4 hrs (A) or 5 days (B) after surgery. Mice were sacrificed 5 days after tissue injection. C. Left uterine horn (LUH) of an intact, disease free mouse. Adhesion formation decreased as time from surgery increased. D-E. Gross photomicrographs of adhesions identified at sites distal from the surgical injury in mice receiving human tissues 16 hrs post-surgery. D. Omentum adhered to the colon. E. Large adhesion within the mesentery. Original magnification, 15x.
Wound-related Establishment of Ectopic Endometrial Growth
Endometriosis-like disease in mice was similarly affected by surgical injury. As shown in TABLE 1, endometrial tissues injected within 16 hrs of sham surgery readily established ectopic sites of growth in animals at a 100% success rate. The distribution and average size of ectopic lesions (1.4 mm3 at 5 days of growth) were comparable to previous results using this model (17, 19-21). In contrast to the random distribution of lesions noted in the sham surgery group, endometrial tissue preferentially attached to the injury site in more than 90% of mice receiving human tissue at 0, 4 or 16 hrs postovariectomy. Furthermore, ectopic endometrial lesions in the 16-hour postovariectomy group were more than twice as large as noted in the sham surgical group (5.4 mm3 compared to 1.4 mm3). The preferential attachment of human tissue to the site of injury decreased to 40% when injected 24-36 hrs postovariectomy, concomitant with reduction in the observable size of the ectopic lesions. Human tissues injected into mice beyond 5 days postovariectomy did not preferentially attach to the former site of injury and the observed size of lesions was similar to those in sham surgery animals. We have previously demonstrated that establishment of experimental endometriosis requires rapid lesion vascularization (22). In the current study, microvessel density (MVD), as assessed by immunohistochemical localization of von Willebrand Factor (vWF), was found to be increased within endometriotic lesions developing from tissue injected nearer the time of surgery (TABLE 1).
Impact of Pioglitazone Treatment on Adhesions and Endometriotic-like Lesions
As shown in TABLE 2, 50% of mice receiving human tissue and PIO therapy in vivo lacked adhesions. Remaining PIO treated mice exhibited fewer adhesions, largely at the surgical injury site. PIO treatment of both mice and endometrial tissues further reduced adhesion development (75% adhesion free at time of necropsy) (TABLE 2; FIGURE 2).
Table 2.
Impact of Pioglitazone (PIO) treatment on the development of adhesions
| Experimental Group (Treatment) |
Percent with Experimental Endometriosis |
Percent with Adhesions |
Lesion size (total avg. volume, mm3) |
Microvessel Density (Avg) |
|||
|---|---|---|---|---|---|---|---|
| N | Surgical Site |
Other Sites |
Surgical Site |
Other Sites |
|||
| E + Vehicle | 10 | 100 | 75 | 100 | 50 | 5.125 | 50.8 |
| E + PIO (in vivo only) |
10 | 75 | 25 | 50 | 25 | 1.9 | 19.7 |
| E + PIO (in vitro and in vivo) |
15 | 75 | 10 | 25 | 0 | 0.925 | 5.1 |
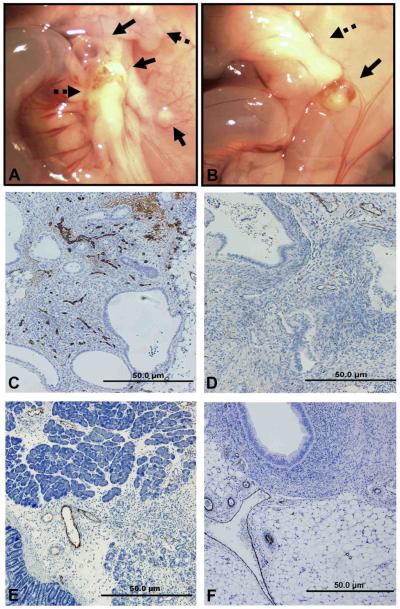
Figure 2. Gross Morphology and Assessment of Microvessel Density in Experimental Endometriosis.
A. Gross photomicrographs of lesions (solid arrows) and adhesions (dotted arrows) at the site of the surgical injury in control (A) mice and in PIO treated mice which also received tissues treated with E+PIO (B). Human endometrial tissues were injected 16 hrs postovariectomy. Assessment of microvessel density (MVD) by immunohistochemical localization of vWF in experimental endometriosis lesions (C-D) and in adhesions (E-F) from the same mice. Control mouse (C and E) received E treated tissues while PIO-treated mouse received tissues treated with E+PIO (D and F). Original magnification, 100x.
As expected, all mice receiving E-treated endometrial tissue but no therapeutic intervention, exhibited ectopic endometrial lesions, predominately at the peritoneal injury site, and adhesive disease was frequently noted. Although adhesions were the primary focus of the current study, each PIO therapeutic regimen (in vivo only or in vitro + in vivo) resulted in fewer animals (75%) exhibiting endometriosis-like disease at sacrifice both the size and number of lesions was reduced compared to control mice. Accurately assessing the therapeutic efficacy of PIO for experimental endometriosis will require longer treatments compared to adhesion prevention.
Finally, MVD was assessed in ectopic lesions as described above. Specifically, immunohistochemical localization of vWF was performed in excised lesions from mice receiving human tissues 16 hrs postovariectomy. As shown in TABLE 2, in PIO-treated mice, which received tissues pretreated with PIO 16 hrs postsurgery, MVD was greatly reduced compared to that observed in control mice receiving tissues 16 hrs postsurgery (FIGURE 2 and TABLE 2).
Assessing MVD in adhesions following PIO treatment was difficult since PIO treatment reduced adhesion development, limiting the samples available for asessement; however, vWF-positive blood vessels were noted in all adhesions examined and MVD was not significantly different in PIO-treated and untreated animals (FIGURE 2).
Discussion
Resident immune cells populate the human endometrium at menstruation (23) and the inflammatory nature of menstrual tissue transferred to the peritoneal cavity likely affects the development of endometriosis (3, 24). Using intraperitoneal endometrial tissue injection to mimic retrograde menstruation, our studies suggest that surgery near the time of menstruation may contribute to peritoneal inflammation, potentially affecting the development of endometriosis and adhesive disease. Specifically, in our model, peritoneal adhesion formation was maximally enhanced by a surgical wound and the presence of endometrial fragments. Mice receiving endometrial tissue injection at 0 to 16 hrs postovariectomy developed multiple adhesions at the wound site and elsewhere in the peritoneal cavity whereas no adhesions distal to the surgical injury site itself were noted in the model after 36 hrs from the time of surgery. Since sham-operated mice lacked adhesions, the presence of endometrial fragments within the peritoneum did not independently trigger adhesion formation. Similarly, in the absence of human tissue injection, the surgical procedure alone also failed to promote adhesions. Importantly, the study reported herein only examined the effects of ovariectomy since this surgical procedure is a standard component of our previously established experimental endometriosis model (15). Therefore, the potential impact of other, perhaps more common, peritoneal surgical procedures will need to be examined in future studies to determine whether our results are specific to the surgical removal of ovaries or to pelvic surgery in general.
Compared to infection-related adhesions, endometriosis-associated adhesions exhibit greater numbers of inflammatory cells (25), likely reflecting the proinflammatory influence of ectopic endometrial growth on the entire peritoneal microenvironment. Importantly, a recent surgical injury can equally affect the manner in which experimental endometriosis develops. Specifically, endometrial tissue preferentially attached at the injury site when injected 0 to 16 hours after surgery while tracking to the wound site is limited at later times. Certainly, proinflammatory cytokines affect both wound healing and angiogenesis during menstruation (26) and surgery (27) and thus the presence of these cytokines likely contributed to our findings.
In previous studies, we have demonstrated that, establishing experimental endometriosis requires that human tissue fragments acquire a peritoneal vascular supply (22). Interestingly, in the current study, the extent of angiogenesis in the ectopic lesions reflected the timing of human tissue injection relative to the ovarietomy. Microvessel density (MVD) was highest in ectopic lesions established near the time of surgery and decreased thereafter. Although the MVD of adhesions appeared less dependent upon the timing of human tissue injection postsurgery, the risk of adhesion development diminished quickly after 16 hrs suggesting an “inflammatory threshold” may be required for adhesion formation.
In the last series of studies in our endometriosis-adhesion model we examined a potential therapeutic agent for adhesion prevention. PIO, a thiazolidinedione with PPAR-γ agonist activity, is currently in use as an anti-diabetic medication. PPAR-γ receptors are expressed in numerous cell types including immune and endothelial cells (28-29); thus, treatment with thiazolidinediones can have both anti-inflammatory and anti-angiogenic effects. Rosiglitazone, another thiazolidinedione, has previously been found to prevent/reduce adhesions in a rat uterine horn adhesion model (14). In our study, we found that treatment of mice with PIO both prior to surgery and for 5 days postsurgery reduced the extent of lesion formation as well as the development of postsurgical adhesions.
In summary, we report that the proinflammatory microenvironment of a recent, peritoneal surgical procedure combined with the presence of endometrial tissue fragments is associated with the development of adhesive disease, even at sites distal to the surgical injury. Current models of experimental adhesion formation require direct trauma to the mesothelium (30-31) and thus future studies with our model may provide useful information on the mechanisms of adhesion formation not directly related to local tissue trauma. Although translation of our chimeric experimental model findings to women is premature at this juncture, it would be difficult to conduct a prospective human study to determine whether avoiding surgery near the time of menstruation is prudent. Therefore, the use of our chimeric model represents a useful experimental approach to understanding the association of peritoneal inflammation to the related disease processes of endometriosis and post surgical adhesion formation. Certainly, our therapeutic intervention with PIO provides preliminary evidence that similar approaches may aid in developing new medical strategies for adhesion prevention.
Capsule.
In this study, we describe a novel model for examining the role of a recent surgical injury on the development of endometriosis-related adhesions.
Supplementary Material
Supplemental Figure 1: Timing of postsurgical tissue injection and collection. Schematic diagram representing the timing of surgery prior to tissue injection. Surgeries were adjusted to allow different mice to receive shared human endometrial tissue injected at the same fixed timepoint. Euthanasia of all mice were performed at 5 days (horizontal bars) following tissue injection, and peritoneal ectopic lesions and adhesions were assessed.
Acknowledgements
We greatly appreciate and acknowledge the women who donated endometrial biopsies for these studies as well as the clinicians within the Vanderbilt Women’s Reproductive Health Clinic for performing these critical biopsies.
Supported by: NICHD R01HD055648 (KGO), RO3 HD052012 (KBT), K23HD043952 (DIL) and the Endometriosis Association (KGO)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part: Society for Gynecologic Investigation 56th Annual Meeting, March 17-19, 2009, Glasgow, Scotland
References
- 1.Bulun SE. Progesterone resistance and endometrial disease. Preface. Semin Reprod Med. 2010;28:3. doi: 10.1055/s-0029-1242987. [DOI] [PubMed] [Google Scholar]
- 2.Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83:529–37. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Herington JL, Bruner-Tran KL, Osteen KG. Matrix Metalloproteinases and Endometriosis. In: I AAaM, editor. Endometriosis-Adenomyosis. Paschalides Med Publication; Athens: 2010. [Google Scholar]
- 4.Alpay Z, Saed GM, Diamond MP. Postoperative adhesions: from formation to prevention. Semin Reprod Med. 2008;26:313–21. doi: 10.1055/s-0028-1082389. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman DM. Adhesion Related Disease- Adhesion Related Deaths. 2003 www.adhesion.org.
- 6.Imudia AN, Kumar S, Saed GM, Diamond MP. Pathogenesis of Intra-abdominal and pelvic adhesion development. Semin Reprod Med. 2008;26:289–97. doi: 10.1055/s-0028-1082387. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad G, Duffy JM, Farquhar C, Vail A, Vandekerckhove P, Watson A, et al. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD000475.pub2. CD000475. [DOI] [PubMed] [Google Scholar]
- 8.Darmas B. Use of barrier products in the prevention of adhesion formation following surgery. J Wound Care. 2008;17:405–8. 11. doi: 10.12968/jowc.2008.17.9.30939. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Quintero VH, Cruz-Pachano FE. Preventing adhesions in obstetric and gynecologic surgical procedures. Rev Obstet Gynecol. 2009;2:38–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Aytan H, Caliskan AC, Yener T, Demirturk F, Aytan P, Yenisehirli A. A novel antibiotic, linezolid, reduces intraperitoneal adhesion formation in the rat uterine horn model. Acta Obstet Gynecol Scand. 2009;88:781–6. doi: 10.1080/00016340903002873. [DOI] [PubMed] [Google Scholar]
- 11.Mendes JB, Campos PP, Rocha MA, Andrade SP. Cilostazol and pentoxifylline decrease angiogenesis, inflammation, and fibrosis in sponge-induced intraperitoneal adhesion in mice. Life Sci. 2009;84:537–43. doi: 10.1016/j.lfs.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Steinleitner A, Lambert H, Kazensky C, Danks P, Roy S. Pentoxifylline, a methylxanthine derivative, prevents postsurgical adhesion reformation in rabbits. Obstet Gynecol. 1990;75:926–8. [PubMed] [Google Scholar]
- 13.Imamoto E, Yoshida N, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, et al. Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 2004;20:37–47. doi: 10.1002/biof.5520200104. [DOI] [PubMed] [Google Scholar]
- 14.Demirturk F, Aytan H, Caliskan A, Aytan P, Yener T, Koseoglu D, et al. The effect of rosiglitazone in the prevention of intra-abdominal adhesion formation in a rat uterine horn model. Hum Reprod. 2006;21:3008–13. doi: 10.1093/humrep/del258. [DOI] [PubMed] [Google Scholar]
- 15.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–8. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 16.Krikun G, Hu Z, Osteen K, Bruner-Tran KL, Schatz F, Taylor HS, et al. The immunoconjugate “icon” targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am J Pathol. 2010;176:1050–6. doi: 10.2353/ajpath.2010.090757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99:2851–7. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlsson G, Gullberg B, Hafstrom L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20–3. doi: 10.1007/BF00391826. [DOI] [PubMed] [Google Scholar]
- 19.Bruner KL, Eisenberg E, Gorstein F, Osteen KG. Progesterone and transforming growth factor-beta coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis. Steroids. 1999;64:648–53. doi: 10.1016/s0039-128x(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 20.Bruner-Tran KL, Yeaman GR, Crispens MA, Igarashi TM, Osteen KG. Dioxin may promote inflammation-related development of endometriosis. Fertil Steril. 2008;89:1287–98. doi: 10.1016/j.fertnstert.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruner-Tran KL, Zhang Z, Eisenberg E, Winneker RC, Osteen KG. Down-regulation of endometrial matrix metalloproteinase-3 and -7 expression in vitro and therapeutic regression of experimental endometriosis in vivo by a novel nonsteroidal progesterone receptor agonist, tanaproget. J Clin Endocrinol Metab. 2006;91:1554–60. doi: 10.1210/jc.2005-2024. [DOI] [PubMed] [Google Scholar]
- 22.Hull ML, Charnock-Jones DS, Chan CL, Bruner-Tran KL, Osteen KG, Tom BD, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88:2889–99. doi: 10.1210/jc.2002-021912. [DOI] [PubMed] [Google Scholar]
- 23.Yeaman GR, Collins JE, Fanger MW, Wira CR, Lydyard PM. CD8+ T cells in human uterine endometrial lymphoid aggregates: evidence for accumulation of cells by trafficking. Immunology. 2001;102:434–40. doi: 10.1046/j.1365-2567.2001.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osteen KG, Bruner-Tran KL, Eisenberg E. Endometrial biology and the etiology of endometriosis. Fertil Steril. 2005;84:33–4. doi: 10.1016/j.fertnstert.2005.01.124. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 25.Tulandi T, Chen MF, Al-Took S, Watkin K. A study of nerve fibers and histopathology of postsurgical, postinfectious, and endometriosis-related adhesions. Obstet Gynecol. 1998;92:766–8. doi: 10.1016/s0029-7844(98)00298-1. [DOI] [PubMed] [Google Scholar]
- 26.Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update. 2000;6:16–27. doi: 10.1093/humupd/6.1.16. [DOI] [PubMed] [Google Scholar]
- 27.Sido B, Teklote JR, Hartel M, Friess H, Buchler MW. Inflammatory response after abdominal surgery. Best Pract Res Clin Anaesthesiol. 2004;18:439–54. doi: 10.1016/j.bpa.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Inoue I, Shino K, Noji S, Awata T, Katayama S. Expression of peroxisome proliferator-activated receptor alpha (PPAR alpha) in primary cultures of human vascular endothelial cells. Biochem Biophys Res Commun. 1998;246:370–4. doi: 10.1006/bbrc.1998.8622. [DOI] [PubMed] [Google Scholar]
- 29.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 30.Aksakal O, Yilmaz B, Gungor T, Sirvan L, Sut N, Inan I, et al. A randomised controlled trial on melatonin and rosiglitazone for prevention of adhesion formation in a rat uterine horn model. Arch Gynecol Obstet. 2009 doi: 10.1007/s00404-009-1240-8. [DOI] [PubMed] [Google Scholar]
- 31.Batukan C, Ozgun MT, Basbug M, Muderris II. Sildenafil reduces postoperative adhesion formation in a rat uterine horn model. Eur J Obstet Gynecol Reprod Biol. 2007;135:183–7. doi: 10.1016/j.ejogrb.2006.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Timing of postsurgical tissue injection and collection. Schematic diagram representing the timing of surgery prior to tissue injection. Surgeries were adjusted to allow different mice to receive shared human endometrial tissue injected at the same fixed timepoint. Euthanasia of all mice were performed at 5 days (horizontal bars) following tissue injection, and peritoneal ectopic lesions and adhesions were assessed.