Abstract
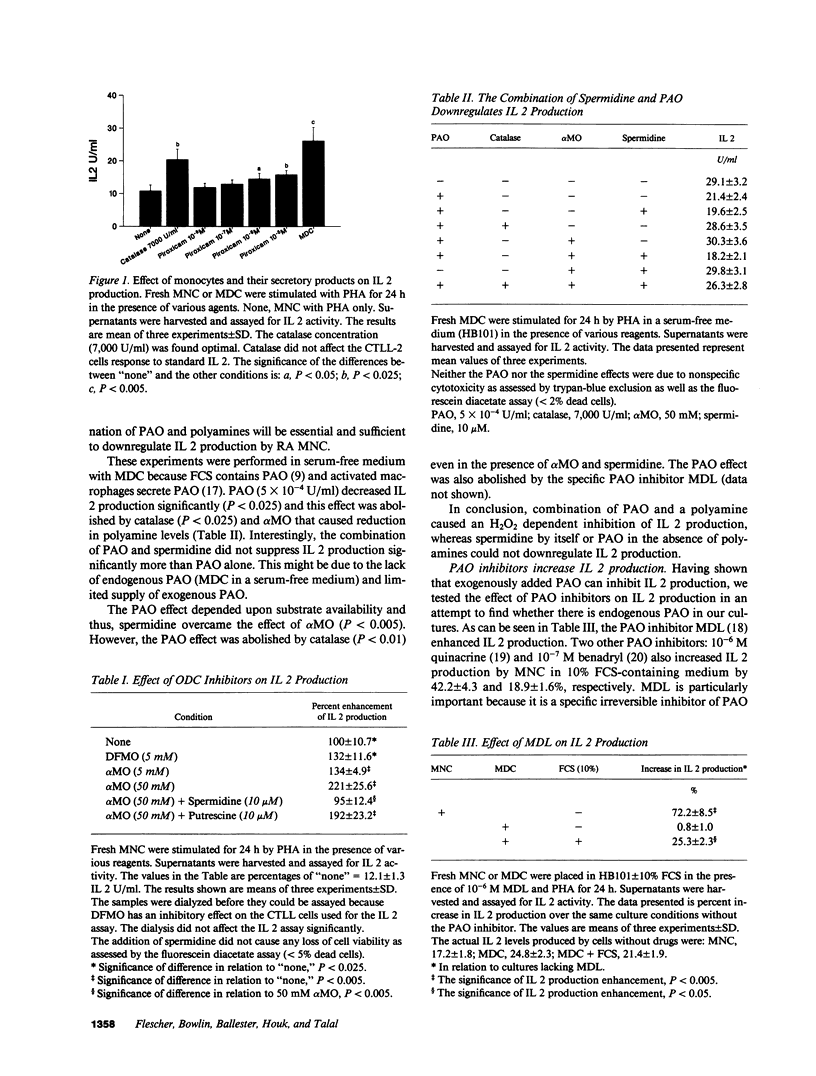
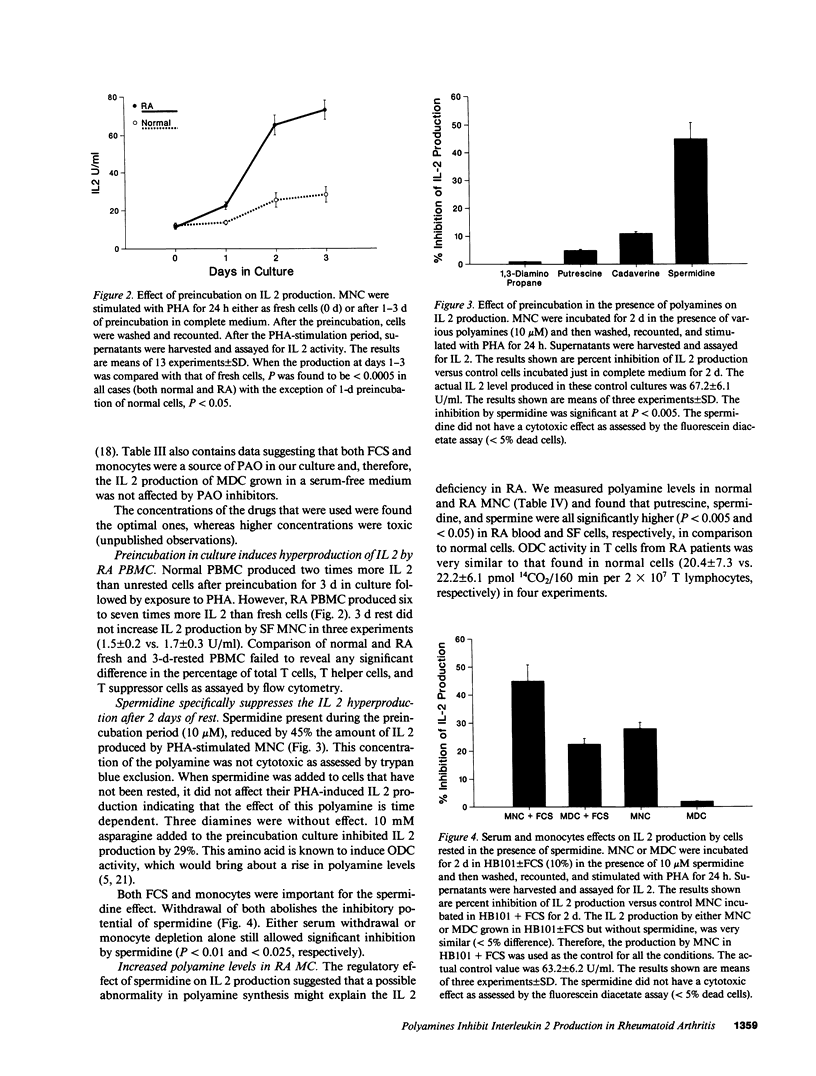
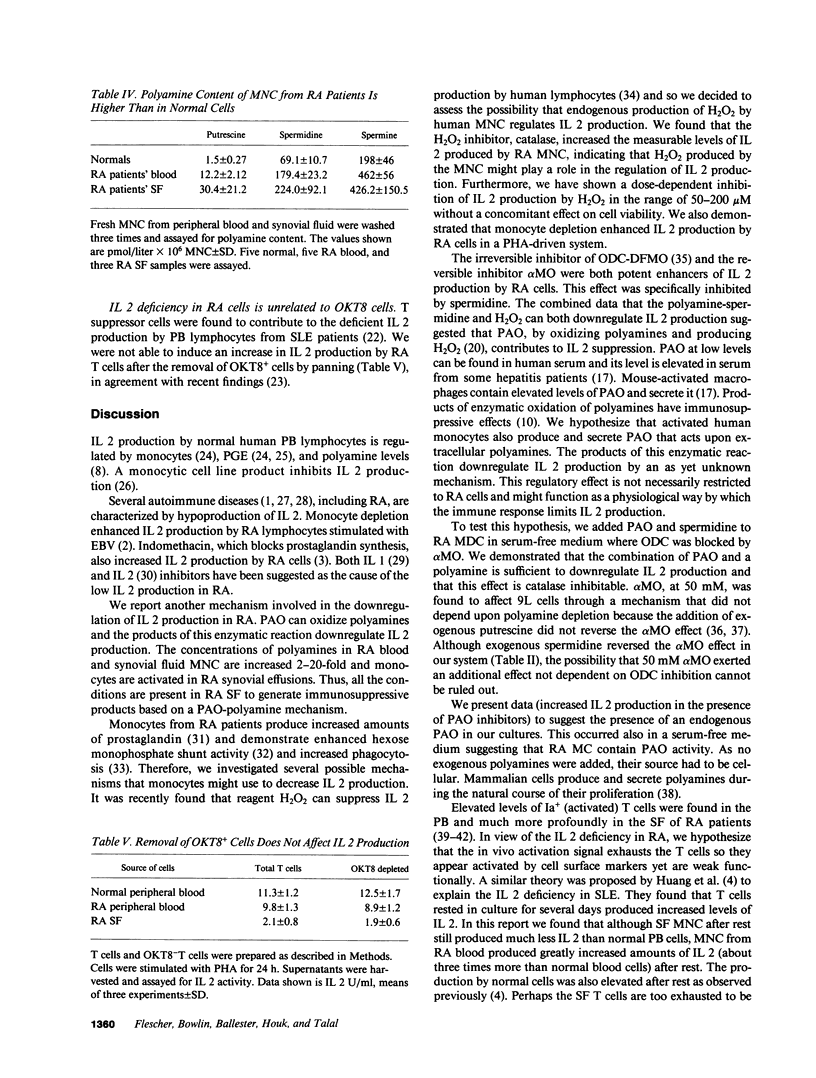
Polyamines downregulate immune reactivity. RA is associated with decreased IL 2 production. In this study, we present evidence to suggest that excessive polyamines can contribute to the IL 2 deficiency in RA. Blocking polyamine production with inhibitors of ornithine decarboxylase results in increased IL 2 production by RA PBMC. Moreover, polyamine oxidase (PAO) inhibitors and catalase also increase IL 2 production by RA PBMC. This effect of PAO inhibition is monocyte mediated. After 3 d in culture, RA PBMC produce three times more IL 2 than do normal PBMC. This rise is prevented by exogenous spermidine but only in the presence of monocytes. The concentration of polyamines in RA PBMC and synovial fluid MNC is 2-20-fold higher than in normal cells. Thus, polyamines and their oxidation products downregulate IL 2 production by RA PBMC and may account for the decreased T cell effector function seen in this disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Roberts T. K. Role of spermine in the cytotoxic effects of seminal plasma. Am J Reprod Immunol Microbiol. 1987 Jan;13(1):4–8. doi: 10.1111/j.1600-0897.1987.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Bey P., Bolkenius F. N., Seiler N., Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985 Jan;28(1):1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- Bowlin T. L., McKown B. J., Sunkara P. S. Ornithine decarboxylase induction and polyamine biosynthesis are required for the growth of interleukin-2- and interleukin-3-dependent cell lines. Cell Immunol. 1986 Apr 1;98(2):341–350. doi: 10.1016/0008-8749(86)90294-7. [DOI] [PubMed] [Google Scholar]
- Bowlin T. L., McKown B. J., Sunkara P. S. The effect of alpha-difluoromethylornithine, an inhibitor of polyamine biosynthesis, on mitogen-induced interleukin 2 production. Immunopharmacology. 1987 Apr;13(2):143–147. doi: 10.1016/0162-3109(87)90051-8. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Byrd W. J., Jacobs D. M., Amoss M. S. Synthetic polyamines added to cultures containing bovine sera reversibly inhibit in vitro parameters of immunity. Nature. 1977 Jun 16;267(5612):621–623. doi: 10.1038/267621a0. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Chouaib S., Fradelizi D. The mechanism of inhibition of human IL 2 production. J Immunol. 1982 Dec;129(6):2463–2468. [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Dougados M., Amor B. Cyclosporin A in rheumatoid arthritis: preliminary clinical results of an open trial. Arthritis Rheum. 1987 Jan;30(1):83–87. doi: 10.1002/art.1780300111. [DOI] [PubMed] [Google Scholar]
- Fidelus R. K., Laughter A. H., Twomey J. J. The role of mitogens and lymphokines in the induction of ornithine decarboxylase (ODC) in T lymphocytes. J Immunol. 1984 Mar;132(3):1462–1465. [PubMed] [Google Scholar]
- Flescher E., Bowlin T. L., Talal N. Polyamine oxidation down-regulates IL-2 production by human peripheral blood mononuclear cells. J Immunol. 1989 Feb 1;142(3):907–912. [PubMed] [Google Scholar]
- Førre O., Bjerkhoel F., Salvesen C. F., Berg K. J., Rugstad H. E., Saelid G., Mellbye O. J., Kåss E. An open, controlled, randomized comparison of cyclosporine and azathioprine in the treatment of rheumatoid arthritis: a preliminary report. Arthritis Rheum. 1987 Jan;30(1):88–92. doi: 10.1002/art.1780300112. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K., Uchida S. T cytotoxic and helper cells are markedly increased, and T suppressor and inducer cells are markedly decreased, in rheumatoid synovial fluids. Arthritis Rheum. 1987 Jul;30(7):737–743. doi: 10.1002/art.1780300703. [DOI] [PubMed] [Google Scholar]
- Hoch S., Schur P. H. Monocyte receptor function in patients with rheumatoid arthritis. Arthritis Rheum. 1981 Oct;24(10):1268–1267. doi: 10.1002/art.1780241006. [DOI] [PubMed] [Google Scholar]
- Huang Y. P., Miescher P. A., Zubler R. H. The interleukin 2 secretion defect in vitro in systemic lupus erythematosus is reversible in rested cultured T cells. J Immunol. 1986 Dec 1;137(11):3515–3520. [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Jahn B., Burmester G. R., Stock P., Rohwer P., Kalden J. R. Functional and phenotypical characterization of activated T cells from intra-articular sites in inflammatory joint diseases. Possible modulation of the CD3 antigen. Scand J Immunol. 1987 Dec;26(6):745–754. doi: 10.1111/j.1365-3083.1987.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Ju S. T., DeKruyff R. H., Dorf M. E. Selective activation of helper and cytolytic T-cell functions of L3T4+ clones with either antireceptor antibody or phorbol ester and ionophore. Cell Immunol. 1987 May;106(2):260–272. doi: 10.1016/0008-8749(87)90170-5. [DOI] [PubMed] [Google Scholar]
- Kanamoto R., Boyle S. M., Oka T., Hayashi S. Molecular mechanisms of the synergistic induction of ornithine decarboxylase by asparagine and glucagon in primary cultured hepatocytes. J Biol Chem. 1987 Oct 25;262(30):14801–14805. [PubMed] [Google Scholar]
- Kay N. E., Douglas S. D. Monocyte metabolic activation in patients with rheumatoid arthritis. Proc Soc Exp Biol Med. 1979 Jul;161(3):303–306. doi: 10.3181/00379727-161-40541. [DOI] [PubMed] [Google Scholar]
- Kluin-Nelemans H. C., van der Linden J. A., Gmelig Meyling F. H., Schuurman H. J. HLA-DR positive T lymphocytes in blood and synovial fluid in rheumatoid arthritis. J Rheumatol. 1984 Jun;11(3):272–276. [PubMed] [Google Scholar]
- Krakauer T. A macrophage-derived factor that inhibits the production and action of interleukin 2. J Leukoc Biol. 1985 Sep;38(3):429–439. doi: 10.1002/jlb.38.3.429. [DOI] [PubMed] [Google Scholar]
- Labib R. S., Tomasi T. B., Jr Enzymatic oxidation of polyamines. Relationship to immunosuppressive properties. Eur J Immunol. 1981 Mar;11(3):266–269. doi: 10.1002/eji.1830110318. [DOI] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Quismorio F. P., Jr, Horwitz D. A. Correction of interleukin-2 production in patients with systemic lupus erythematosus by removal of spontaneously activated suppressor cells. J Clin Invest. 1985 Feb;75(2):762–768. doi: 10.1172/JCI111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Dinarello C. A., Carson D. A., Vaughan J. H. Release of lymphokines after infection with Epstein Barr virus in vitro. II. A monocyte-dependent inhibitor of interleukin 1 downregulates the production of interleukin 2 and interferon-gamma in rheumatoid arthritis. J Immunol. 1986 May 15;136(10):3643–3648. [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Robinson C. A., Dinarello C. A., Carson D. A., Vaughan J. H. Basis for defective responses of rheumatoid arthritis synovial fluid lymphocytes to anti-CD3 (T3) antibodies. J Clin Invest. 1986 Sep;78(3):713–721. doi: 10.1172/JCI112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Kashiwado T., Ziff M. Inhibitor of interleukin-2 in rheumatoid synovial fluid. Arthritis Rheum. 1987 Feb;30(2):121–129. doi: 10.1002/art.1780300201. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Murota N., Yamaoka K., Sato K., Yamada T., Nishido T., Okuda M. Interleukin 2 defect in the peripheral blood and the lung in patients with Sjögren's syndrome. Clin Exp Immunol. 1986 Sep;65(3):497–505. [PMC free article] [PubMed] [Google Scholar]
- Mondovì B., Turini P., Befani O., Sabatini S. Purification of bovine plasma amine oxidase. Methods Enzymol. 1983;94:314–318. doi: 10.1016/s0076-6879(83)94056-9. [DOI] [PubMed] [Google Scholar]
- Partsch G., Desser H., Tausch G. Die Untersuchung der aliphatischen Diamine und des Histamins im Serum von Patienten mit chronischer Polyarthritis. Z Rheumatol. 1978 Sep-Oct;37(9-10):329–334. [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz M., Deimann W., Gram N., Hunstein W., Gemsa D. Characterization of blood mononuclear cells of rheumatoid arthritis patients. I. Depressed lymphocyte proliferation and enhanced prostanoid release from monocytes. Clin Immunol Immunopathol. 1982 Dec;25(3):405–416. doi: 10.1016/0090-1229(82)90205-7. [DOI] [PubMed] [Google Scholar]
- Seyfried C. E., Morris D. R. Methods for the study of the physiological effects of inhibitors of polyamine biosynthesis in mitogen-activated lymphocytes. Methods Enzymol. 1983;94:373–389. doi: 10.1016/s0076-6879(83)94067-3. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Maschler R., Maurer H. R., Allen J. C. Inhibition of cells in culture by polyamines does not depend on the presence of ruminant serum. Cell Tissue Kinet. 1983 May;16(3):269–276. [PubMed] [Google Scholar]
- Staite N. D., Messner R. P., Zoschke D. C. Inhibition of human T lymphocyte E rosette formation by neutrophils and hydrogen peroxide. Differential sensitivity between helper and suppressor T lymphocytes. J Immunol. 1987 Oct 1;139(7):2424–2430. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Thoen J., Førre O., Waalen K., Kåss E. Phenotypes of T lymphocytes from peripheral blood and synovial fluid of patients with rheumatoid arthritis and juvenile rheumatoid arthritis. Evidence in favour of normal helper and suppressor functions of T lymphocytes from patients with juvenile rheumatoid arthritis. Scand J Rheumatol. 1987;16(4):247–256. doi: 10.3109/03009748709102925. [DOI] [PubMed] [Google Scholar]


