Abstract
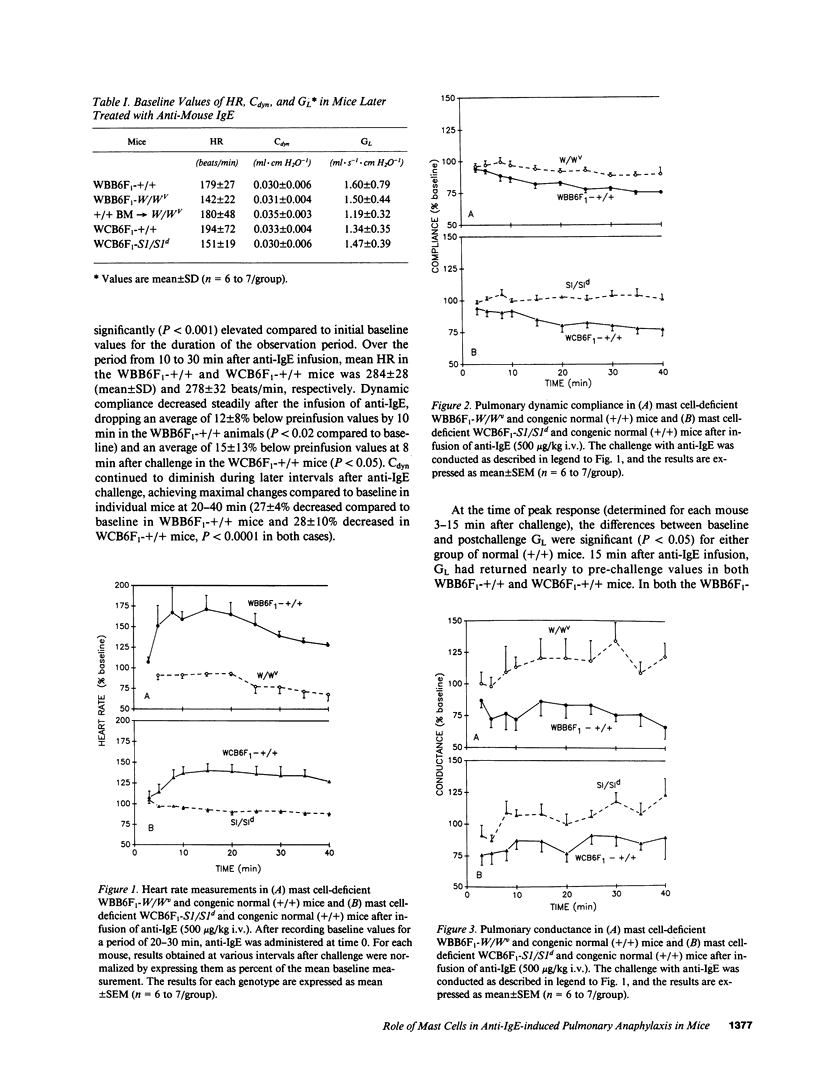
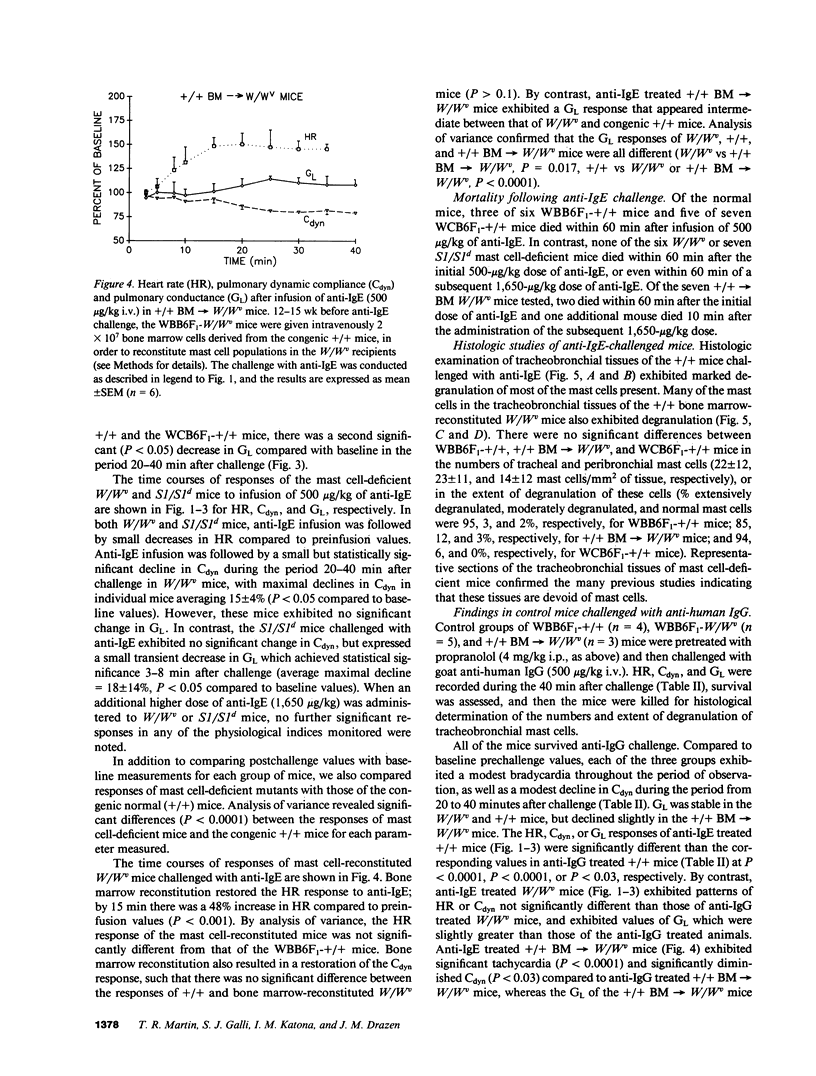
We used genetically mast cell-deficient WBB6F1-W/Wv and WCB6F1-S1/S1d mice and the congenic normal (+/+) mice to investigate the effects of intravenous infusion of goat antimouse IgE on heart rate (HR), pulmonary dynamic compliance (Cdyn), pulmonary conductance (GL), and survival. In WBB6F1-+/+ and WCB6F1-+/+ mice, anti-IgE induced extensive degranulation of tracheobronchial mast cells, as well as significant elevation of HR, significant reductions in Cdyn and GL and, in some cases, death. In contrast, W/Wv and S1/S1d mice exhibited little or no pathophysiological responses and no mortality after challenge with anti-IgE. In W/Wv mice reconstituted with mast cells by intravenous administration of bone marrow cells derived from congenic +/+ mice (+/+ BM----W/Wv mice), anti-IgE induced extensive mast cell degranulation, as well as pathophysiological responses and mortality similar to those observed in WBB6F1-+/+ mice. These findings suggest a critical role for mast cells in the development of the cardiopulmonary changes and mortality associated with anti-IgE-induced anaphylaxis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMDUR M. O., MEAD J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol. 1958 Feb;192(2):364–368. doi: 10.1152/ajplegacy.1958.192.2.364. [DOI] [PubMed] [Google Scholar]
- Barrett K. E., Metcalfe D. D. Heterogeneity of mast cells in the tissues of the respiratory tract and other organ systems. Am Rev Respir Dis. 1987 May;135(5):1190–1195. doi: 10.1164/arrd.1987.135.5.1190. [DOI] [PubMed] [Google Scholar]
- Brown J. K., Leff A. R., Frey M. J., Reed B. R., Lazarus S. C., Shields R., Gold W. M. Characterization of tracheal mast cell reactions in vivo. Inhibition by a beta-adrenergic agonist. Am Rev Respir Dis. 1982 Nov;126(5):842–848. doi: 10.1164/arrd.1982.126.5.842. [DOI] [PubMed] [Google Scholar]
- Casey F. B., Abboa-Offei B. E. Pulmonary function changes in normal rats induced by antibody against rat IgE. Clin Exp Immunol. 1979 Jun;36(3):473–478. [PMC free article] [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Donnell M. Pulmonary mechanics in normal rats. J Appl Physiol Respir Environ Exerc Physiol. 1977 Dec;43(6):942–948. doi: 10.1152/jappl.1977.43.6.942. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Austen K. F. Leukotrienes and airway responses. Am Rev Respir Dis. 1987 Oct;136(4):985–998. doi: 10.1164/ajrccm/136.4.985. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Nabel G., Pyne K., Cantor H., Dvorak H. F., Galli S. J. Ultrastructural identification of the mouse basophil. Blood. 1982 Jun;59(6):1279–1285. [PubMed] [Google Scholar]
- Froese A. Receptors for IgE on mast cells and basophils. Prog Allergy. 1984;34:142–187. [PubMed] [Google Scholar]
- Galli S. J., Arizono N., Murakami T., Dvorak A. M., Fox J. G. Development of large numbers of mast cells at sites of idiopathic chronic dermatitis in genetically mast cell-deficient WBB6F1-W/Wv mice. Blood. 1987 Jun;69(6):1661–1666. [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M. What do mast cells have to do with delayed hypersensitivity? Lab Invest. 1984 Apr;50(4):365–368. [PubMed] [Google Scholar]
- Galli S. J., Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984 Nov 9;226(4675):710–713. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987 Apr;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. New approaches for the analysis of mast cell maturation, heterogeneity, and function. Fed Proc. 1987 Apr;46(5):1906–1914. [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Gold W. M., Meyers G. L., Dain D. S., Miller R. L., Bourne H. R. Changes in airway mast cells and histamine caused by antigen aerosol in allergic dogs. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):271–275. doi: 10.1152/jappl.1977.43.2.271. [DOI] [PubMed] [Google Scholar]
- Guerzon G. M., Paré P. D., Michoud M. C., Hogg J. C. The number and distribution of mast cells in monkey lungs. Am Rev Respir Dis. 1979 Jan;119(1):59–66. doi: 10.1164/arrd.1979.119.1.59. [DOI] [PubMed] [Google Scholar]
- Guirgis H. M., Townley R. G. The effect of pertussis and beta adrenergic-blocking agents on mast cells. J Allergy Clin Immunol. 1976 Aug;58(2):241–249. doi: 10.1016/0091-6749(76)90129-9. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D., Crowle P. K. Immune response potential of mast cell-deficient W/Wv mice. Int Arch Allergy Appl Immunol. 1986;80(1):85–94. doi: 10.1159/000234031. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D. Systemic anaphylaxis in mast-cell-deficient mice of W/Wv and Sl/Sld genotypes. Exp Cell Biol. 1987;55(2):63–68. doi: 10.1159/000163399. [DOI] [PubMed] [Google Scholar]
- Hanley S. P. Prostaglandins and the lung. Lung. 1986;164(2):65–77. doi: 10.1007/BF02713630. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Jacoby W., Cammarata P. V., Findlay S., Pincus S. H. Anaphylaxis in mast cell-deficient mice. J Invest Dermatol. 1984 Oct;83(4):302–304. doi: 10.1111/1523-1747.ep12340431. [DOI] [PubMed] [Google Scholar]
- Katona I. M., Urban J. F., Jr, Scher I., Kanellopoulos-Langevin C., Finkelman F. D. Induction of an IgE response in mice by Nippostrongylus brasiliensis: characterization of lymphoid cells with intracytoplasmic or surface IgE. J Immunol. 1983 Jan;130(1):350–356. [PubMed] [Google Scholar]
- Kitamura Y., Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979 Mar;53(3):492–497. [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- LIMA A. O., VAZ N. M., NETTO M. B., JANINI J. B. DISRUPTION OF TISSUE MAST CELLS IN MICE. Int Arch Allergy Appl Immunol. 1964;25:65–75. doi: 10.1159/000229509. [DOI] [PubMed] [Google Scholar]
- MOTA I. Failure of rat and rabbit antiserum to passively sensitize normal and pertussis-treated rats and mice so as to induce mast cell damage and histamine release on later contact with antigen. Immunology. 1962 Jan;5:11–19. [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Gerard N. P., Galli S. J., Drazen J. M. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol (1985) 1988 Jun;64(6):2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- Mekori Y. A., Chang J. C., Wershil B. K., Galli S. J. Studies of the role of mast cells in contact sensitivity responses. Passive transfer of the reaction into mast cell-deficient mice locally reconstituted with cultured mast cells: effect of reserpine on transfer of the reaction with DNP-specific cloned T cells. Cell Immunol. 1987 Oct 1;109(1):39–52. doi: 10.1016/0008-8749(87)90290-5. [DOI] [PubMed] [Google Scholar]
- Mekori Y. A., Galli S. J. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J Immunol. 1985 Aug;135(2):879–885. [PubMed] [Google Scholar]
- Metcalfe D. D., Kaliner M., Donlon M. A. The mast cell. Crit Rev Immunol. 1981 Sep;3(1):23–74. [PubMed] [Google Scholar]
- Nosál R., Pecivová J., Drábiková K. On the interaction of beta-adrenoceptor-blocking drugs with isolated mast cells. Agents Actions. 1985 Sep;16(6):478–484. doi: 10.1007/BF01983650. [DOI] [PubMed] [Google Scholar]
- Pepys J., Hargreave F. E., Chan M., McCarthy D. S. Inhibitory effects of disodium cromoglycate on allergen-inhalation tests. Lancet. 1968 Jul 20;2(7560):134–137. doi: 10.1016/s0140-6736(68)90419-4. [DOI] [PubMed] [Google Scholar]
- Peters S. P., Schulman E. S., Schleimer R. P., MacGlashan D. W., Jr, Newball H. H., Lichtenstein L. M. Dispersed human lung mast cells. Pharmacologic aspects and comparison with human lung tissue fragments. Am Rev Respir Dis. 1982 Dec;126(6):1034–1039. doi: 10.1164/arrd.1982.126.6.1034. [DOI] [PubMed] [Google Scholar]
- Piper P. J. Formation and actions of leukotrienes. Physiol Rev. 1984 Apr;64(2):744–761. doi: 10.1152/physrev.1984.64.2.744. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Sirois P., Borgeat P. From slow reacting substance of anaphylaxis (SRS-A) to leukotriene D4 (LTD4). Int J Immunopharmacol. 1980;2(4):281–293. doi: 10.1016/0192-0561(80)90028-4. [DOI] [PubMed] [Google Scholar]
- Urbina C., Ortiz C., Hurtado I. A new look at basophils in mice. Int Arch Allergy Appl Immunol. 1981;66(2):158–160. doi: 10.1159/000232814. [DOI] [PubMed] [Google Scholar]
- Wanner A., Abraham W. M. Experimental models of asthma. Lung. 1982;160(5):231–243. doi: 10.1007/BF02719297. [DOI] [PubMed] [Google Scholar]
- Wershil B. K., Murakami T., Galli S. J. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol. 1988 Apr 1;140(7):2356–2360. [PubMed] [Google Scholar]



