Abstract
In previous studies, low blood levels of n-3 fatty acids (FA) have been associated with increased risk of cardiac death, and the omega-3 index (red blood cell (RBC) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) expressed as weight percentage of total FA) has recently been proposed as a new risk factor for death from coronary artery disease, especially following sudden cardiac arrest (SCA). As blood samples often haven been harvested after the event, the aim of our study was to evaluate the stability of RBC fatty acids following SCA. The total FA profile, including the omega-3 index, was measured three times during the first 48 h in 25 survivors of out-of-hospital cardiac arrest (OHCA), in 15 patients with a myocardial infarction (MI) without SCA and in 5 healthy subjects. We could not demonstrate significant changes in the FA measurements in any of the groups, this also applied to the omega-6/omega-3 ratio and the arachidonic acid (AA)/EPA ratio. Furthermore, we compared the omega-3 index in 14 OHCA-patients suffering their first MI with that of 185 first-time MI-patients without SCA; mean values being 4.59% and 6.48%, respectively (p = 0.002). In a multivariate logistic regression analysis, a 1% increase of the omega-3 index was associated with a 58% (95% CI: 0.25–0.76%) reduction in risk of ventricular fibrillation (VF). In conclusion, the omega-3 index remained stable after an event of SCA and predicted the risk of VF.
Keywords: n-3 fatty acids, Eicosapentaenoic acid, Docosahexaenoic acid, Red blood cell membranes, The omega-3 index, Fatty acid stability, Sudden cardiac arrest, Ventricular fibrillation, Myocardial infarction
Introduction
The first clinical evidence of a possible protective effect of n-3 fatty acids (FA) against sudden cardiac death (SCD) came from the publication of the GISSI-Prevenzione trial in 1999 [1]. In this study, supplementation with 1 g/day of n-3 FA following a myocardial infarction (MI) resulted in a 45% reduction of sudden deaths during 3.5 years of follow-up. This finding has been supported by the demonstration of low levels of blood n-3 FA in patients suffering SCD [2, 3] or a fatal cardiac event [4]. In addition, our research group has recently presented evidence for an increased risk of ventricular fibrillation (VF) during the acute ischemic phase of an MI in patients with low levels of cellular n-3 FA [5]. In our study, as well as in a previous investigation [3], blood samples were harvested after the episode of sudden cardiac arrest (SCA). To obtain a reliable result with this method, we have to assume that the blood composition of FA remains stable following the event, or else we need to correct for changes induced by the event itself in selected case–controls.
Previous studies have demonstrated significant changes in FA composition of serum [6] and plasma lipids [7] in the early stage of an MI. Recently, the red blood cell (RBC) FA composition has been found to reflect the cardiac omega-3 content [8, 9]. Even though stable in rats for 24 h after the induction of an MI [7], the only evaluation of post-MI RBC FA in humans demonstrated a statistically significant change in the investigated FA during a 7 day period [10]. As most episodes of cardiac arrest appear during the early course of an MI, these findings are of interest when assessing the FA profile of SCD/SCA-patients.
In patients with SCA, the event itself, as well as the resuscitation, might potentially affect the measurements. Stress-induced or injected adrenalin can activate phospholipases, releasing FA from cell membrane phospholipids leading to falsely low post-resuscitation measurements. The only evaluation of this question has so far been performed in animal studies, in which no significant effect of adrenalin on the FA composition in RBC [3] or serum lipids [11] has been observed. FA stability after an episode of cardiac arrest has previously not been reported in humans, and this was the primary aim of our study.
Based on the proposed post-MI changes in FA, we wanted to compare the time-related profile of RBC FA in SCA-patients with that of MI-patients without a cardiac arrest. We also wanted to evaluate whether the level of RBC eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) (the omega-3 index) might be related to VF, as previously demonstrated in another group of SCA patients [5].
Materials and Methods
Study Subjects and Design
This study was performed at Stavanger University Hospital, Stavanger, Norway. The inclusion criteria were; age >18 years and out-of-hospital cardiac arrest (OHCA) of assumed cardiac origin. Patients with permanent return of spontaneous circulation (ROSC) had 6 mL of EDTA- blood drawn on hospital admission. Further blood sampling was repeated after 8–12 h and after 24–48 h. At this point, most patients were on a respirator at the intensive care unit, and blood samples were collected from an already established arterial crane. For the few patients who woke up immediately after resuscitation, blood sampling was performed as part of the hospital’s routine. Echocardiography was performed shortly after admission for the evaluation of the ejection fraction (EF). Survivors gave written informed consent before discharge. If the patient stayed unconscious until death, the family was asked for consent on the patient’s behalf.
From February 2007 until June 2009, blood samples for evaluation of RBC FA were collected from 25 patients with documented VF. Patients were divided into three groups based on the mechanism of their ventricular arrhythmia; (1) SCA without a present MI, (2) SCA with a first-time MI and (3) SCA with recurrent MI. The presence or absence of an MI was determined from the release pattern of troponin-T (TnT), using ST-segment analysis of the electrocardiogram (ECG) for the definition of ST-elevation MI (STEMI) or non-ST-elevation MI (NSTEMI). For one case, there were only two in-hospital samples available, and this patient was only included in the analysis of risk.
For the evaluation of MI related FA changes, 15 subjects with an acute MI (AMI) without SCA were recruited. In this group, MI was defined by a maximal TnT >0.03 μg/L and a typical release pattern. EDTA-blood from most of these patients was collected as part of the hospital’s routine at admission or at coronary angiography and during the subsequent 48 h, employing the same time points as described for the SCA-patients. After collection of blood and baseline characteristics, the identity of these patients was destroyed and all samples treated anonymously. The selection of the MI-patients was not sufficiently random for them to serve as controls for the assessment of risk.
To rule out a biological variation of the FA profile, we also analyzed a subsequent set of three blood samples in 5 healthy subjects, 3 men and 2 women, with an age of 34–72 years. The first and last (after 24 h) blood samples were drawn after an overnight fast, the second sample was in the fed state 8–12 h after the first one.
For risk evaluation, we compared the admission omega-3 index in the SCA-patients with first-time MI with our previous control-population of 185 first-time MI patients from the Risk factors in Acute Coronary Syndrome (RACS, ClinicalTrials.gov identifier: NCT00521976) study [5]). In that study, patients with chest pain or otherwise suspected acute coronary syndrome (ACS) were included at the same hospital from November 2002 until September 2003. Blood samples for the analysis of the omega-3 index were harvested immediately after admission, without subsequent sampling during hospitalization. Controls were selected based on the absence of VF or sustained ventricular tachycardia (VT) for at least 30 days of follow-up.
The present study was approved by the Regional Board of Research Ethics and the Norwegian Health Authorities and conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Laboratory Methods
Storage of blood samples prior to preparation was allowed for 24 h at room-temperature and 48 h in a refrigerator. RBC for analysis of FA were prepared from EDTA-blood after centrifugation at 2,500g for 10 min in room temperature. Plasma was extracted and the buffy coat discarded, after which sedimented RBC were washed twice with phosphate buffered saline (PBS), followed each time by centrifugation at 2,500g for 3 min. All samples were stored at –70 °C until extraction of FA could be performed. At this temperature, the composition of RBC FA has been demonstrated to remain stable for at least 4 years [12].
For the FA analysis a 50-μL sample of thawed packed RBC was placed on a filter paper disc (Whatman grade 1, 3.0 cm diameter) that had been pre-treated with butylated hydroxytoluene (50 mg/L) according to Marangoni et al. [13]. Dried blood spots were shipped to the US and the FA composition analyzed in the laboratory of W. S. Harris by flame ionization gas chromatography (GC9A, Shimadazu Corporation, Columbia, MD, USA), as previously described [5]. FA in dried RBC-samples remain stable during storage and shipment in cooled conditions for at least 1 week, as previously described [14]. We have also shown that dried RBC-samples can be stored at −70 °C for 1.5 years with no reduction in the omega-3 index (n = 22) (unpublished data). The coefficient of variation (CV) for the omega-3 index was 6%. Laboratory personnel performing the analyses were blinded with respect to clinical events.
Statistical Analyses
Characteristics of cases and controls are given as means ± standard deviations (SD) for normally distributed variables and as medians with interquartile range (25th–75th percentile) when the assumption of normality was violated. Differences between groups at baseline were tested by the Mann–Whitney U Test and the Kruskal–Wallis Test for non-normally distributed variables with the independent samples t test and the one-way between group analysis of variance (ANOVA) as the parametric alternative. Categorical variables were evaluated using the Chi-square test, or in the case of few expected observations, the p value was derived from Fisher’s exact probability test. The sample sizes of the subgroups of SCA-patients were, however, too small to test for differences in inter-group frequencies.
For the evaluation of FA stability following an event of SCA and/or AMI we used one-way repeated measures ANOVA to compare the omega-3 index and individual FA at admission, after 8–12 h and after 24–48 h in the four different groups of patients; (1) SCA without an AMI (n = 6), (2) SCA with an AMI (both first and recurrent) (n = 18), (3) AMI without SCA (n = 15) and (4) healthy subjects (n = 5). The analysis was performed for each group with time as the only within-subject factor at three levels. The Mauchly’s test of sphericity was used to check that the correlations and variances of the observations remained constant at all time points. The within-subject CV was calculated from the mean and SD of the three subsequent omega-3 index measurements, according to the method described by Harris and Thomas [15].
To evaluate the association between the omega-3 index and risk of VF we performed logistic regression analyses adjusting for age and sex. In addition, high-sensitivity C-reactive protein (hsCRP), EF and previous angina pectoris were included as potential confounders in a backward elimination procedure, and stayed in the final model if they were significant in the first step. Based on the existing knowledge of STEMI and anterior location as potential risk factors for VF during the course of an AMI [16, 17], we performed separate analyses for STEMI-patients only and for patients with anterior location of their infarction. We chose not to include maximal TnT as a measure of infarct size due to the possibility of this parameter being differentially affected by the mechanical manipulation of the heart, by coronary hypoperfusion during resuscitation [18, 19] as well as by later percutaneous coronary intervention (PCI) therapy [20]. The Odds Ratio (OR) for VF is presented with 95% confidence interval (CI). The statistical analyses were performed with the statistical package SPSS version 17.0, with p values derived from the logistic regressions using the Wald chi-square test. All tests were 2-sided with a significance level of 5%.
Results
Characteristics of SCA-Patients
Out of the 25 patients admitted after an event of OHCA, 19 experienced their VF during the initial course of an AMI. The remaining 6 patients had no evidence of a present MI and their arrhythmia was classified as primary VF with the need for an implantable cardioverter defibrillator (ICD) prior to discharge. Patient characteristics for the different SCA-groups are given in Table 1.
Table 1.
Characteristics of patients suffering SCA
| First myocardial infarction (n = 14) | Recurrent myocardial infarction (n = 5) | Primary arrhythmia (n = 6) | p value | |
|---|---|---|---|---|
| Men | 12 (86%) | 5 (100%) | 5 (83%) | nd |
| Women | 2 (14%) | 0 | 1 (17%) | |
| Age (median, years) | 52.5 (46.8–59.5) | 68.0 (58.0–77.5) | 73.5 (62.5–81.5) | 0.006 |
| BMI (mean ± SD, kg/m2) | 26.6 ± 3.9b | 26.4 ± 3.3c | 26.9 ± 4.3 | 0.017 |
| Symptoms prior to SCA | ||||
| Chest pain | 10 (71%) | 3 (60%) | 0 | nd |
| Dyspnea | 0 | 0 | 1 (17%) | |
| Asymptomatic | 0 | 2 (40%) | 4 (67%) | |
| Unknown | 4 (29%) | 0 | 1 (17%) | |
| ECG findings | ||||
| STEMI | 11 (79%) | 3 (60%) | 0 | nd |
| NSTEMI | 3 (21%) | 2 (40%) | 0 | |
| Infarct location | ||||
| Anterior wall | 10 (71%) | 3 (60%) | 0 | nd |
| Inferior wall | 3 (21%) | 1 (20%) | 0 | |
| Lateral wall | 1 (7%) | 1 (20%) | 0 | |
| TnT max (median, μg/L) | 4.55 (1.21–6.99) | 4.53 (2.61–11.99) | 0.16 (0.04–0.74) | 0.005 |
| Ejection fraction (median, %) | 58 (36–60)d | 35 (30–50) | 40 (20–43)e | ns |
| Coronary angiography | ||||
| Normal | 0 | 0 | 2 (33%) | nd |
| 1-vessel disease | 9 (64%) | 1 (20%) | 1 (17%) | |
| 2-vessel disease | 3 (21%) | 2 (40%) | 0 | |
| 3-vessel disease | 2 (14%) | 2 (40%) | 3 (50%) | |
| Coronary intervention | ||||
| LAD | 9 (64%) | 3 (60%) | 1 (17%) | nd |
| RCA | 2 (14%) | 1 (20%) | 1 (17%) | |
| CX | 3 (21%) | 1 (20%) | 0 | |
| Hypothermic treatment | 10 (71%) | 4 (80%) | 5 (83%) | nd |
| Implantation of ICD | 0 | 0 | 6 (100%) | nd |
| Death prior to discharge | 2 (14%) | 2 (40%) | 0 | nd |
| Previous history | ||||
| Angina pectoris | 3 (21%) | 0 | 1 (17%) | nd |
| Myocardial infarction | 0 | 5 (100%) | 5 (83%) | |
| Heart failure | 0 | 1 (20%) | 5 (83%)a | |
| Previous CABG | 0 | 1 (20%) | 2 (33%) | |
| Previous PTCA | 0 | 3 (60%) | 2 (33%) | |
| Hypertension | 4 (29%) | 2 (40%) | 4 (67%) | |
| Aortic stenosis | 0 | 0 | 0 | |
| Mitral insufficiency | 0 | 2 (40%) | 4 (67%) | |
| Diabetes mellitus | 0 | 0 | 0 | |
| Hypercholesterolemia | 10 (71%) | 3 (60%) | 2 (33%) | |
| Current smoking | 4 (29%) | 1 (20%) | 1 (17%) | |
| Ex-smoker | 6 (50%)d | 3 (75%)c | 4 (67%) | |
| Family history | 7 (70%)f | 2 (50%)c | 4 (67%) | |
| Medication prior to admission | ||||
| Beta-blocker | 1 (7%) | 1 (25%)c | 3 (50%) | nd |
| Ca-blocker | 1 (7%) | 1 (25%)c | 2 (33%) | |
| ACE/AT II-inhibitor | 1 (7%) | 2 (50%)c | 6 (100%) | |
| Diuretics | 1 (7%) | 0c | 6 (100%) | |
| ASA | 1 (7%) | 4 (100%)c | 0 | |
| Warfarin | 0 | 0c | 6 (100%) | |
| Statin | 2 (14%) | 3 (75%)c | 6 (100%) | |
| Antiarrhythmics | 0 | 0c | 0 | |
| Baseline blood samples (median) | ||||
| Hemoglobin (g/L) | 14.7 (13.5–15.8) | 14.0 (13.0–17.0) | 13.3 (11.1 15.1) | ns |
| Sodium (mmol/L) | 140 (136–142) | 143 (142–144) | 138 (136–144) | 0.045 |
| Calcium (mmol/L) | 4.0 (3.9–4.6) | 4.0 (3.8–4.6) | 3.5 (3.1–4.6) | ns |
| Creatinine (μmol/L) | 92 (80–115) | 110 (89–118) | 107 (81–154) | ns |
| Total-cholesterol (mmol/L) | 6.4 (4.8–6.9) | 3.8 (3.6–4.1) | 3.7 (2.9–5.1) | 0.014 |
| HDL-cholesterol (mmol/L) | 1.4 (1.0–1.7) | 1.3 (0.9–1.8) | 1.4 (0.7–1.8) | ns |
| Triglyceride (mmol/L) | 2.2 (1.0–3.2)g | 1.5 (0.6–1.7)h | 1.1 (1.0–1.4)h | ns |
| Glucose (mmol/L) | 15.1 (8.7–22.5)b | 12.0 (9.4 -18.9) | 13.8 (8.1 14.9)e | ns |
| hsCRP (mg/L) | 1.7 (1.1–3.1) | 1.8 (1.1–2.9) | 3.2 (2.3–28.3) | ns |
| Number of fish meals | ||||
| <1 × per month | 1 (11%)b | 0 | 0 | nd |
| 2–3 × per month | 4 (44%)b | 2 (100%)i | 3 (50%) | |
| 1 × per week | 2 (22%)b | 0 | 1 (17%) | |
| 2–3 × per week | 1 (11%)b | 0 | 2 (33%) | |
| >3 × per week | 1 (11%)b | 0 | 0 | |
| Omega-3 supplementation | ||||
| Cod-liver oil | 0b | 0h | 2 (33%) | nd |
| Fish-oil capsules | 1 (11%)b | 1 (33%)h | 1 (17%) | |
| Omega-3 index at admission (mean ± SD, %) | 4.59 ± 1.58 | 5.26 ± 1.59 | 7.15 ± 1.77 | 0.014 |
Categorical data are given as n (%). Median values of continuous data given with 25th and 75th percentiles in parentheses (interquartile range)
Nd no data, p values not available, too small groups to test for differences for categorical variables. ns not significant
ACE angiotensin converting enzyme, ASA acetylsalicylic acid, AT II angiotensin II receptor, BMI body mass index, CABG coronary artery bypass grafting, HDL high density lipoprotein, hsCRP high-sensitivity C-reactive protein, ICD implantable cardioverter defibrillator, LAD left anterior descending artery, RCA right coronary artery, CX circumflex, NSTEMI non-ST-elevation myocardial, PTCA percutaneous transluminal coronary angioplasty, SCA sudden cardiac arrest, SD standard deviation, STEMI ST-elevation myocardial infarction, TnT troponin-T
aFor one of the patients the diagnosis of dilated cardiomyopathy was first established after admission and this information is therefore not included in the baseline characteristics; b n = 9; c n = 4; d n = 12; e n = 5; f n = 10; g n = 13; h n = 3; i n = 2
Fourteen out of the 19 AMI-patients, 12 men and 2 women, experienced their first MI. These patients were relatively young (median age 52.5 years, range 41–78 years) and had a predominance of STEMI located to the anterior wall of the heart and mainly one-vessel disease evaluated by coronary angiography (Table 1). Retrospectively, 3 patients had evidence of pre-infarction angina, but none of them had received any treatment for this condition.
Characteristics of Control Subjects
The characteristics of the 185 control subjects (134 men and 51 women) are presented in Table 2. As compared to the group of SCA-patients with first-time MI they were significantly older, had a higher frequency of NSTEMI at admission, and at baseline hsCRP was significantly higher and s-glucose significantly lower. For the remaining baseline characteristics, including infarct location, previous history, presence of risk factors, use of medication, EF and degree of coronary artery disease (CAD), there were no statistically significant differences among the two groups.
Table 2.
Characteristics of cases and controls
| Case-patients (n = 14) | Control patients (n = 185) | p value | |
|---|---|---|---|
| Men | 12 (86%) | 134 (72%) | ns |
| Women | 2 (14%) | 51 (28%) | |
| Age (median, years) | 52.5 (46.8–59.5) | 64 (53.5–73.0) | 0.003 |
| BMI (mean ± SD, kg/m2) | 26.6 ± 3.9a | 25.9 ± 4.5 | ns |
| ECG findings at admission | |||
| STEMI | 11 (79%) | 85 (46%)b | 0.026 |
| NSTEMI | 3 (21%) | 98 (54%)b | |
| Infarct location | |||
| Anterior wall | 10 (71%) | 85 (45%) | ns |
| Inferior wall | 3 (21%) | 64 (35%) | |
| Lateral wall | 1 (7%) | 0 | |
| Unidentifiable | 0 | 36 (20%) | |
| Ejection fraction (median, %) | 58 (36–60)c | 55 (50–60)d | ns |
| Coronary angiography | |||
| 1-vessel disease | 9 (64%) | 55 (39%)e | ns |
| 2-vessel disease | 3 (21%) | 39 (28%)e | |
| 3-vessel disease | 2 (14%) | 47 (33%)e | |
| Death prior to discharge | 2 (14%) | 1 (0.5%) | 0.013 |
| Previous history | |||
| Angina pectoris | 3 (21%) | 56 (30%) | ns |
| Heart failure | 0 | 12 (7%) | ns |
| Previous CABG | 0 | 6 (3%) | ns |
| Previous PTCA | 0 | 8 (4%) | ns |
| Hypertension | 4 (29%) | 61 (33%) | ns |
| Diabetes mellitus | 0 | 21 (11%) | ns |
| Hypercholesterolemia | 10 (71%) | 92 (50%) | ns |
| Current smoking | 4 (29%) | 84 (45%) | ns |
| Ex-smoker | 6 (50%)c | 55 (40%)f | ns |
| Family history | 7 (70%)g | 121 (69%)h | ns |
| Medication prior to admission | |||
| Beta-blocker | 1 (7%) | 31 (17%) | ns |
| Ca-blocker | 1 (7%) | 22 (12%)i | ns |
| ACE/AT II-inhibitor | 1 (7%) | 32 (17%) | ns |
| Diuretics | 1 (7%) | 17 (9%) | ns |
| ASA | 1 (7%) | 32 (17%) | ns |
| Warfarin | 0 | 8 (4%) | ns |
| Statin | 2 (14%) | 36 (20%) | ns |
| Baseline blood samples (median) | |||
| Hemoglobin (g/dL) | 14.7 (13.5–15.8) | 14.1 (13.1–15.0) | ns |
| Creatinine (μmol/L) | 92 (80–115) | 89 (75–98) | ns |
| Total-cholesterol (mmol/L) | 6.4 (4.8–6.9) | 5.6 (5.0–6.2) | ns |
| HDL-cholesterol (mmol/L) | 1.4 (1.0–1.7) | 1.2 (1.0–1.5) | ns |
| Triglycerides (mmol/L) | 2.2 (1.0–3.2)j | 1.4 (1.0–2.0) | ns |
| Glucose (mmol/L) | 15.1 (8.7–22.5)a | 6.5 (5.6–8.6)b | <0.001 |
| hsCRP (mg/L) | 1.7 (1.1–3.1) | 4.4 (2.0–13.5)b | 0.002 |
| Omega-3 index (mean ± SD, %) | 4.59 ± 1.58 | 6.48 ± 2.20 | 0.002 |
Categorical data given as n (%). Median values of continuous data given with 25th and 75th percentiles in parentheses (interquartile range)
ACE angiotensin converting enzyme, ASA acetylsalicylic acid, AT II angiotensin II receptor, BMI body mass index, CABG coronary artery bypass grafting, HDL high density lipoprotein, hsCRP high-sensitivity C-reactive protein, NSTEMI non-ST-elevation myocardial, PTCA percutaneous transluminal coronary angioplasty, SD standard deviation, STEMI ST-elevation myocardial infarction
a n = 9; b n = 183; c n = 12; d n = 178; e n = 141; f n = 139; g n = 10; h n = 175; i n = 184; j n = 13
Serial Measurements of Individual FA and the Omega-3 Index
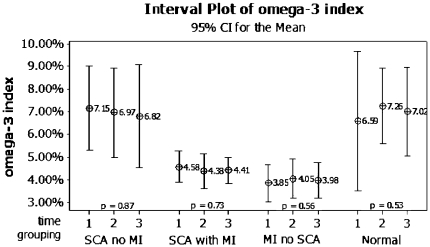
A plot of the means of the omega-3 index with 95% CI in SCA patients with and without an AMI, in AMI-patients without SCA and in healthy subjects is presented in Fig. 1. Among patients with SCA, mean time from event to first sample was 1.3 ± 0.6 h. The time from admission until first, second and third sample was 0.5 ± 0.4, 11.1 ± 2.3 and 39.3 ± 6.0 h, respectively. After removing from the analysis one patient with chest pain for at least 74 h before the occurrence of cardiac arrest, patients with SCA during the course of their AMI had a mean duration of symptoms of 3.3 ± 5.7 h (range 0.6–21.0 h) at the time of the first sample. For AMI-patients without SCA, time from symptom onset until first sample was 3.4 ± 2.5 h (range 0.8–9.7 h) and from admission to subsequent sampling 0.3 ± 0.4, 10.2 ± 3.1 and 35.6 ± 6.8 h, respectively. Healthy subjects had samples taken at time 0, after 8.1 ± 0.1 and 23.4 ± 0.4 h. The repeated measures ANOVA (sphericity assumed) showed no statistically significant time-dependent effect on the omega-3 index in any of the groups. The within-subject CV differed from 16.7% in SCA-patients with an AMI to 10.4% in SCA-patients with no AMI. The corresponding values for AMI-patients without SCA and for healthy subjects were 11.7 and 12.9%, respectively.
Fig. 1.
Mean of the omega-3 index with 95% confidence interval (CI) at admission (time 1), after 8–12 h (time 2) and 24–48 h (time 3). Values given for sudden cardiac arrest (SCA) patients with and without an acute myocardial infarction (MI), MI-patients without SCA and healthy subjects (normal). p values for variance of the omega-3 index in each group derived from the ANOVA analysis with time as the only within-subject factor at three levels
Similar comparisons were performed for the individual FA. As shown in Table 3 the FA profile remained stable irrespective of sampling time in each subject group. Accordingly, the ratio between the omega-6 and omega-3 FA was also found to be stable, including the arachidonic acid (AA) to EPA ratio.
Table 3.
Fatty acid profiles from red blood cells
| Patient group | At admission | 8–12 h | 24–48 h | p |
|---|---|---|---|---|
| SCA without MI (n = 6) | ||||
| Myristic acid (C14:0) | 3.56 (2.12–5.01) | 5.00 (2.21–7.80) | 5.29 (3.39–7.20) | 0.276 |
| Palmitic acid (C16:0) | 23.10 (21.55–24.66) | 22.59 (20.48–24.69) | 22.98 (21.44–24.52) | 0.843 |
| Stearic acid (C18:0) | 21.01 (18.36–23.66) | 20.14 (17.42–22.87) | 20.43 (18.43–22.44) | 0.660 |
| Oleic acid (C18:1n-9) | 15.68 (13.93–17.43) | 15.69 (14.40–16.98) | 15.11 (14.08–16.14) | 0.544 |
| Linoleic acid (C18:2n-6) | 8.32 (6.34–10.31) | 8.19 (6.38–9.99) | 8.04 (6.44–9.63) | 0.427 |
| α-Linolenic acid (C18:3n-3) | 0.10 (0.03–0.17) | 0.08 (0.04–0.12) | 0.08 (0.03–0.12) | 0.560 |
| AA (C20:4n-6) | 12.03 (9.96–14.11) | 12.07 (9.72–14.43) | 12.14 (10.16–14.12) | 0.980 |
| EPA (C20:5n-3) | 1.42 (0.78–2.07) | 1.36 (0.64–2.08) | 1.24 (0.47–2.01) | 0.429 |
| DPA (C22:5n-3) | 2.35 (1.83–2.86) | 2.34 (1.80–2.88) | 2.26 (1.92–2.60) | 0.876 |
| DHA (C22:6n-3) | 5.73 (4.41–7.05) | 5.61 (4.25–6.97) | 5.58 (3.93–7.23) | 0.949 |
| Total n-3 FA | 7.15 (5.30–9.01) | 6.97 (4.98–8.96) | 6.82 (4.54–9.09) | 0.866 |
| n-6 FA/n-3 FA | 2.62 (1.93–3.31) | 2.68 (1.99–3.37) | 2.82 (1.98–3.66) | 0.669 |
| SCA with MI (n = 18) | ||||
| Myristic acid (C14:0) | 4.27 (3.21–5.32) | 4.90 (3.88–5.92) | 4.98 (3.27–6.70) | 0.569 |
| Palmitic acid (C16:0) | 24.28 (22.95–25.60) | 24.44 (23.09–25.78) | 25.01 (24.07–25.94) | 0.356 |
| Stearic acid (C18:0) | 20.47 (19.32–21.61) | 20.71 (19.79–21.64) | 20.93 (20.05–21.82) | 0.552 |
| Oleic acid (C18:1n-9) | 15.34 (14.43–16.25) | 15.27 (14.59–15.96) | 15.22 (14.38–16.06) | 0.933 |
| Linoleic acid (C18:2n-6) | 9.55 (9.02–10.09) | 9.37 (8.81–9.92) | 9.27 (8.61–9.93) | 0.144 |
| α-Linolenic acid (C18:3n-3) | 0.10 (0.08–0.12) | 0.10 (0.07–0.13) | 0.09 (0.06–0.11) | 0.470 |
| AA (C20:4n-6) | 11.12 (9.87–12.37) | 10.56 (9.49–11.63) | 10.24 (9.49–10.99) | 0.101 |
| EPA (C20:5n-3) | 0.79 (0.60–0.97) | 0.70 (0.47–0.92) | 0.74 (0.54–0.95) | 0.283 |
| DPA (C22:5n-3) | 1.91 (1.67–2.15) | 1.87 (1.61–2.12) | 1.76 (1.51–2.01) | 0.218 |
| DHA (C22:6n-3) | 3.91 (3.34–4.48) | 3.80 (3.19–4.42) | 3.68 (3.21–4.15) | 0.591 |
| Total n-3 FA | 4.70 (4.03–5.37) | 4.50 (3.72–5.28) | 4.43 (3.82–5.03) | 0.576 |
| n-6 FA/n-3 FA | 3.95 (3.48–4.42) | 4.00 (3.45-4.56) | 4.04 (3.46–4.62) | 0.891 |
| MI without SCA (n = 15) | ||||
| Myristic acid (C14:0) | 5.91 (4.59–7.23) | 6.33 (4.92–7.74) | 7.13 (5.61–8.66) | 0.380 |
| Palmitic acid (C16:0) | 25.79 (24.90–26.67) | 25.40 (24.59–26.22) | 24.99 (24.23–25.75) | 0.135 |
| Stearic acid (C18:0) | 21.79 (21.03–22.54) | 21.42 (20.76–22.09) | 21.18 (20.41–21.95) | 0.214 |
| Oleic acid (C18:1n-9) | 14.82 (14.11–15.54) | 14.61 (13.98–15.23) | 14.43 (13.81–15.06) | 0.499 |
| Linoleic acid (C18:2n-6) | 9.22 (8.32–10.11) | 9.20 (8.32–10.08) | 8.82 (8.00–9.64) | 0.053 |
| α-Linolenic acid (C18:3n-3) | 0.12 (0.09–0.15) | 0.15 (0.10–0.20) | 0.16 (0.10–0.23) | 0.335 |
| AA (C20:4n-6) | 8.23 (7.40–9.06) | 8.46 (7.65–9.27) | 8.52 (7.54–9.49) | 0.616 |
| EPA (C20:5n-3) | 0.68 (0.44–0.92) | 0.69 (0.43–0.95) | 0.63 (0.43–0.84) | 0.361 |
| DPA (C22:5n-3) | 1.65 (1.47–1.84) | 1.66 (1.51–1.80) | 1.69 (1.57–1.82) | 0.804 |
| DHA (C22:6n-3) | 3.18 (2.55–3.80) | 3.36 (2.69–4.03) | 3.34 (2.71–3.98) | 0.421 |
| Total n-3 FA | 3.85 (3.03–4.67) | 4.05 (3.18–4.92) | 3.98 (3.18–4.77) | 0.562 |
| n-6 FA/n-3 FA | 4.09 (3.35–4.83) | 4.03 (3.21–4.85) | 3.88 (3.21–4.56) | 0.239 |
| Healthy subjects (n = 5) | ||||
| Myristic acid (C14:0) | 7.00 (2.87–11.13) | 5.18 (1.64–8.71) | 4.45 (2.88–6.02) | 0.207 |
| Palmitic acid (C16:0) | 23.90 (21.87–25.93) | 23.47 (22.48–24.46) | 23.73 (22.25–25.20) | 0.904 |
| Stearic acid (C18:0) | 21.09 (18.69–23.49) | 20.88 (20.03–21.72) | 21.06 (20.06–22.06) | 0.956 |
| Oleic acid (C18:1n-9) | 13.86 (12.63–15.08) | 15.34 (13.08–15.59) | 14.70 (13.72–15.69) | 0.232 |
| Linoleic acid (C18:2n-6) | 9.74 (8.58–10.91) | 10.27 (8.74–11.81) | 10.14 (9.08–11.21) | 0.312 |
| α-Linolenic acid (C18:3n-3) | 0.17 (0.01–0.33) | 0.12 (0.08–0.15) | 0.13 (0.09–0.17) | 0.613 |
| AA (C20:4n-6) | 9.04 (7.23–10.85) | 9.99 (8.12–11.86) | 9.98 (7.85–12.11) | 0.409 |
| EPA (C20:5n-3) | 1.53 (0.53–2.53) | 1.73 (0.87–2.59) | 1.69 (0.79–2.59) | 0.393 |
| DPA (C22:5n-3) | 2.26 (1.55–2.97) | 2.53 (2.20–2.85) | 2.48 (1.94–3.03) | 0.340 |
| DHA (C22:6n-3) | 5.06 (2.85–7.27) | 5.53 (4.47–6.59) | 5.33 (4.14–6.51) | 0.599 |
| Total n-3 FA | 6.59 (3.50–9.69) | 7.26 (5.56–8.96) | 7.02 (5.06–8.97) | 0.532 |
| n-6 FA/n-3 FA | 2.67 (1.24–4.10) | 2.40 (1.61–3.19) | 2.47 (1.74–3.20) | 0.505 |
Means of red blood cell membrane fatty acids (FA) (given as percent of total FA) with 95% confidence interval (CI) at admission, after 8–12 h and 24–48 h. Values given for sudden cardiac arrest (SCA) patients with and without an acute myocardial infarction (MI), MI-patients without SCA and healthy subjects. p values for variance of the FA in each group derived from the ANOVA analysis with time as the only within-subject factor at three levels
AA arachidonic acid, EPA eicosapentaenoic acid, DPA docosapentaenoic acid, DHA docosahexaenoic acid
Omega-3 Index and Risk of VF
The mean omega-3 index among SCA-patients with first-time AMI was 4.59% as compared to 6.48% in controls. In the main logistic regression analysis age, sex, hsCRP and EF were included as covariates, 12 cases and 177 controls were enrolled based on available measurements. The omega-3 index turned out to be a highly significant predictor of VF during the course of a first-time AMI with an OR of 0.42 (95% CI; 0.24–0.75, p = 0.003). Of the other covariates included in the analysis, only EF (OR 0.94, 95% CI: 0.89–0.99) and hsCRP (OR 0.61, 95% CI: 0.39–0.95) contributed to the risk of VF.
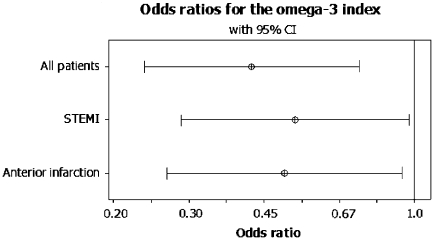
When restricting the analysis to patients admitted with STEMI and to those with anterior location of their infarction, the omega-3 index still remained an independent predictor of VF with an OR of 0.53 (95% CI: 0.29–0.96), p = 0.037, in STEMI-patients (n = 91) and 0.50 (95% CI: 0.27–0.93), p = 0.028, among those with anterior infarctions (n = 92) (Fig. 2).
Fig. 2.
Result of the multivariate logistic regression analysis in the total population (all patients) and in the subgroups of ST-elevation myocardial infarctions (STEMI) and anterior infarct location adjusting for age, sex, high sensitivity C-reactive protein and ejection fraction. Odds ratios (ORs) given with 95% confidence interval (CI)
Discussion
We have demonstrated that the RBC FA, including the omega-3 index, remained stable after SCA and during the post-MI period. In healthy subjects, we have also ruled out any significant biological variability, largely confirming previous observations on reproducibility over time [15].
There has been diverging results in previous studies regarding the effect of an AMI on FA composition of phospholipids [6, 7, 10]. Our findings are consistent with results from an experimental study in rats by Shearer et al. [7]. Kark et al. [10] sampled 20 MI-patients up to 7 days after the event. Decreasing values of EPA were evident after 42 h, whereas DHA started to increase 7 h after admission. In that study there were no statistical analyses for composite indexes, but we would expect that increasing values of DHA (the major contributor to the sum of EPA + DHA) would reflect an increasing omega-3 index after the initial 7 h of admission, a finding not confirmed in our study. The observed decrease in EPA after 42 h could, however, have been missed by our investigation, as our mean time to last sample was 35.6 h following admission. The time between onset of symptoms and first blood sample was similar in the two studies. In contrast to the study of Kark et al., our STEMI patients were treated invasively, but there is no indication that this difference in the treatment strategy may influence the FA composition in phospholipids.
Unique to our study is a separate evaluation of the effect of SCA on FA composition in humans. The so-far existing research has been performed on primates [3] and rats [11]. In the study by Siscovick et al. [3] RBC membrane levels of EPA + DHA (corresponding to our omega-3 index) were altered only slightly (0.33%) from the pre-mortem to the post-mortem state, suggesting a non-significant effect on these FA by the cardiac arrest itself. Jurand et al. [11] injected rats with noradrenaline, but could not demonstrate any variation in serum FA. Our study confirms the findings in these animal studies, and is strengthened by similar results obtained in SCA-patients both with and without an AMI. Although the latter group only consisted of 6 patients, these subjects represent a population in which we could evaluate the effect of the cardiac arrest itself without any interference from an AMI. Also, there was no evidence of a decreased omega-3 index at admission in patients given adrenaline prior to hospitalization (5.60%, n = 14) as compared to those not receiving this medication (5.01%, n = 11).
Our findings suggest that any sample obtained within the first 48 h after an event of cardiac arrest, is representative of the total FA status, including the omega-3 index and AA/EPA ratio, of the individual prior to the event. Proof of that assumption would, however, require a measurement just prior to the event, but that would hardly be achievable in a clinical setting. The other option would be blood samples obtained in advance in large prospective cohort studies, but in those settings dietary changes may have taken place between the time of sampling and the event. As a second-best choice, our study was designed to harvest the first sample as close to the event as possible, based on the assumption that it may take some time for the cellular changes to appear. The first sample taken at admission was harvested at a mean time of only 1.3 h after the SCA event. Furthermore, the mean time from symptom onset was 3.3 h in AMI-patients with SCA as compared to 3.4 h in AMI-patients without a cardiac arrest. A possible effect of the cardiac arrest on the FA profile, has been one of the main objections to case–control studies demonstrating an increased risk of SCD/SCA with low levels of n-3 FA [3, 5]. However, these objections are not supported by our present results.
We had previously demonstrated that a 1% increase of the omega-3 index reflects a 48% reduction in risk of VF during the ischemic phase of an AMI [5]. In the present case–control study we have recruited a similar group of SCA-patients with first-time MI, with the use of the same control subjects in both study settings. As in our previous investigation, we found a large difference in the omega-3 index values between cases and controls at admission. Furthermore, the logistic regression analysis confirms an inverse relation between the omega-3 index and risk of VF; a 1% increase in this index reflecting a 58% reduced risk of VF (95% CI: 25–76%). Even though EF and hsCRP contributed to the risk of VF, the omega-3 index was the strongest predictor. The omega-3 index also remained an independent predictor of risk, but was slightly attenuated, in the subgroup analyses of STEMI-patients and anterior infarctions. Contrary to other investigations, both of our studies have documented ventricular fibrillation at the time of resuscitation, supporting the proposed antiarrhythmic mechanism of n-3 FA [21–23], especially in ischemia-induced VF. Theoretically, the antiarrhythmic mechanism of n-3 FA is based on an increase of the arrhythmic threshold in the ischemic zone of the myocardium, due to an indirect effect of the FA on sodium and calcium ion-channels in the myocardial membrane [22, 24].
The group of SCA patients without the presence of an AMI is highly different from the first-time MI population. Their high omega-3 index of 7.15% does not contradict the above finding, as their VF is mainly considered to be a result of myocardial scarring and heart failure. For arrhythmias generated by such mechanisms, n-3 FA might not be protective, as suggested by the discrepant results of n-3 FA in ICD-patients [25, 26]. Also, chronically ill patients may have changed their lifestyle according to an assumed protection by n-3 FA, ingesting an increased amount of fish and omega-3 supplements.
Limitations
Our evaluation of a time-dependent change in the FA profile following an event of SCA and/or AMI is limited by the relative low number of patients in each category, reducing our power to confidently exclude a small change. Statistically, power calculations are difficult to perform with repeated sampling, but the use of serial samples without any detectable change in healthy individuals and in different patient groups strengthen our conclusion. As our patient populations had highly different omega-3 index values, the present study furthermore supports the stability of this analysis in patients with both high and low n-3 FA levels.
The main objection to the present case-control study might be the different time-periods for inclusion of cases and controls. Whereas controls were participants in the RACS-study [5], sampled between November 2002 and September 2003, our new cases had their blood samples taken between February 2007 and June 2009. Both studies were, however, performed at Stavanger University Hospital, the only hospital available for the CAD and SCA populations in this region of Norway. The source-population should therefore be similar for both studies and there is no reason to believe that their way of living, including diet, would have changed significantly during a 5 year period. The results of our study are, however, only valid for the range of omega-3 index values included in the analyses.
Furthermore, we do not know for certain whether the omega-3 index in admitted SCA-patients differ from those of OHCA-patients where ROSC was not obtained. Also, the urgent situation during resuscitation and admission sometimes resulted in missing samples for admitted SCA-patients.
Conclusion
Our study supports an association between low levels of n-3 FA and the risk of VF during the ischemic phase of an AMI in hospitalized patients. The results are strengthened by our demonstration of the stability of RBC FA within the first 48 h after an episode of SCA or AMI, suggesting that the validity of risk assessment is maintained within this time frame.
Acknowledgments
Study grants from the Regional Health Authorities in Western Norway, The Research Foundation at Stavanger University Hospital and The Laerdal Foundation for Acute Medicine are gratefully acknowledged. The FA analyses were performed by William S. Harris who is a consultant to companies with interests in omega-3 FA, including GlaxoSmithKline, Reliant Pharmaceuticals and the Monsanto Company. No pharmaceutical company supported this study financially. There are otherwise no financial or other relationships associated with the manuscript that might lead to a conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AA
Arachidonic acid
- ACS
Acute coronary syndrome
- AMI
Acute myocardial infarction
- CAD
Coronary artery disease
- DHA
Docosahexaenoic acid
- ECG
Electrocardiography
- EF
Ejection fraction
- EPA
Eicosapentaenoic acid
- FA
Fatty acid(s)
- hsCRP
High-sensitivity C-reactive protein
- ICD
Implantable cardioverter defibrillator
- MI
Myocardial infarction
- NSTEMI
Non-ST-elevation myocardial infarction
- OHCA
Out-of-hospital cardiac arrest
- PCI
Percutaneous coronary intervention
- RBC
Red blood cell(s)
- ROSC
Return of spontaneous circulation
- SCA
Sudden cardiac arrest
- SCD
Sudden cardiac death
- STEMI
ST-elevation myocardial infarction
- TnT
Troponin-T
- VF
Ventricular fibrillation
- VT
Ventricular tachycardia
References
- 1.Investigators GISSI-Prevenzione Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- 2.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 3.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.274.17.1363. [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 5.Aarsetøy H, Pönitz V, Nilsen OB, Grundt H, Harris WS, Nilsen DWT. Low levels of cellular omega-3 increase the risk of ventricular fibrillation during the acute ischaemic phase of a myocardial infarction. Resuscitation. 2008;78:258–264. doi: 10.1016/j.resuscitation.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Kirkeby K. Disturbances in serum lipids and in their fatty acid composition following acute myocardial infarction. Acta med scand. 1972;192:523–528. doi: 10.1111/j.0954-6820.1972.tb04860.x. [DOI] [PubMed] [Google Scholar]
- 7.Shearer GC, Chen J, Chen Y, Harris WS. Myocardial infarction does not affect fatty acid profiles in rats. Prostaglandins Leukot Essent Fatty Acids. 2009;81:411–416. doi: 10.1016/j.plefa.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 9.Owen AJ, Peter-Przyborowska BA, Hoy AJ, McLennan PL. Dietary fish-oil dose and time-response effects on cardiac phospholipid fatty acid composition. Lipids. 2004;39:955–961. doi: 10.1007/s11745-004-1317-0. [DOI] [PubMed] [Google Scholar]
- 10.Kark JD, Manor O, Goldman S, Berry EM. Stability of red blood cell membrane fatty acid composition after acute myocardial infarction. J Clin Epidemiol. 1995;48:889–895. doi: 10.1016/0895-4356(94)00218-F. [DOI] [PubMed] [Google Scholar]
- 11.Jurand J, Oliver M. Effects of acute myocardial infarction and of noradrenaline infusion on fatty acid composition of serum lipids. Atherosclerosis. 1970;11:157–170. doi: 10.1016/0021-9150(70)90013-4. [DOI] [PubMed] [Google Scholar]
- 12.Hodson L, Skeaff CM, Wallace AJ, Arribas GL. Stability of plasma and erythrocyte fatty acid composition during cold storage. Clin Chem Acta. 2002;321:63–67. doi: 10.1016/S0009-8981(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 13.Marangoni F, Colombo C, Galli C. A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: applicability to nutritional and epidemiological studies. Anal Biochem. 2004;326:267–272. doi: 10.1016/j.ab.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Aarsetoey H, Pönitz V, Grundt H, Staines H, Harris WS, Nilsen DWT. (N-3) fatty acid content of red blood cells does not predict risk of future cardiovascular events following an acute coronary syndrome. J Nutr. 2009;139:507–513. doi: 10.3945/jn.108.096446. [DOI] [PubMed] [Google Scholar]
- 15.Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem. 2010;43:338–340. doi: 10.1016/j.clinbiochem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Gheeraert PJ, De Buyzere ML, Taeymans YM. Risk factors for primary ventricular fibrillation during acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2006;27:2499–2510. doi: 10.1093/eurheartj/ehl218. [DOI] [PubMed] [Google Scholar]
- 17.Gheeraert PJ, Henriques JPS, De Buyzere ML. Out-of-hospital ventricular fibrillation in patients with acute myocardial infarction coronary angiographic determinants. Am Coll Cardiol. 2000;35:144–150. doi: 10.1016/S0735-1097(99)00490-8. [DOI] [PubMed] [Google Scholar]
- 18.Grubb NR, Fox KAA, Cawood CP. Resuscitation from out-of-hospital cardiac arrest: implication for cardiac enzyme estimation. Resuscitation. 1996;33:35–41. doi: 10.1016/S0300-9572(96)00971-9. [DOI] [PubMed] [Google Scholar]
- 19.Müller M, Hirschl MM, Herkner H. Creatine kinase-MB fraction and cardiac troponin-T to diagnose acute myocardial infarction after cardiopulmonary resuscitation. J Am Coll Cardiol. 1996;28:1220–1225. doi: 10.1016/S0735-1097(96)00316-6. [DOI] [PubMed] [Google Scholar]
- 20.Morrow DA, Cannon CP, Jesse RL, Newby K, Ravkilde J, Storrow AB, et al. National academy of clinical biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 21.Matthan NR, Jordan H, Chung M, Lichtenstein AH, Lathrop DA, Lau J. A systematic review and meta-analysis of the impact of omega-3 fatty acids on selected arrhythmia outcomes in animal models. Metabolism. 2005;54:1557–1565. doi: 10.1016/j.metabol.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Leaf A, Xiao YF, Kang JX, Billman GE. Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. Pharmacol Ther. 2003;98:355–377. doi: 10.1016/S0163-7258(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 23.Reiffel JA, McDonald A (2006) Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol 98(Suppl.):50i–60i [DOI] [PubMed]
- 24.Xiao YF, Ke Q, Chen Y, Morgan JP, Leaf A. Inhibitory effect of n-3 fish oil fatty acids on cardiac Na+/Ca2+ exchange currents in HEK293t cells. Biochem Biophys Res Commun. 2004;321:116–123. doi: 10.1016/j.bbrc.2004.06.114. [DOI] [PubMed] [Google Scholar]
- 25.Brouwer IA, Raitt MH, Dullemeijer C, Kraemer DF, Zock PL, Morris C, et al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur Heart J. 2009;30:820–826. doi: 10.1093/eurheartj/ehp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalf RG, Sanders P, James MJ, Cleland LG, Young GD. Effect of dietary n-3 polyunsaturated fatty acids on the inducibility of ventricular tachycardia in patients with ischemic cardiomyopathy. Am J Cardiol. 2008;101:758–761. doi: 10.1016/j.amjcard.2007.11.007. [DOI] [PubMed] [Google Scholar]