Abstract
We investigate olfactory associative learning in larval Drosophila. A reciprocal training design is used, such that one group of animals receives a reward in the presence of odor X but not in the presence of odor Y (Train: X+ // Y), whereas another group is trained reciprocally (Train: X // Y+). After training, differences in odor preference between these reciprocally trained groups in a choice test (Test: X -- Y) reflect associative learning. The current study, after showing which odor pairs can be used for such learning experiments, 1) introduces a one-odor version of such reciprocal paradigm that allows estimating the learnability of single odors. Regarding this reciprocal one-odor paradigm, we show that 2) paired presentations of an odor with a reward increase odor preference above baseline, whereas unpaired presentations of odor and reward decrease odor preference below baseline; this suggests that odors can become predictive either of reward or of reward absence. Furthermore, we show that 3) innate attractiveness and associative learnability can be dissociated. These data deepen our understanding of odor-reward learning in larval Drosophila on the behavioral level, and thus foster its neurogenetic analysis.
Keywords: discrimination, Drosophila, innate behavior, intensity, learning, olfaction, taste
Introduction
The Drosophila larva has recently received renewed (for classical accounts, see Rodrigues and Siddiqi 1978; Rodrigues 1980) attention in neurogenetic analyses of behavior (reviewed in Gerber and Stocker 2007; Vosshall and Stocker 2007; Stocker 2008; Buch and Pankratz 2009; Gerber et al. 2009). Indeed, the larva offers a fortunate combination of genetic tractability, simplicity in terms of cell number, and low-complexity but arguably still interesting behavioral faculties: Larvae can learn to associate either visual stimuli (Gerber et al. 2004) or odors (Scherer et al. 2003) with food reward, leading to conditioned approach toward the respectively rewarded cue. Also, larvae can be trained to associate odors with either gustatory (Gerber and Hendel 2006; Niewalda et al. 2008) or electric shock (Aceves-Pina and Quinn 1979; Khurana et al. 2009; Pauls et al. 2010) punishment. Associative processing in these paradigms, in particular odor-reward training, has subsequently been described in some parametric detail regarding, for example, the number of training trials, the temporal stability of the memory trace, larval gender and age (Neuser et al. 2005), the effectivity of different kinds of gustatory reward as well as their respective concentrations (Niewalda et al. 2008; Schipanski et al. 2008) and the role of outcome expectations in the actual behavioral expression of the olfactory memory trace (Gerber and Hendel 2006).
However, a number of gaps remain that limit the utility of these larval odor-reward learning paradigms, particularly in olfaction research. This is because the paradigm uses a 2-group reciprocal and differential conditioning regimen: One group of larvae receives training such that one odor (X) is rewarded (+), and another odor (Y) is not (X+ // Y); in contrast, the other group receives reciprocal training, such that the contingencies are X // Y+. Then, both groups are tested for their relative preference between X versus Y (X -- Y). If the reciprocally trained groups differ with respect to their relative preference in this test, this difference must be due to the different contingencies of the odors and the reward, that is, it must be due to associative learning. Although such an interpretation in principle is compelling, exactly which associations are formed must remain uncertain: Do the larvae associate X with reward during X+ // Y training and/or do they associate Y with reward during X // Y+ training? In other words, one cannot use a 2-odor differential conditioning paradigm to assess the learnability of individual odors. As a consequence, the paradigm is not suitable for generalization types of experiment, where only one odor is trained, and a nontrained odor is tested. Also, using a 2-odor differential conditioning paradigm does not allow testing for intensity learning, where one odor, at a particular intensity, is trained and that same odor is tested, at either the trained intensity or at a higher or a lower intensity (for adult flies: Yarali et al. 2009). Last but not least, when using a 2-odor differential conditioning paradigm for neurogenetic analyses, one has to control for the mutants’ ability to detect both odors. To overcome these limitations and to reduce the workload for mutant analyses, Saumweber (2007) has introduced a one-odor version of the paradigm (subsequently employed in Selcho et al. [2009] as well as the companion paper of Chen et al. [2011]); notably, the paradigm maintains the reciprocity required to draw conclusions about the associative nature of the learning process.
In the current study, we describe and elaborate on this reciprocal one-odor paradigm, asking which odors can be used in such a paradigm, what the relation is between the relative innate attractiveness of odors and their learnability, and which kinds of association actually are formed. In a companion paper, we demonstrate the utility of this paradigm to describe a behavioral “space” for odor quality in larval Drosophila (Chen et al. 2011).
Materials and methods
We use third-instar, feeding-stage larvae from the Canton-S wild-type strain (Michels et al. 2005), aged 5 days after egg laying. Flies are kept in mass culture and maintained at 25 °C, 60–70% relative humidity and a 14/10 h light:dark cycle. Experiments are performed in red light under a fume hood at 21–26 °C room temperature.
As olfactory stimuli, we use 3-octanol (3OCT, CAS: 589-98-0; purity: 97%, Merck), 1-octanol (1OCT, CAS: 111-87-5; purity: 99%, Sigma-Aldrich), n-amyl acetate (AM, CAS: 628-63-7, purity: 99%, Merck), and linalool (LIN, CAS: 78-70-6; purity: 97%, Merck); in cases when odors are presented in diluted form, paraffin oil (CAS: 8012-95-1; Sigma-Aldrich) (PAR) is used as diluent. Odors are applied by adding 10 μL of odor substance into teflon containers (inner diameter 5 mm) which can be closed by a perforated lid (7 holes, 0.5-mm diameter). As we dilute odors in PAR, we first test whether PAR might be behaviorally active. Therefore, we test innate preference (see next paragraph, Innate preference tests) between a container with 10 μL of PAR versus an empty container (EM); larvae behave indifferently in this situation (supplementary Figure 1A; one-sample sign [OSS] test: P = 0.791, N = 16); we therefore use the EM condition in all cases where a no-odor reference is needed.
Petri dishes (Sarstedt) with 85-mm inner diameter are filled with 1% agarose (electrophoresis grade; Roth), allowed to solidify, covered with their lids, and then left untreated until the following day. As positive reinforcer, we use 2 mol fructose (FRU, purity: 99%, Roth) added to 1 L of 1% agarose 10 min after boiling. Before experiments, we replace the regular lids of the Petri dishes with lids perforated in the center by fifteen 1-mm holes to improve aeration.
Innate preference tests
To test the innate odor preference of larvae, we take experimentally naïve animals and give them the choice between an odor and an empty container; that is, throughout this paper we use the term “innate” in the sense of “experimentally naïve.” To test for relative innate preference, we offer a choice between 2 different odors (for sketches, see Figure 1A). A spoonful of food medium containing larvae is taken from the food bottle and transferred to a glass vial. Thirty animals are collected, briefly washed in tap water and as a group transferred to the assay plates.
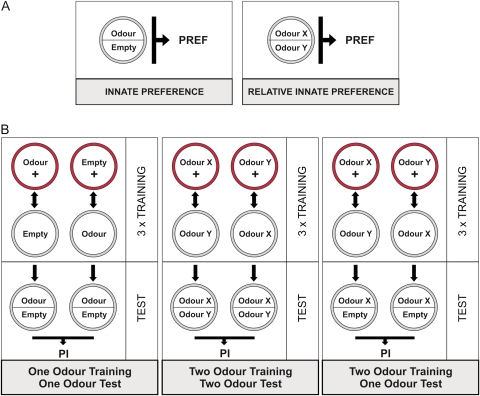
Figure 1.
Schematics of experimental regimen. (A) Innate preference test (left) using one odor, and relative innate preference test (right) using 2 odors. (B) The 3 types of learning experiment. Please note that in half of the cases, the sequence of training trials was as indicated, whereas in the other half, it was reversed (e.g., with regard to the left most column the “Empty” trial was run first, to be followed by a “Odor +” trial). This figure appears in color in the online version of Chemical Senses.
Immediately before testing, 2 containers loaded differently (i.e., either one container was loaded with odor and the other one was empty: innate preference; or they were loaded with different odors: relative innate preference) are placed onto the assay plate on opposite sides of the plate, 7 mm from the edges, to create a choice situation. Sidedness is changed after each experiment; that is, for half of the cases, a given load is placed to the left and for half of the cases to the right side. Then, the lid is closed and the larvae are allowed to move about the assay plate. After 3 min, the number of animals on either side is determined.
We calculate an odor preference ranging from −1 to 1 by determining the number of animals observed on the one side minus the number of animals observed on the other side, divided by the total number of larvae. We introduce a 0.5-mm middle stripe for a neutral zone. Larvae that remain in the neutral zone are added to the total:
| (1) |
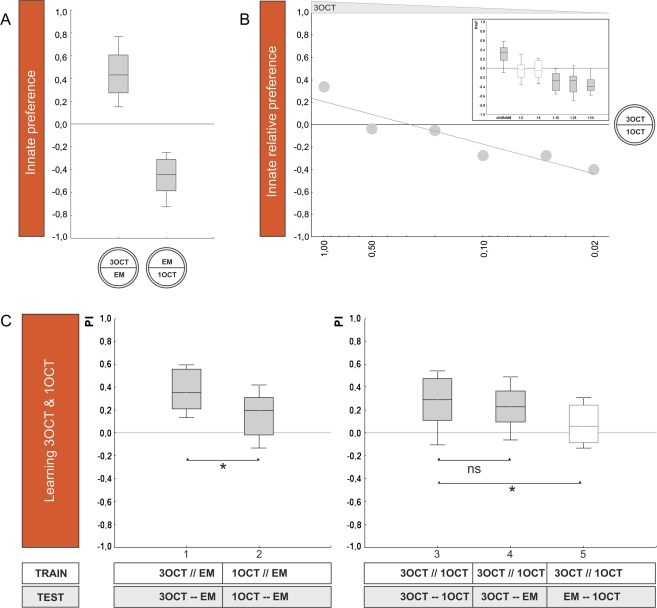
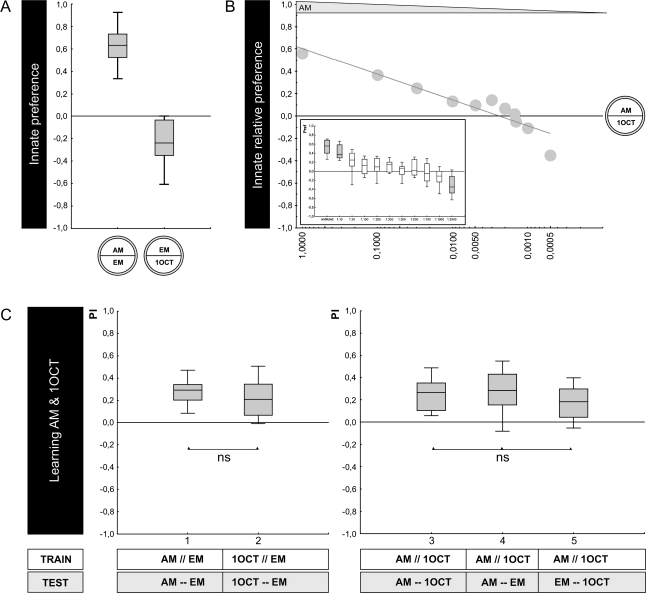
For any given odor pair to be considered, we designate the side of that odor that “wins” in relative innate preference as the X-side; for example, when the relative innate preference between 3OCT versus 1OCT is tested, 3OCT wins (Figure 2B). Therefore, 3OCT is assigned as X and 1OCT as Y, such that positive values indicate a relative innate preference for 3OCT and negative values a relative innate preference for 1OCT. To maintain this assignment also when innate preference of the individual odors is measured versus an empty odor container, the winning odor is designated as X and the empty container as Y, whereas in the case when the innate preference of the losing odor is assayed, that odor is designated as Y and the empty container as X; thus, in Figure 2A, preference for 3OCT is shown by positive values and preference for 1OCT by negative values.
Figure 2.
Comparing innate preference to associative learnability: 3OCT–1OCT. (A) Innate preference: Experimentally naïve animals are given the choice between an odor and a no odor condition (EM). Larvae approach both undiluted 3OCT and undiluted 1OCT when tested against EM and do so to the same extent. The shading of the boxes indicates significant difference from chance level (OSS, P < 0.05/2). (B) Relative innate preference: Larvae prefer undiluted 3OCT over undiluted 1OCT; as 3OCT is diluted, preferences shift to 1OCT. The median scores from the inset figure are plotted against 3OCT dilution. The shading of the boxes indicates significant difference from chance level (OSS, P < 0.05/6. (C) Learnability: In the 2 cases to the left, larvae are trained and tested with either 3OCT versus EM (group 1) or with 1OCT versus EM (group 2); the shading of the boxes indicates P < 0.05/2 (OSS), whereas *indicates P < 0.05 (MWU). Larvae show higher performance indices for 3OCT than for 1OCT. In all 3 cases to the right, larvae are trained differentially with 3OCT versus 1OCT but are tested with either both odors (group 3), with only 3OCT versus an empty container (group 4), or with only 1OCT versus an empty container (group 5). The shading of the boxes indicates P < 0.05/3 (OSS); ns and *indicate P > 0.05/2 and P < 0.05/2 (MWU), respectively. When the odor with higher learnability is omitted at test (group 3 vs. group 5), scores are reduced. Data are presented as box plots with the median as bold line, box boundaries as 25/75% quartiles and whiskers as 10/90% quantiles. This figure appears in color in the online version of Chemical Senses.
Learning experiments
Here, we describe the principle of the learning experiments; deviations are then mentioned along with the Results (for sketches, see Figure 1B). We train groups of 30 larvae and compare olfactory choice performance after either of 2 reciprocal training regimen: for one regimen, animals receive odor X with a reward (+) and odor Y without reward (Train: X+ // Y); for the second regimen, animals are trained reciprocally (Train: X // Y+). Then, animals are tested for their choice between odor X versus odor Y on a pure assay plate (Test: X -- Y), unless mentioned otherwise. Associative learning is indicated by systematic differences in test performance between the reciprocal treatment conditions. This conclusion is compelling as during training animals from both training regimen have identical exposure to both odors and the reward—what differs between them is solely the contingency between these stimuli. The reciprocally trained groups were run alternately, which allows pairing of data (see Hendel et al. 2005) for the calculation of a performance index (PI; see below).
Specifically, immediately before a trial, 2 containers loaded both with the same odor are placed on opposite sites of the assay plate, which may or may not contain the reward; as described above, the lid is replaced by a perforated one. Thirty animals are collected, transferred to the assay plate, and the lid is closed. After 5 min, animals are transferred to a fresh assay plate with the alternative odor-substrate combination. This training cycle is repeated 3 times, using fresh assay plates for each trial.
After such training, animals are tested for their odor choice. Unless mentioned otherwise, they are placed in the middle of a fresh agarose-only assay plate with a container of odor X on one side and one of odor Y on the other side (Test: X -- Y). After 3 min, the number of animals on the odor X side, on the middle stripe, and on the odor Y side is counted. After this test is completed, the next group of animals is run and trained reciprocally. For both groups, we then calculate an odor preference ranging from −1 to 1. To this end, we determine the number of animals observed on the odor X side minus the number of animals observed on the odor Y side, divided by the total number of larvae, which includes animals on the middle stripe:
| (2) |
To determine whether these preferences are different depending on training regimen, we take the data from the alternately run, reciprocally trained groups, and calculate a PI ranging from −1 to 1 as:
| (3) |
Thus, positive PIs indicate conditioned approach toward the previously rewarded odor, whereas negative values would reflect conditioned avoidance. After the data for one such PI value in one group are collected, the corresponding data for a PI value of the other groups are gathered, that is, data from all groups to be compared statistically are obtained in parallel. Groups differ with respect to the kinds of odors used as X and Y during training and/or test (see Figure 1B and Results).
In a conservative approach, we use nonparametric analyses throughout. We use OSS tests for comparisons of PIs or preference values against zero; Kruskal–Wallis (KW) tests and Mann–Whitney U (MWU) tests are used for comparisons between groups. We correct the level of significance if multiple comparisons are made by dividing the P level of 0.05 by the number of comparisons made (Bonferroni-correction) to maintain an experiment-wide error rate at 5%. Data are presented as box plots with the median as bold line, box boundaries as the 25/75% quantiles, and whiskers as the 10/90% quantiles. Experimenters were blind with respect to whether the training plates contained the reward; this was decoded only after the experiment.
Results
Our strategy is to first “titrate” odor dilutions such that relative innate olfactory preference is equal for a given odor pair. Then, the learning experiments are performed at this “titration point” of dilution to see whether equal relative innate preference goes along with equal learnability.
3OCT–1OCT: innate and relative preferences
We find that both 3OCT and 1OCT support attraction and thus are detectable by the larvae (Figure 2A; OSS tests: P < 0.05/2; N = 18, 20 respectively); the level of attraction is equal for both odors (Figure 2A; MWU-test: U = 173.5; P = 0.85; sample sizes as above). When we test relative preference between these 2 undiluted odors, however, larvae prefer 3OCT over 1OCT (Figure 2B; OSS test: P < 0.05/6; N = 20), suggesting that one cannot predict relative preference between 2 odors based on preference scores for either odor tested against the no-odor option. Indeed, innate relative preferences under conditions of choice are systematically larger than the differences in single-odor preferences would predict (see supplementary Figure 7); therefore, relative measures of innate preference between 2 odors can resolve differences in “valuation” that single-odor preference measures cannot resolve. In any event, along a dilution series for 3OCT, larvae become indifferent between 3OCT and 1OCT at a dilution of 3OCT:paraffin of 1:2 and remain indifferent down to a 1:5 dilution (Figure 2B; OSS tests: P > 0.05/6; N = 18, 16, respectively); diluting 3OCT yet further leads to preference for 1OCT (Figure 2B; OSS tests: P < 0.05/6; N = 20, 18, 18). Based on the plot in Figure 2B, we chose a 1:2 dilution of 3OCT to balance innate preference relative to undiluted 1OCT. These respective intensities are therefore used in the next experiment to see whether under such conditions of equal relative innate preference both odors would be equal in their associative learnability as well.
3OCT–1OCT: learning
Using a one-odor associative learning paradigm introduced by Saumweber (2007), we find that both odors, at the respective intensities, can be learned (Figure 2C; OSS tests: P < 0.05/2; N = 21, 29). Interestingly, learnability is not equal for both odors: Larvae trained and tested with 3OCT show higher associative performance indices than is the case for 1OCT (Figure 2C; MWU-test: U = 152.0; P < 0.05; sample sizes as above). Correspondingly, if we train larvae differentially, that is, reward one of the odors but not the other and omit one of the odors during test, the omission of the less-learnable odor 1OCT is of negligible effect (as compared with the scores found when both trained odors indeed are present at test: Figure 2C; MWU-test: U = 210.0; P > 0.05/2; N = 23, 21). If, however, the higher learnability odor 3OCT is omitted at test, associative performance indices are substantially reduced (Figure 2C; MWU-test: U = 123.5; P < 0.05/2; N = 23, 21) (considering all 3 groups in a KW test yields: H = 8.62; degrees of freedom = 2; P < 0.05; sample sizes as above). This argues that 3OCT is easier to learn than 1OCT—although there is no difference in their relative innate attractiveness.
Two other cases of dissociation: AM–3OCT …
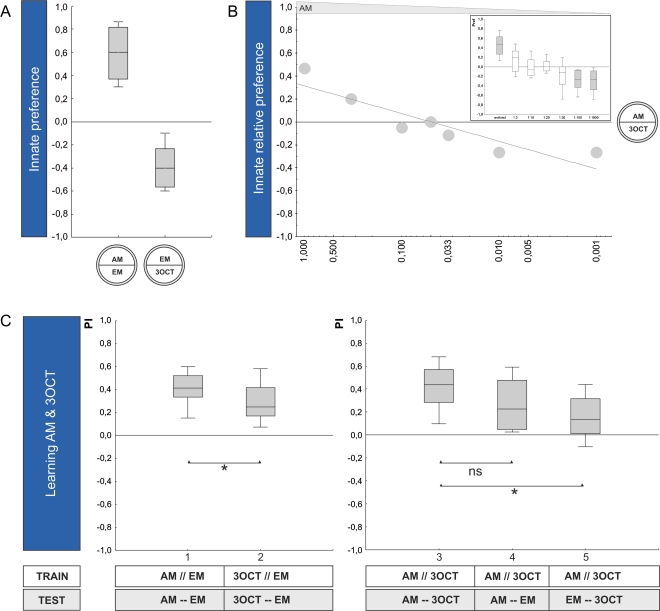
Using the same 2-step strategy, we find that also for n-amyl acetate (AM) and 3-octanol (3OCT) innate relative preference and learnability are dissociated (for all statistics, see legend of Figure 3). That is, after titrating innate relative preference (Figure 3B), larvae still show higher associative performance indices for AM than for 3OCT in the learning task (group 1 vs. 2, Figure 3C). When AM as the higher learnability odor is omitted at test (group 3 vs. 5, Figure 3C), associative performance indices are strongly reduced, whereas omitting the less-learnable odor 3OCT leaves associative performance indices statistically unaffected (group 3 vs. 4, Figure 3C).
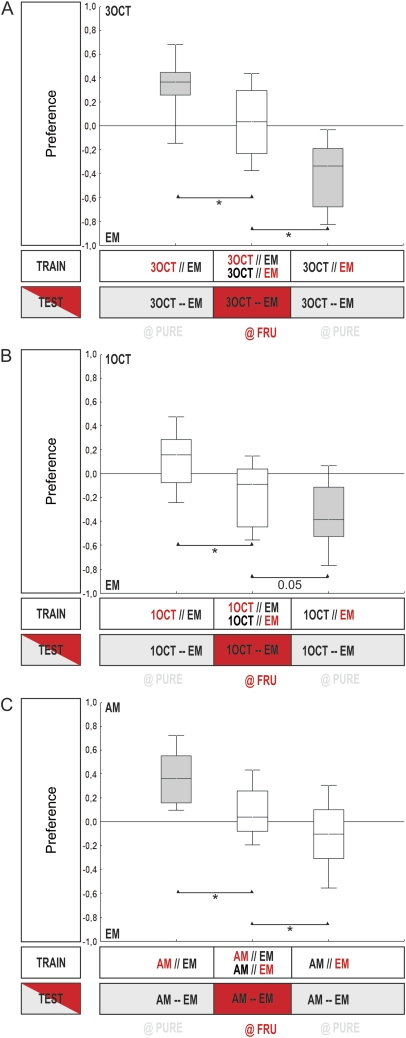
Figure 3.
Comparing innate behavior to associative learnability: AM–3OCT. (A) Innate preference: Experimentally naïve animals are given the choice between an odor and a no odor condition (EM). Larvae approach both undiluted AM and undiluted 3OCT when tested against EM (OSS tests: P < 0.05/2; N = 16, 16) and, statistically, do so to the same extent (MWU-test: U = 85.5; P = 0.11; N = 16, 16). The shading of the boxes indicates significant difference from chance level (OSS tests above). (B) Relative innate preference: When undiluted AM is tested against undiluted 3OCT in a relative choice situation, larvae strongly prefer AM over 3OCT (OSS test: P < 0.05/7; N = 22). Along a dilution series of AM, this preference for AM is lost at AM: paraffin dilutions between 1:3 and 1:30 (OSS tests: P > 0.05/7; N = 18, 16, 16, 18). At dilutions of 1:100 and 1:1000, larvae prefer 3OCT over AM (OSS tests: P < 0.05/7; N = 14, 20). The shading of the boxes indicates significant difference from chance level (OSS tests above). Based on these results, we chose a 1:20 dilution of AM for testing AM and 3OCT for learnability. (C) Learnability: Both odors, at the respective intensities, are learnable (OSS tests: P < 0.05/2; N = 22, 21). Higher associative performance indices are seen for AM than for 3OCT (MWU-test: U = 149.0; P < 0.05; sample sizes as above), even though their innate relative preference had been titrated against (see B). When after differential conditioning between AM and 3OCT, the better-learnable AM is omitted at test, associative performance indices are reduced (group 3 vs. 5, MWU-test: U = 122.0; P < 0.05/2; N = 24, 23); if, in turn, the less-learnable odor 3OCT is omitted, scores are not significantly reduced when using the warranted Bonferroni correction (group 3 vs. 4, MWU-test: U = 184.0; P = 0.05; N = 24, 23) (a KW test across the 3 groups yields: H = 11.14; degrees of freedom = 2; P < 0.05; sample sizes as above). Other details as in the legend of Figure 2. This figure appears in color in the online version of Chemical Senses.
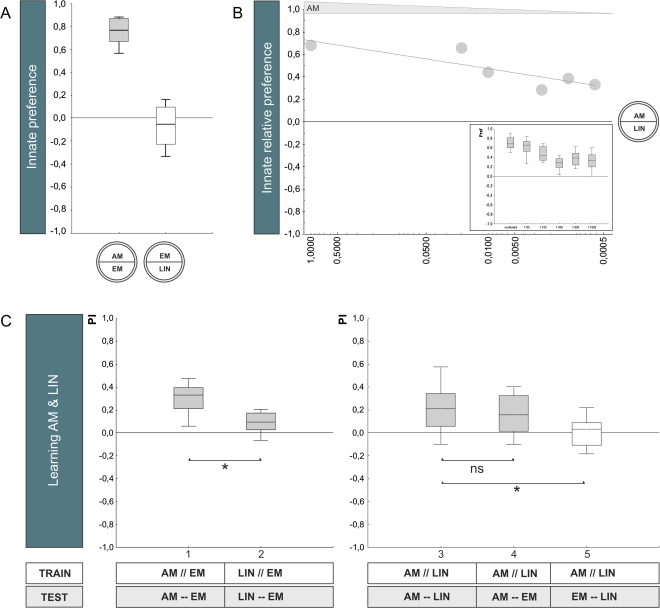
… and AM–LIN
The same pattern of results is found for n-amyl acetate (AM) and linalool (LIN) as well, yet in a more extreme way (for all statistics, see legend of Figure 4): Although LIN does not support innate behavior (Figure 4A,B) (but see Honjo and Furukubo-Tokunaga [2005; 2009] and Fishilevich et al. [2005]), it does support learnt behavior (Figure 4C; group 2). Clearly, associative performance scores for LIN are much lower than for AM (Figure 4C; group 1 vs. 2); correspondingly, when larvae are differentially trained between AM versus LIN and the low-learnability odor LIN is omitted at test, associative performance indices remain unaltered (Figure 4C; group 3 vs. 4), whereas omission of the high-learnability odor AM strongly reduces these scores (Figure 4C; group 3 vs. 5).
Figure 4.
Comparing innate behavior to associative learnability: AM–LIN. (A) Innate preference: Experimentally naïve animals are given the choice between an odor and a no odor condition (EM). Larvae approach undiluted AM but not undiluted LIN, when tested against EM (OSS tests: P < 0.05/2 for AM and P = 0.135 for LIN; N = 21, 46). Correspondingly, preference for AM is higher than for LIN (MWU-test: U = 2; P < 0.05). The shading of the box indicates significant difference from chance level (OSS tests above). (B) Relative innate preference: Larvae prefer undiluted AM over undiluted LIN (OSS test: P < 0.05/6; N = 10); as AM is diluted, preference for AM is reduced but remains significant (OSS tests: P < 0.05/6 in all cases; N = 10, 14, 12, 14, 34). The median scores from the inset figure are plotted against AM dilution. The shading of the boxes indicates significant difference from chance level (OSS tests above). Based on these results, we chose a 1:1600 dilution of AM and undiluted LIN in the learning experiments in (C). (C) Learnability: In the 2 cases to the left, larvae are trained and tested with either AM versus EM (group 1) or with LIN versus EM (group 2); both groups show significant learning effects (OSS tests: P < 0.05/2; N = 26, 24). Larvae show higher associative performance indices for AM than for LIN (MWU-test: U = 84; P < 0.05; sample sizes as above). In all 3 cases to the right, larvae are trained differentially with AM versus LIN but are tested with either both odors (group 3) or with only AM versus an empty container (group 4) or with only LIN versus an empty container (group 5). When AM as the odor with higher learnability is omitted at test, scores are reduced (group 3 vs. 5, MWU-test: U = 213.5; P < 0.05/2; N = 30, 28), whereas omission of the low-learnability odor LIN at test is without effect (group 3 vs. 4, MWU-test: U = 425.5; P = 0.57; N = 30, 31) (a KW test across the 3 groups reveals: H = 13.38; degrees of freedom = 2; P < 0.05; sample sizes as above). Other details as in the legend of Figure 2. This figure appears in color in the online version of Chemical Senses.
This case of a lack of innate behavior toward LIN on the one hand and effective learnability on the other hand raises the question whether also PAR as solvent may, despite being ineffective in innate behavior (supplementary Figure 1A), nevertheless support learnt behavior. This, however, is not the case (supplementary Figure 1B).
No dissociation of innate relative preference and learnability for AM–1OCT
In contrast to the 3 cases reported above, for n-amyl acetate (AM) and 1-octanol (1OCT), we find that adjusting innate relative preference does adjust for equal learnability (see legend of Figure 5 for all statistics). That is, after adjusting for innate relative preference (Figure 5A,B), associative performance indices do not significantly differ, although one may note a tendency for lower scores for 1OCT (Figure 5C). Interestingly, under these conditions of about equal learnability, after differential conditioning, the omission of either odor at test remains without significant effect (Figure 5C).
Figure 5.
Comparing innate behavior to associative learnability: AM–1OCT. (A) Innate preference: Experimentally naïve animals are given the choice between an odor and a no odor condition (EM). Larvae approach both undiluted AM and undiluted 1OCT when tested against EM (OSS tests: P < 0.05/2; N = 16, 28, respectively); for AM, preference is stronger than for 1OCT (MWU-test: U = 48; P < 0.05, sample sizes as above). The shading of the boxes indicates significant difference from chance level (OSS tests above). (B) Relative innate preference: Larvae prefer undiluted AM over undiluted 1OCT (OSS test: P < 0.05/11; N = 16). When a dilution series of AM is performed, larvae show relative attraction for AM down to a 1:10 dilution (OSS tests: P < 0.05/11; N = 16, 16). At AM dilutions between 1:30 and 1:1000, larvae behave indifferently (OSS tests: P > 0.05/11; N = 16, 20, 18, 20, 20, 20, 20, 26). At a yet lower dilution (1:2000), larvae prefer 1OCT over AM (OSS test: P < 0.05/11; N = 28). The median scores from the inset figure are plotted against AM dilution. The shading of the boxes indicates significant difference from chance level (OSS tests above). Based on these results, we chose a 1:700 dilution of AM and undiluted 1OCT for the learning experiments in (C). (C) Learnability: In the 2 cases to the left, larvae are trained and tested with either AM versus EM (group 1) or with 1OCT versus EM (group 2); larvae show significant (OSS tests: P < 0.05/2; N = 26, 26) and equal (MWU-test: U = 259; P = 0.15; sample sizes as above) associative performance indices in these groups. In all 3 cases to the right, larvae are trained differentially with AM versus 1OCT but are tested with either both odors (group 3) or with only AM versus an empty container (group 4) or with only 1OCT versus an empty container (group 5). Omitting either one of the odors at test does not significantly reduce scores (KW test: H = 5.31; degrees of freedom = 2; P = 0.07, N = 35, 35, 34). Other details as in the legend of Figure 2.
A closer look at the one-odor paradigm
When considering the preference scores used to calculate associative performance indices, we noted that there is repulsion for nonrewarded odors (for 3OCT as example, see supplementary Figure 2, group 2 as well as supplementary Figure 3, group 4). In other words, unpaired presentations of 3OCT and reward induce repulsion toward 3OCT (corresponding effects are seen for the other odors, at least as trends: supplementary Figure 3–5, groups 2 and 4). This is in accordance with formal models of the effects of reinforcement and nonreinforcement in associative learning (Rescorla and Wagner 1972). These models suggest that during a reward-only trial an association is formed between the “experimental context” and the reward. In an ensuing odor-only trial, this context-reward association is activated and predicts the reward—but the reward is not actually present. This leads to a negative prediction error, that is, to “frustration” because a lot of reward is predicted yet no reward is received. If at this moment an odor is presented, the odor becomes a signal for no-reward (rather than remaining neutral, that is, not being a signal for anything). This kind of model is accounting for an astonishingly wide array of experimental data in animals and man (Rescorla 1988), and key neuronal elements implementing this prediction error have been identified (Schulz 2006).
However, what is the “true baseline” of odor preference after one-odor training? This is a relevant question because one may need to reckon with effects of handling, odor exposure, or reward exposure (in addition to the documented associative effects). In other words, regarding our study the question remains whether paired odor-reward training indeed increases odor preference above baseline and whether unpaired odor/reward training really decreases odor preference below baseline. To answer this question, we take advantage of the observation that learnt olfactory behavior is abolished in the presence of the reward (Gerber and Hendel 2006). That is, a learnt odor is tracked down by the larvae because they expect to find the reward in its vicinity; if the sought—for reward is actually present, such conditioned search behavior is abandoned. This effect we replicate here, using 1:2 diluted 3OCT, undiluted 1OCT, and 1:700 diluted AM (supplementary Figure 6A). Thus, the olfactory behavior of larvae trained as normal but tested on a reward-containing assay plate does not contain conditioned behavioral components. The actual preference scores under these testing conditions can therefore serve to determine the BASELINE of odor preference after one-odor training (supplementary Figure 6B). Such an approach reveals an increase relative to BASELINE after paired odor-reward training (Figure 6A; 3OCT: MWU-test: U = 139.5; P < 0.05/2; N = 17, 32; Figure 6B; 1OCT: MWU-test: U = 228.5; P < 0.05/2; N = 29, 31; Figure 6C; AM: MWU-test: U = 227.5; P < 0.05/2; N = 28, 38), as well as a decrease after unpaired odor/reward training (Figure 6A; 3OCT: MWU-test: U = 92; P < 0.05/2; N = 16, 32; Figure 6B; 1OCT: MWU-test: U = 151; P = 0.05; N = 29, 31; Figure 6C; AM: MWU-test: U = 368; P < 0.05/2; N = 29, 38). This is also confirmed by a reanalysis of corresponding data from Gerber and Hendel (2006; supplementary Figure 6C). Thus, after paired odor-reward training, the odor is a signal for reward, whereas after unpaired odor/reward training, the odor can be a signal for no-reward.
Figure 6.
A closer look at the one-odor learning paradigm. (A) Paired odor-reward training (3OCT // EM) increases odor preference above BASELINE, whereas unpaired training (3OCT // EM) decreases odor preference below BASELINE (red lettering indicates the rewarded condition during training). The red filled rectangles below the plots indicate that for determining the BASELINE, testing was performed in the presence of fructose, which abolishes conditioned behavior (for details, see text and supplementary Figure 6). Shading of the boxes indicates P < 0.05/3 (OSS) and *indicates P < 0.05/2 (MWU). (B and C) display the corresponding results regarding 1OCT and AM, respectively. This figure appears in color in the online version of Chemical Senses.
Discussion
The current study contributes 3 significant aspects about odor-reward associative learning in larval Drosophila, which will be discussed in detail below:
We introduce a one-odor version of the paradigm, reducing the complexity of the task and enabling between—odor comparisons of learnability; this will help to avoid confounding differences in learnability in analyses of the larval “perceptual odor space” (Chen et al. 2011).
Using this one-odor paradigm, we show that relative innate attractiveness and associative learnability can be dissociated. This dissociation may shed some light on a particular anatomical feature of the insect olfactory pathway, namely that the projection neurons have 2 target areas, the lateral horn, and the mushroom bodies. Furthermore, it raises caveats for the kinds of behavioral control procedures to be employed in cases of disrupted odor-reward learning.
It reveals that paired presentations of an odor with a reward increase, whereas unpaired presentations of odor and reward decrease odor preference—and can even lead to conditioned odor aversion; this poses a challenge to current models of how, neurobiologically, such learning comes about.
The one-odor paradigm
We introduce a one-odor version of the reciprocal conditioning paradigm and apply it regarding 4 odors. For 3OCT, 1OCT and AM, the resulting associative performance indices appear substantial, whereas for LIN, scores are rather low (the respective groups 1, 2 in Figures 2C, 3C, 4C, and 5C). Notably, for LIN, we do not find any measurable innate preference. This is in contrast to the results of Honjo and Furukubo-Tokunaga (2005; location cited Figure 1B). Those authors found spontaneous preference toward LIN (as did Fishilevich et al. 2005) and strong training-dependent changes of LIN preferences, effects actually stronger than for most of the other 18 odors tested; also, it is reported that 3OCT and 1OCT are not learnt. These discrepancies may reflect differences between the wild-type strains used and/or in the case of the learning experiments, differences in the behavioral paradigms employed. Indeed, the learning paradigms differ substantially: 1) the experiments by Honjo and Furukubo-Tokunaga (2005) use a nonreciprocal experimental design, allowing for confounding nonassociative effects (for discussion, see Gerber and Stocker 2007); 2) the authors use only one 30-min odor-reward training trial, whereas in our case, larvae are trained 3 times for 5 min for each reciprocal odor-substrate combination; 3) the authors bath the larvae in a liquid film of 1 M sucrose solution, whereas in our case 2 M fructose is presented in solidified agarose; 4) the authors spot undiluted odor on a filter disk and place it on the inside of the lid during training, whereas we use Teflon containers filled with odor; 5) the authors use 50–100 larvae, as compared with only 30 larvae in our case. Thus, extrapolations between our learning results with the ones of Honjo and Furukubo-Tokunaga (2005) appear problematic.
In any event, thanks to the availability of a reciprocal one-odor paradigm, we can estimate the associative learnability of individual odors. It reveals, for 3OCT and 1OCT as an example, that although their innate relative preference had been balanced (Figure 2B), their learnability can be substantially different (groups 1 and 2 in Figure 2C). Correspondingly, after 2-odor training, omission of the low-learnability odor 1OCT at test does not reduce associative performance indices (group 4 in Figure 2C), whereas omission of the high-learnability odor 3OCT does (group 5 in Figure 2C).
Also, the introduced one-odor paradigm will simplify analyses of “learning mutants” because the mutants’ ability to smell needs to be controlled for only the one odor chosen for the experiment. Finally, it will allow for generalization-type behavioral experiments, such that one given odor is trained, but another odor is tested. This will enable descriptions of the olfactory “coding space” of the larva (Chen et al. 2011), supplementing previous approaches based on cross-adaptation (Boyle and Cobb 2005; Kreher et al. 2008).
We note that performance indices for the 2-odor paradigm are not different from the performance indices in the one-odor paradigm (see the respective groups 1 vs. 3 in Figures 2C, 3C, 4C, and 5C). Although these respective experimental groups were not treated in parallel (such that statistical comparisons between them are not formally valid), this makes us wonder whether the difference between 2 odors (groups 3) may be as obvious to the larvae as is the difference of a given odor to a no-odor condition (groups 1). If this were so, one should find little generalization between the odors used in this study. From our current experiments (Chen et al. 2011), this does seem to be the case: If after training with AM, larvae are tested with, for example, 1OCT, performance indices are less than one-third of the performance indices we observe when the trained odor AM is used for testing. This level of specificity is not apparently less than what we observe in adult flies (Niewalda T, personal communication).
Dissociation of innate attractiveness and associative learnability
The most dramatic example of dissociation between innate attractiveness and associative learnability is LIN: although there is no evidence for an innate preference for LIN (right panel Figure 4A), larvae can associate LIN with a sugar reward (group 2 in Figure 4C)! Also, although for 3OCT and 1OCT, relative innate preference was balanced (Figure 2B), the associative performance indices in the one-odor paradigm are substantially higher for 3OCT than for 1OCT (groups 1 and 2 in Figure 2C). A corresponding pattern of results is found for AM and 3OCT as odor pair (Figure 3). The possibility of such discrepancies should be considered in odor-quality generalization experiments where it may be important that 2 olfactory inputs cannot be discriminated by the larvae on the basis of intensity information or when testing for odor-quality discrimination in single-receptor mutants; in such kinds of experiment, it would seem wise to equate the odors for learnability rather than innate relative preference.
Given the dissociation between associative learnability and innate relative preference, one may ask on which level of processing these 2 kinds of olfactory behavior are dissociated. In the larva, 25 different olfactory receptor genes (Ors) (Kreher et al. 2008)—11 of them apparently larva-specific—are expressed. One of these is Or83b, encoding a chaperone-like protein that is required for proper receptor function and is expressed in all larval olfactory sensory neurons (Fishilevich et al. 2005). In addition, the olfactory sensory neurons typically express one Or gene which by virtue of the ligand profile of the encoded receptor protein determines the receptive range of the sensory neuron (Kreher et al. 2005; Fishilevich et al. 2005) (2 sensory neurons are exceptions as they coexpress Or33b/ Or47a and Or94a/ Or94b, respectively [Fishilevich et al. 2005; Kreher et al. 2008]). Each olfactory sensory neuron then projects to only one of the 21 glomeruli in the larval antennal lobe, which in turn receives input from only that one sensory neuron (Ramaekers et al. 2005); within the larval antennal lobe, the lateral connectivity via local interneurons (Python and Stocker 2002; Ramaekers et al. 2005) likely further shapes the pattern of activated antennal lobe glomeruli (regarding adult flies: Ng et al. 2002; Wilson et al. 2004; Wilson and Laurent 2005; Shang et al. 2006; Olsen et al. 2007). For the present discussion, it seems significant that the 21 projection neurons, each receiving input in only one antennal lobe glomerulus, convey the information further toward 2 target areas in the brain: First, they connect directly to the lateral horn from which in turn premotor neurons originate. Secondly, they make a “detour” via the mushroom body where they connect to typically one or 2 out of the approximately 30–40 glomeruli in the larval mushroom body calyx (Marin et al. 2005; Masuda-Nakagawa et al. 2005, 2009; Ramaekers et al. 2005). In turn, each mushroom body neuron, connecting to multiple calyx glomeruli, gets input from multiple projection neurons (Masuda-Nakagawa et al. 2005, 2009). By analogy to the situation in adults, it is likely in the mushroom bodies that reward information is received as well, and where the odor-reward associative memory trace is established (Gerber et al. 2004; and see reviews by Heisenberg 2003; Gerber et al. 2009). In any event, the about 600 mushroom body neurons connect to relatively few (a dozen maybe) output neurons that in turn entertain connections to the motor system. Thus, the larval motor systems receive 2 kinds of olfactory information: direct input via the lateral horn pathway and indirect input via the mushroom body loop. This architecture is shared by most if not all insects. We therefore speculate that the dissociation between innate relative preference on the one hand and associative learnability on the other hand may come about by innate preference behavior being steered via the direct lateral horn pathway, whereas learnt behavior may require the readout of the olfactory memory trace in the mushroom body loop (regarding the adult, see Heimbeck et al. 2001).
The effect of unpaired presentations of odor and reward
We have shown that training can endow the larvae with predictive information about either the presence and also about the absence of reward. That is, as is generally acknowledged, paired odor-reward training increases odor preference above baseline, arguing that the trained odor is a predictor of reward. However, unpaired odor/reward training decreases odor preference below baseline—suggesting that the odor may predict the absence of reward (for related reports in the bee: Bitterman et al. 1983; Hellstern et al. 1998). How can such “absence predictions” come about?
Formal models of the effects of reinforcement and nonreinforcement in associative learning (Rescorla and Wagner 1972) assume that 3 factors are required for conditioning, namely contiguity, contingency, and prediction error (Schulz 2006). In the case of odor-reward training, contiguity is given by the temporal coincidence of the odor and the reward, and contingency is complete because both stimuli, if they do occur, do occur together. Lastly, a positive prediction error ensues during training when—initially “out of the blue,” the reward is received in the presence of the odor; as training progresses, the odor becomes more and more predictive of the reward, and the prediction error is getting smaller until the learning process ceases. Although these kinds of model are powerful in accounting for a wide array of behavioral and neural data, including differences in learnability (“salience”), they require an important modification to accommodate absence predictions due to unpaired odor/reward training. That is, within this type of theoretical framework one needs to suggest that during a reward-only trial, an association is formed between the experimental context and the reward. In an ensuing odor-only trial within the same context, this context-reward association is activated and predicts the reward—but the reward is not actually present. This leads to a negative prediction error because less reward is experienced than is predicted. If at this moment of frustration an odor is presented, the odor becomes a signal for no-reward (rather than remaining neutral, i.e., not being a signal for anything).
Clearly, regarding the larva this scenario now invites experimental scrutiny, including directly testing whether context-reward associations can be formed by the larvae, where in their brain these associations take place, and whether they can indeed account for learning by unpaired odor/reward training—or not.
Funding
This study was supported by the Deutsche Forschungsgemeinschaft (SPP 1392 Integrative analyses of olfaction [to B.G.] and a PhD fellowship of the Excellence Initiative Grant Graduate School for Life Science Würzburg [to T.S.]) and the Federal Ministry of Science and Technology (Bernstein Network Insect inspired robotics [to B.G.]). B.G. is a Heisenberg Fellow of the Deutsche Forschungsgemeinschaft.
Supplementary Material
Acknowledgments
We are indebted to Katharina Gerber, Katja Tschirner, Anne Haberberger, Julia Ehmer, Anette Huber, Xuebin Mao, Miriam Koblowsky, Sabrina Angermeyer, Jenny Bretzger, Andreas Strehle, and Andreas Hellmann for help with the behavioral experiments. We are grateful to Ayse Yarali, Birgit Michels, Martin Heisenberg, and Yi-chun Chen for support and comments.
References
- Aceves-Pina EO, Quinn WG. Learning in normal and mutant Drosophila larvae. Science. 1979;206:93–96. doi: 10.1126/science.206.4414.93. [DOI] [PubMed] [Google Scholar]
- Bitterman ME, Menzel R, Fietz A, Schafer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J Comp Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- Boyle J, Cobb M. Olfactory coding in Drosophila larvae investigated by cross-adaptation. J Exp Biol. 2005;208:3483–3491. doi: 10.1242/jeb.01810. [DOI] [PubMed] [Google Scholar]
- Buch S, Pankratz MJ. Making metabolic decisions in Drosophila. Fly. 2009;3:74–77. doi: 10.4161/fly.3.1.7795. [DOI] [PubMed] [Google Scholar]
- doi: 10.1093/chemse/bjq123. Chen Y-c, Mishra D, Schmitt L, Schmuker M, Gerber B. 2011. A behavioral odor similarity “space” in larval Drosophila. Chem Senses. 36:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Dominigos AI, Asahina K, Naef F, Vosshall LB, Louis M. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Gerber B, Hendel T. Outcome expectations drive learned behaviour in larval Drosophila. Proc Biol Sci. 2006;273:2965–2968. doi: 10.1098/rspb.2006.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B, Scherer S, Neuser K, Michels B, Hendel T, Stocker RF, Heisenberg M. Visual learning in individually assayed Drosophila larvae. J Exp Biol. 2004;207:179–188. doi: 10.1242/jeb.00718. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ. 2009;47:139–185. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- Heimbeck G, Bugnon V, Gendre N, Keller A, Stocker RF. A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:15336–15341. doi: 10.1073/pnas.011314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Hellstern F, Malaka R, Hammer M. Backward inhibitory learning in honeybees: a behavioral analysis of reinforcement processing. Learn Mem. 1998;4:429–444. doi: 10.1101/lm.4.5.429. [DOI] [PubMed] [Google Scholar]
- Hendel T, Michels B, Neuser K, Schimpanski A, Kaun K, Sokolowski MB, Marohn F, Michel R, Heisenberg M, Gerber B. The carrot, not the stick: appetitive rather than aversive gustatory stimuli support associative olfactory learning in individually assayed Drosophila larvae. J Comp Physiol A. 2005;191:265–279. doi: 10.1007/s00359-004-0574-8. [DOI] [PubMed] [Google Scholar]
- Honjo K, Furukubo-Tokunaga K. Induction of cAMP response element-binding protein-dependent medium-term memory by appetitive gustatory reinforcement in Drosophila larvae. J Neurosci. 2005;25:7905–7913. doi: 10.1523/JNEUROSCI.2135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Furukubo-Tokunaga K. Distinctive neuronal networks and biochemical pathways for appetitive and aversive memory in Drosophila larvae. J Neurosci. 2009;29:852–862. doi: 10.1523/JNEUROSCI.1315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Abu Baker MB, Siddiqi O. Odour avoidance learning in the larva of Drosophila melanogaster. J Biosci. 2009;34:621–631. doi: 10.1007/s12038-009-0080-9. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Dennis Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59(1):110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher SA, Kwon AY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Marin EC, Watts RJ, Tanaka NK, Ito K, Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132:725–737. doi: 10.1242/dev.01614. [DOI] [PubMed] [Google Scholar]
- Masuda-Nakagawa LM, Gendre N, O'Kane CJ, Stocker RF. Localized olfactory representation in mushroom bodies of Drosophila larvae. Proc Natl Acad Sci U S A. 2009;106:10314–10319. doi: 10.1073/pnas.0900178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Nakagawa LM, Tanaka NK, O'Kane CJ. Stereotypic and random patterns of connectivity in the larval mushroom body calyx of Drosophila. Proc Natl Acad Sci U S A. 2005;102:19027–19032. doi: 10.1073/pnas.0509643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels B, Diegelmann S, Tanimoto H, Schwenkert I, Buchner E, Gerber B. A role of synapsin for associative learning: the Drosophila larva as a study case. Learn Mem. 2005;12:224–231. doi: 10.1101/lm.92805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuser K, Husse J, Stock P, Gerber B. Appetitive olfactory learning in Drosophila larvae: effects of repetition, reward strength, age, gender, assay type, and memory span. Anim Behav. 2005;69:891–898. [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenböck G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Niewalda T, Singhal N, Fiala A, Saumweber T, Wegener S, Gerber B. Salt processing in larval Drosophila: choice, feeding, and learning shift from appetitive to aversive in a concentration-dependent way. Chem Senses. 2008;33:685–692. doi: 10.1093/chemse/bjn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls D, Pfitzenmaier JER, Krebs-Wheaton R, Selcho M, Stocker RF, Thum AS. Electric shock-induced associative olfactory learning in Drosophila larvae. Chem Senses. 2010;35:335–346. doi: 10.1093/chemse/bjq023. [DOI] [PubMed] [Google Scholar]
- Python F, Stocker RF. Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons. J Comp Neurol. 2002;445:374–387. doi: 10.1002/cne.10188. [DOI] [PubMed] [Google Scholar]
- Ramaekers A, Magnenat E, Marin EC, Gendre N, Jefferis GSXE, Luo L, Stocker RF. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr Biol. 2005;15:982–992. doi: 10.1016/j.cub.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black A, Prokasy WR, editors. Classical conditioning II. New York: Academic Press; 1972. pp. 64–99. [Google Scholar]
- Rodrigues V. Olfactory behavior of Drosophila melanogaster. In: Siddiqi O, Babu P, Hall LM, Hall JC, editors. Development and neurobiology of Drosophila. New York: Plenum; 1980. pp. 361–371. [Google Scholar]
- Rodrigues V, Siddiqi O. Genetic analysis of chemosensory pathway. Proc Indian Acad Sci Sect B Biol Sci. 1978;87:147–160. [Google Scholar]
- Saumweber T. Associative learning is impaired upon lack of the presynaptic protein SAP47. Diploma Thesis. Würzburg: University of Würzburg; 2007. [Google Scholar]
- Selcho M, Pauls D, Han KA, Stocker RF, Thum AS. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS One. 2009;4(6):e5897. doi: 10.1371/journal.pone.0005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipanski A, Yarali A, Niewalda T, Gerber B. Behavioral analyses of sugar processing in choice, feeding, and learning in larval Drosophila. Chem Senses. 2008;33:563–573. doi: 10.1093/chemse/bjn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S, Stocker RF, Gerber B. Olfactory learning in individually assayed Drosophila larvae. Learn Mem. 2003;10:217–225. doi: 10.1101/lm.57903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Shang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2006;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. Brain development in Drosophila melanogaster. New York: Springer Verlag; 2008. Design of the larval chemosensory system. Advances in experimental medicine and biology. 628: 69–81. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–33. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Yarali A, Ehser S, Hapil FZ, Huang J, Gerber B. Odour intensity learning in fruit flies. Proc Biol Sci. 2009;276:3413–3420. doi: 10.1098/rspb.2009.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.