Abstract
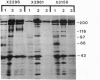
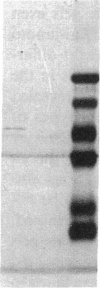
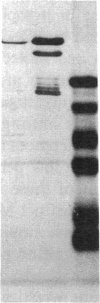
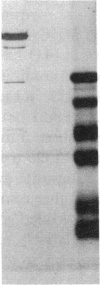
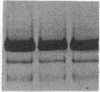
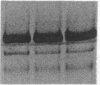
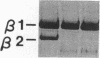
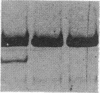
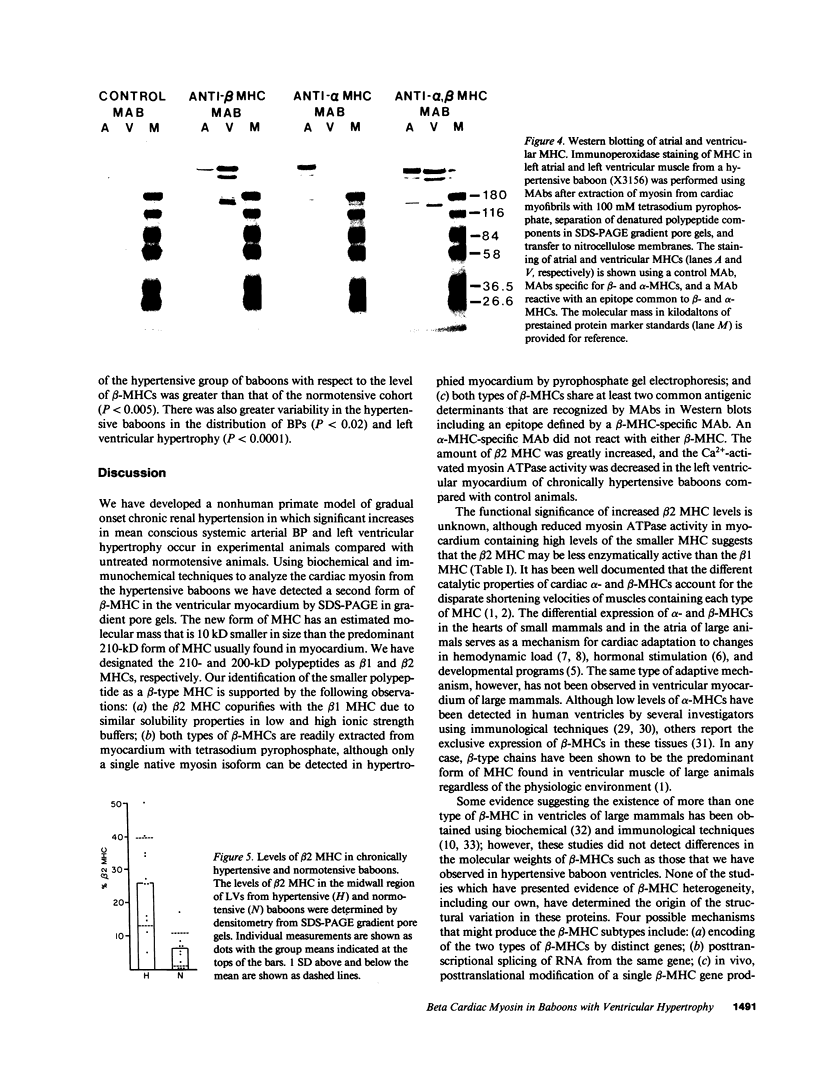
We have identified two distinct beta-myosin heavy chains (MHCs) present in baboon myocardium by electrophoresis in gradient pore gels and by Western blots with anti-MHC MAb. The two beta-MHCs have molecular masses of 210 and 200 kD and share several antigenic determinants including an epitope recognized by a beta-MHC-specific MAb. A fivefold increase in the level of the 200-kD beta-MHC was observed in the hypertrophied left ventricles of baboons with chronic (5.3 +/- 0.7 yr) renal hypertension. A 60% increase (P less than 0.01) in BP and a 100% increase (P less than 0.001) in left ventricular mass to body weight ratio occurred in hypertensive baboons compared with normotensive animals. The Ca2+-activated myosin ATPase activity in hypertrophied left ventricles was decreased by 35% (P less than 0.05) compared with controls. Normal levels of the 200-kD MHC were detected in the right ventricles and intraventricular septa of the hypertensive animals. These data suggest that cardiac MHCs of primates may exist in alternative molecular forms that are indistinguishable by nondenaturing gel electrophoresis and that increased concentration of a second beta-MHC is associated with ventricular hypertrophy (r = 0.55). The functional significance and mechanisms that control the concentration of beta-MHC subspecies remain to be determined.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop V. S., Shade R. E., Haywood J. R., Hamm C. Sinoaortic denervation in the nonhuman primate. Am J Physiol. 1987 Feb;252(2 Pt 2):R294–R298. doi: 10.1152/ajpregu.1987.252.2.R294. [DOI] [PubMed] [Google Scholar]
- Bouvagnet P., Leger J., Pons F., Dechesne C., Leger J. J. Fiber types and myosin types in human atrial and ventricular myocardium. An anatomical description. Circ Res. 1984 Dec;55(6):794–804. doi: 10.1161/01.res.55.6.794. [DOI] [PubMed] [Google Scholar]
- Bouvagnet P., Léger J., Dechesne C. A., Dureau G., Anoal M., Léger J. J. Local changes in myosin types in diseased human atrial myocardium: a quantitative immunofluorescence study. Circulation. 1985 Aug;72(2):272–279. doi: 10.1161/01.cir.72.2.272. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brower M. S., Brakel C. L., Garry K. Immunodetection with streptavidin-acid phosphatase complex on Western blots. Anal Biochem. 1985 Jun;147(2):382–386. doi: 10.1016/0003-2697(85)90286-6. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro U., Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Crawford M. H., Walsh R. A., Cragg D., Freeman G. L., Miller J. Echocardiographic left ventricular mass and function in the hypertensive baboon. Hypertension. 1987 Sep;10(3):339–345. doi: 10.1161/01.hyp.10.3.339. [DOI] [PubMed] [Google Scholar]
- Cummins P. Contractile protein transitions in human cardiac overload: reality and limitations. Eur Heart J. 1984 Dec;5 (Suppl F):119–127. doi: 10.1093/eurheartj/5.suppl_f.119. [DOI] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Dechesne C. A., Léger J., Bouvagnet P., Mairhofer H., Léger J. J. Local diversity of myosin expression in mammalian atrial muscles. Variations depending on age and thyroid state in the rat and the rabbit. Circ Res. 1985 Nov;57(5):767–775. doi: 10.1161/01.res.57.5.767. [DOI] [PubMed] [Google Scholar]
- Dechesne C., Leger J., Bouvagnet P., Claviez M., Leger J. J. Fractionation and characterization of two molecular variants of myosin from adult human atrium. J Mol Cell Cardiol. 1985 Aug;17(8):753–767. doi: 10.1016/s0022-2828(85)80037-7. [DOI] [PubMed] [Google Scholar]
- Flink I. L., Morkin E. Sequence of the 20-kilodalton heavy chain peptide from the carboxyl-terminus of bovine cardiac myosin subfragment-1. J Clin Invest. 1984 Aug;74(2):639–646. doi: 10.1172/JCI111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L., Pauletto P., Pessina A. C., Sartore S., Schiaffino S. Isomyosin distribution in normal and pressure-overloaded rat ventricular myocardium. An immunohistochemical study. Circ Res. 1981 Oct;49(4):1003–1009. doi: 10.1161/01.res.49.4.1003. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Rodgers M. E. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- Henkel R. D., VandeBerg J. L., Walsh R. A. A microassay for ATPase. Anal Biochem. 1988 Mar;169(2):312–318. doi: 10.1016/0003-2697(88)90290-4. [DOI] [PubMed] [Google Scholar]
- Hoh J. F. Light chain distribution of chicken skeletal muscle myosin isoenzymes. FEBS Lett. 1978 Jun 15;90(2):297–300. doi: 10.1016/0014-5793(78)80390-1. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Adelstein R. S. Characterization of myosin heavy chains in cultured aorta smooth muscle cells. A comparative study. J Biol Chem. 1987 May 25;262(15):7282–7288. [PubMed] [Google Scholar]
- Klotz C., Aumont M. C., Leger J. J., Swynghedauw B. Human cardiac myosin ATPase and light subunits. A comparative study. Biochim Biophys Acta. 1975 Apr 29;386(2):461–469. doi: 10.1016/0005-2795(75)90289-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latham R. D., Rubal B. J., Sipkema P., Westerhof N., Virmani R., Robinowitz M., Walsh R. A. Ventricular/vascular coupling and regional arterial dynamics in the chronically hypertensive baboon: correlation with cardiovascular structural adaptation. Circ Res. 1988 Oct;63(4):798–811. doi: 10.1161/01.res.63.4.798. [DOI] [PubMed] [Google Scholar]
- Latham R. D., Rubal B. J., Westerhof N., Sipkema P., Walsh R. A. Nonhuman primate model for regional wave travel and reflections along aortas. Am J Physiol. 1987 Aug;253(2 Pt 2):H299–H306. doi: 10.1152/ajpheart.1987.253.2.H299. [DOI] [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Martin B. J., Low R. B., Alpert N. R. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res. 1982 Jun;50(6):856–864. doi: 10.1161/01.res.50.6.856. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- McGill H. C., Jr, Carey K. D., McMahan C. A., Marinez Y. N., Cooper T. E., Mott G. E., Schwartz C. J. Effects of two forms of hypertension on atherosclerosis in the hyperlipidemic baboon. Arteriosclerosis. 1985 Sep-Oct;5(5):481–493. doi: 10.1161/01.atv.5.5.481. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Bouveret P., Gorza L., Schiaffino S., Clark W. A., Zak R., Swynghedauw B., Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res. 1983 Jul;53(1):52–62. doi: 10.1161/01.res.53.1.52. [DOI] [PubMed] [Google Scholar]
- Morkin E. Stimulation of cardiac myosin adenosine triphosphatase in thyrotoxicosis. Circ Res. 1979 Jan;44(1):1–7. doi: 10.1161/01.res.44.1.1. [DOI] [PubMed] [Google Scholar]
- Pope B., Hoh J. F., Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980 Sep 8;118(2):205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Rovner A. S., Thompson M. M., Murphy R. A. Two different heavy chains are found in smooth muscle myosin. Am J Physiol. 1986 Jun;250(6 Pt 1):C861–C870. doi: 10.1152/ajpcell.1986.250.6.C861. [DOI] [PubMed] [Google Scholar]
- Rushbrook J. I., Stracher A. Comparison of adult, embryonic, and dystrophic myosin heavy chains from chicken muscle by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and peptide mapping. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4331–4334. doi: 10.1073/pnas.76.9.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., Dreizen P. Canine cardiac myosin with special referrence to pressure overload cardiac hypertrophy. I. Subunit composition. J Biol Chem. 1978 Dec 10;253(23):8648–8658. [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H., Kuro-o M., Koyama H., Kurabayashi M., Sugi M., Takaku F., Furuta S., Yazaki Y. Heterogeneity of beta-type myosin isozymes in the human heart and regulational mechanisms in their expression. Immunohistochemical study using monoclonal antibodies. J Clin Invest. 1988 Jan;81(1):110–118. doi: 10.1172/JCI113281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H., Sugi M., Kuro-o M., Ueda S., Takaku F., Furuta S., Shirai T., Yazaki Y. Isozymic changes in myosin of human atrial myocardium induced by overload. Immunohistochemical study using monoclonal antibodies. J Clin Invest. 1984 Aug;74(2):662–665. doi: 10.1172/JCI111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda P. K., Zak R., Rabinowitz M. Purification of messenger ribonucleic acids for fast and slow myosin heavy chains by indirect immunoprecipitation of polysomes from embryonic chick skeletal muscle. Biochemistry. 1980 Apr 29;19(9):1955–1965. doi: 10.1021/bi00550a035. [DOI] [PubMed] [Google Scholar]
- Wisenbaugh T., Allen P., Cooper G., 4th, Holzgrefe H., Beller G., Carabello B. Contractile function, myosin ATPase activity and isozymes in the hypertrophied pig left ventricle after a chronic progressive pressure overload. Circ Res. 1983 Sep;53(3):332–341. doi: 10.1161/01.res.53.3.332. [DOI] [PubMed] [Google Scholar]