Abstract
Relapse to hazardous levels of alcohol consumption following treatment for an alcohol use disorders is common. Investigation of the neurobiological correlates of resumption of hazardous drinking is necessary to clarify the mechanisms contributing to relapse. Fifty-seven treatment-seeking alcohol dependent participants (ALC) completed arterial spin labeling perfusion MRI of the frontal and parietal gray matter (GM) at 7 ± 3 days of abstinence (baseline). ALC participants were restudied after 35 ± 11 days of abstinence (assessment point 2: AP2). Twenty-eight non-smoking, light drinking controls (nsLD) from the community were studied with perfusion MRI. ALC Participants were followed over 12-months after baseline study and were classified as Abstainers (no alcohol consumption; n = 19) and Resumers (any alcohol consumption; n = 38) at follow-up. Cross-sectional and longitudinal perfusion was compared in Abstainers, Resumers and nsLD. At baseline Resumers demonstrated significantly lower frontal and parietal GM perfusion than nsLD and Abstainers. Abstainers and nsLD were not different on frontal or parietal GM perfusion. No significant longitudinal perfusion changes were observed in Abstainers and Resumers. At AP2, Resumers showed significantly lower frontal GM perfusion than nsLD and Abstainers, while no group differences were observed for parietal GM. Abstainers and nsLD were not different on frontal GM perfusion. The significantly decreased frontal GM perfusion in Resumers compared to both Abstainers and nsLD across the assessment interval suggests premorbid and/or acquired neurobiological abnormalities of the frontal GM in Resumers.
Keywords: alcohol dependence, neuroimaging, brain perfusion, relapse, abstinence, treatment outcome
Introduction
Relapse to hazardous levels of alcohol consumption following treatment for alcohol use disorders (i.e., alcohol dependence or alcohol abuse) is common (Donovan, 1996; Monahan and Finney, 1996; Miller et al., 2001; Maisto and Connors, 2006; Dawson et al., 2007) and appears to result from a complex interplay among multiple biopsychosocial factors (Donovan, 1996; Kalivas and Volkow, 2005; Baler and Volkow, 2006; Bradizza et al., 2006; Moos and Moos, 2006; Walter et al., 2006). Historically, research investigating the factors associated with relapse in alcohol use disorders (AUD) has focused on neuropsychological, psychiatric, sociodemographic and behavioral variables [e.g., (Glenn and Parsons, 1991; McKay, 1999; Driessen et al., 2001; Bottlender and Soyka, 2005; Bradizza et al., 2006; Moos and Moos, 2006; Zywiak et al., 2006; Ritvo and Park, 2007; Kodl et al., 2008; Vengeliene et al., 2008)]. A greater understanding of the neurobiological factors associated with the persistence of AUD is necessary to assist in the identification of mechanisms that confer increased vulnerability for the relapse/remit cycle that adversely affects the quality of life of so many with AUD.
Recent advances in in-vivo neuroimaging methods have facilitated investigation of the neurobiological (e.g., morphological, biochemical and/or metabolic) correlates of relapse in AUD (Volkow et al., 2003, 2004; Fowler et al., 2007; Volkow et al., 2008). In treatment seeking AUD, higher brain activation in the putamen, anterior cingulate, and medial prefrontal cortex after approximately two months of abstinence was related to level of alcohol consumption in those who relapsed subsequent to treatment (Grusser et al., 2004). Higher BOLD response (accessed via fMRI) in the thalamus and striatum in response to affectively positive cues were inversely related to drinking days and overall alcohol intake in those who relapsed after treatment (Heinz et al., 2007). In AUD abstinent for approximately 18 days, lower frontal cerebral blood flow was observed in individuals who relapsed relative to those who remained abstinent for approximately two months following treatment (Noel et al., 2002). In AUD initially studied after 1 to 12 week of abstinence (Wrase et al., 2008), those who relapsed within 6 months following treatment demonstrated significantly lower amygdala volume compared to both individuals who abstained over the same interval and controls. Relapsers demonstrated smaller ventral striatal and hippocampal volumes than controls, but were equivalent to abstainers in these regions. Abstainers exhibited a lower volume than controls only in the ventral striatum. In the AUD group as a whole, smaller amygdala volume was correlated with greater alcohol craving, which appeared to be driven by the relapsers. We recently combined multimodality proton magnetic resonance (MR), neurocognitive, psychiatric, and sociodemographic measures to predict outcome following treatment for AUD (Durazzo et al., 2008). Unipolar mood disorders and neurocognitive measures of processing speed, decreased temporal gray matter and frontal white matter levels of N-acetylaspartate [NAA; a surrogate indicator of neuronal integrity (Moffett et al., 2007)], and decreased concentrations of frontal gray matter choline-containing compounds [Cho; marker of cell membrane turnover and/or synthesis (Ross and Bluml, 2001)] were independent predictors of resumption of hazardous levels of alcohol consumption within 12-months following treatment.
Some previous research (Durazzo et al., 2008; Grusser et al., 2004; Noel et al., 2002) investigating the neurobiological and functional correlates of relapse in AUD suggests abnormalities in the frontal lobe. However, all the previous neuroimaging research was cross-sectional in design. Therefore, it is unclear if the baseline findings that distinguished future abstainers from relapsers were persistent over time during early abstinence. Evaluation for neurobiological change in future abstainers and relapsers during short-term abstinence from alcohol is of critical importance, as several MR studies reported changes in brain neurobiology are most prominent in the first 3 months of abstinence (Shear et al., 1994; Pfefferbaum et al., 1995; Agartz et al., 2003; Ende et al., 2005; Gazdzinski et al., 2005; Yeh et al., 2007). The objective of this report was to determine if individuals who ultimately resumed hazardous levels of alcohol consumption (Resumers) in the weeks to months following stabilization/early recovery treatment show differences from those who remained abstinent (Abstainers) in frontal and parietal gray matter (GM) perfusion at: 1) the time of entry into treatment for AUD (baseline), and 2) during short-term abstinence from alcohol (over approximately 5-weeks). Perfusion in both the frontal and parietal lobes were assessed as neurobiological abnormalities in both of these regions are implicated in the development and persistence of alcohol/substance use disorders (Baler and Volkow, 2006; Verdejo-Garcia and Bechara 2009; Kalivas et al., 2008). We predicted: 1) Resumers demonstrate lower cortical perfusion in the frontal and parietal GM than Abstainers and light-drinking controls at baseline and at 5-weeks of abstinence; 2) Resumers show less recovery of frontal and parietal GM perfusion over approximately 5-weeks of monitored abstinence; 3) lower frontal and parietal GM perfusion at baseline, and lower recovery in these regions are related to greater levels of hazardous alcohol consumption in Resumers after treatment.
Methods
Participants
Fifty-seven outpatient participants (three females) were recruited from the San Francisco VA Medical Center (SFVAMC) Substance Abuse Day Hospital and the Kaiser Permanente Chemical Dependence Recovery Program as part of a parent study evaluating the influence of chronic cigarette smoking on neurobiological and neurocognitive recovery from AUD. Non-smoking, light-drinking controls (nsLD; n = 28) were recruited from the local community and screened for any biomedical or psychiatric condition that is known or suspected to influence brain neurobiology or function. At study enrollment, all participants were between 28 and 66 years of age and all alcohol dependent participants met DSM-IV criteria for alcohol dependence. Fifty-seven alcohol dependent participants completed perfusion MRI at 7 ± 3 days after consumption of their last alcoholic drink (baseline). Forty-one alcohol dependent participants were restudied after approximately 35 ± 11 days of abstinence (assessment point 2 = AP2). nsLD participants completed a baseline study and 13 were restudied approximately 6 months later. The vast majority of the alcohol dependent subjects studied also participated in our earlier work (Durazzo et al., 2008). All participants provided their written informed consent prior to study. All study procedures were approved by the University of California San Francisco and the SFVAMC and were accordance with the ethical standards of the Declaration of Helsinki.
Primary inclusion criteria for the alcohol dependent participants were fluency in English, DSM-IV diagnosis of alcohol dependence or alcohol abuse at baseline (all met criteria for alcohol dependence with physiological dependence), consumption of greater than 150 standard alcoholic drinks (i.e., 13.6 grams of ethanol) per month for at least 8 years prior to enrollment for men, and greater than 80 drinks per month for at least 6 years prior to enrollment for women. See Table 1 for group demographic data. Exclusion criteria for alcohol dependent participants were any history of the following: dependence on any substance other than alcohol or nicotine in the years immediately prior to enrollment, any intravenous drug use in the 5 years prior to baseline study, opioid agonist therapy, intrinsic cerebral masses, HIV/AIDS, cerebrovascular accident, cerebral aneurysm, arteriovenous malformations, peripheral vascular disease, myocardial infarction, uncontrolled chronic hypertension (systolic > 180 and/or diastolic > 120 mmHg), type-I diabetes, chronic obstructive pulmonary disease, non-alcohol related seizures, significant exposure to known neurotoxins, demyelinating and neurodegenerative diseases, Wernicke-Korsakoff syndrome, alcohol-induced persisting dementia, penetrating head trauma, and closed head injury resulting in loss of consciousness > 10 minutes. Psychiatric exclusion criteria were history of schizophrenia-spectrum disorders, bipolar disorder, cyclothymia, post-traumatic stress disorder, obsessive-compulsive disorder and panic disorder. Hepatitis C, type-2 diabetes, hypertension, unipolar mood disorder (major depression, substance-induced mood disorder) were allowed in alcohol dependent given their high prevalence in AUD (Parekh and Klag, 2001; Mertens et al., 2003; Mertens et al., 2005; Stinson et al., 2005; Hasin et al., 2007). Participants were urine-tested for illicit substances before all assessments and no participant tested positive for these substances at any assessment.
Table 1.
Group demographic, alcohol and cigarette consumption, mood and anxiety measures at baseline
| Variable | nsLD (n = 28) | Abstainers (n= 19) | Resumers (n = 38) |
|---|---|---|---|
| Age | 45.5 (8.5) | 48.2 (12.2) | 49.9 (7.8) |
| Education | 16.2 (2.6) | 14.2 (2.3) | 13.5 (1.9) |
| Caucasian (%) | 75 | 82 | 80 |
| AMNART | 118 (7) | 115 (9) | 112 (9) |
| 1-year ave drinks/month | 16 (18) | 326 (187) | 433 (188) |
| 3-year ave drinks/month | 16 (18) | 299 (173)* | 396 (162)* |
| 8-year ave drinks/month | 16 (18) | 268 (139) | 348 (142) |
| Lifetime ave drinks/month | 17 (15) | 202 (110)* | 271 (158)* |
| Months of heavy drinking | NA | 224 (112) | 282 (113) |
| Lifetime years of regular drinking | 29 (8) | 35 (10) | 35 (9) |
| Smokers (%) | 0 | 58 | 61 |
| FTND total | NA | 5.5 (1.7) | 5.1 (2.4) |
| Smoking duration | NA | 29 (12) | 28 (15) |
| Cigarette pack years | NA | 29 (18) | 28 (20) |
| Beck Depression Inventory | 4 (4) | 11 (7)* | 16 (10)* |
| STAI-trait | 38 (7) | 48 (10) | 49 (13) |
| Comorbid psychiatric disorder (%) | NA | 33 | 42 |
| Comorbid medical condition (%) | NA | 46 | 49 |
| Comorbid substance abuse disorder (%) | NA | 12 | 18 |
| Body mass index | 25.8 (3.1) | 26.2 (3.8) | 26.5 (3.9) |
| History of previous treatment for AUD (%) | NA | 57 | 51 |
| Number of previous treatments for AUD | NA | 2.4 | 2.3 |
Note. Resumers > Abstainers, p ≤ .05; AMNART: American National Adult Reading Test; FTND: Fagerstrom Test for Nicotine Dependence; NA: not applicable; STAI: State-Trait Anxiety Inventory; Mean (SD).
The stabilization/early recovery outpatient treatment program durations for alcohol dependent participants ranged from 14-30 days. Ninety percent of the longitudinal alcohol dependent participants were in outpatient treatment programs at the San Francisco VA Medical Center or Kaiser Permanente between baseline and AP2. Alcohol dependent participants attended these programs 3-5 days per week and were given weekly drug screens. Breath alcohol levels were acquired randomly or in the case of suspected or obvious intoxication. Chart review indicated no alcohol dependent participant tested positive for illicit/non-prescribed substances or alcohol between baseline and AP2. No participant was positive for breath alcohol or illicit substances at any assessment.
Perfusion MRI Data Acquisition and Processing
Perfusion MRI was acquired at 1.5 Tesla (Vision, Siemens Medical, Inc., Iselin, NJ) with double inversion with proximal labeling of both tagged and control images (DIPLOMA), a pulsed arterial spin labeling (ASL) sequence, which is optimized to measure perfusion in the frontal and parietal GM (Jahng et al., 2003). The sequence was a single-shot gradient-echo echo-planar imaging with five 8 mm thick slices, oriented approximately -5° from the orbital-meatal angle, 2 mm slices gaps, with the most inferior slice located approximately 20 mm above the circle of Willis. The following parameters were employed for the perfusion sequence: 225 × 300 mm2 FOV, 2.5 s TR, 15 ms TE and a 2.3 × 2.3 mm2 in-plane resolution. The time between the tagging pulses and the imaging pulses (i.e., post-labeling delay) was 1500 ms. This perfusion sequence sufficiently compensates for magnetization transfer, untagged blood modulation and arterial transit time, which can substantially influence the accuracy of perfusion measurement (see Gazdzinski et al., 2006).
3D T1-weighted images were also acquired using a standard magnetization prepared rapid gradient echo sequence (TR/TE/TI = 10/7/300 ms, 150 flip angle, 1.0 × 1.0-mm2 in-plane resolution). The T1-weighted images were segmented into probability maps for gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF). An automated process partitioned the structural images into lobes, sub-cortical nuclei, brainstem and cerebellum (Cardenas et al., 2005), which were then overlaid on the segmented images. In a stepwise co-registration process, the perfusion data was then registered to the probabilistically segmented structural images (see Gazdzinski et al., 2006 for details). Atrophy-corrected GM perfusion values from voxels with at least 80% GM were then separately averaged for the frontal and parietal lobes. Finally, the perfusion values were normalized to reflect 100% GM contribution, neglecting average WM contributions of about 5% or less in each of the groups (see Mon et al., 2009). We excluded analysis of WM perfusion as the perfusion sequence was optimized for GM perfusion imaging of the frontal and parietal lobes. In a reliability and reproducibility study of the DIPLOMA ASL technique, the intra-class correlation coefficient was 0.80 for gray matter perfusion measurements in healthy controls studied twice within a 2 hours period (Jahng et al., 2005). For longitudinal analyses of alcohol dependent, three participants were excluded due to poor data quality at AP2, four relapsed between baseline and AP2, and perfusion data was not acquired on 10 participants.
Clinical Measures
At baseline (7 ± 3 days after last drink for alcohol dependent individuals) participants completed the Clinical Interview for DSM-IV Axis I Disorders, Version 2.0 [SCID-I/P (First et al., 1998)], semi-structured interviews for lifetime alcohol consumption [Lifetime Drinking History; (Sobell et al., 1988; Sobell and Sobell, 1992)] and substance use (in-house questionnaire assessing substance type, and quantity and frequency of use)]. From the Lifetime Drinking History, average number of alcoholic drinks per month over 1, 3, and 8 years prior to enrollment, average number of drinks per month over lifetime, lifetime years of regular drinking (i.e., years in which the participant consumed at least one alcoholic drink per month), age of onset and duration of heavy drinking (more than 100 drinks per month in males and 80 drinks per month in females) were calculated. Premorbid verbal intelligence was estimated with the American National Adult Reading Test (Grober and Sliwinski, 1991). All participants completed standardized questionnaires assessing depressive [Beck Depression Inventory, BDI (Beck, 1978)], and anxiety symptomatology [(State-Trait Anxiety Inventory, form Y-2, STAI (Spielberger et al., 1977)]. For smokers, nicotine dependence was assessed via the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991).
Follow-up Assessment for Alcohol Dependent Cohort
Alcohol dependent participants were initially followed for approximately 12 months following the AP2 studies. Follow-up involved at least one face-to-face and/or telephone contact with participants, review of available medical records, and/or telephone interview of collateral sources. Additionally, 34 of 57 participants were re-evaluated 265 ± 56 days after baseline assessment with all MR, psychiatric and behavioral measures administered at the baseline assessment (results not reported here). Alcohol consumption during this interval was evaluated with the Timeline Follow-Back Interview (Sobell and Sobell, 1992), and the quantity/frequency of any other substance use was recorded during follow-up interviews. The disposition of the remaining 23 participants was obtained via brief face-to-face or telephone interview (n = 12), review of medical records (confined to entries from mental health professionals providing outpatient substance abuse treatment for the participant; n = 9), or telephone interview of collateral sources (family or friend; n = 2).
Alcohol dependent participants were designated as Abstainers (n = 19) if they met all of the following criteria: a) self-reported no alcohol consumption between the baseline assessment and follow-up; b) no report of alcohol consumption between the baseline and follow-up in available medical records; and c) available laboratory indicators of alcohol consumption (e.g., gamma glutamyltransferase) were within normal limits at follow-up. Participants were designated as resumers of alcohol consumption (Resumers; n = 38) if they met any of the following criteria: a) self-reported any alcohol consumption at any time between the baseline assessment and follow up via telephone or in-person interview; b) use of alcohol was indicated in medical records by mental health treatment providers; c) report of alcohol use by a relative or close friend of the participant via telephone or in-person interview. To assist in characterizing the severity of the drinking episode(s) for Resumers, the number of participants who met Project MATCH criteria for relapse (i.e., males: ≥ 3 consecutive days of consumption of ≥ 6 drinks per day; females: ≥ 3 consecutive days of consumption of ≥ 4 drinks per day) were identified. These criteria were applied only to those Resumers who had specific quantity/frequency information regarding their drinking episodes after the baseline assessment (see Table 2).
Table 2.
Alcohol consumption characteristics of Resumers following stabilization/early recovery treatment
| Variable | mean (SD) | minimum | maximum |
|---|---|---|---|
| Duration of abstinence (days): | 166 (75) | 24 | 337 |
| Duration of drinking episode(s) (days) | 59 (60) | 3 | 226 |
| Drinks per day during drinking episode(s) | 14 (8) | 3 | 24 |
| Total drinks during drinking episode(s) | 708 (755) | 9 | 3024 |
| Percent consuming ≥ 6 drinks per drinking day | 90 | ||
| Percent meeting Project MATCH relapse Criteria | 90 |
Note. Duration of abstinence: number of consecutive abstinent days from stabilization/early recovery treatment to first drink; Duration of drinking episode(s): total number of days where at least one alcoholic beverage was consumed.
The 19 Abstainers were initially reassessed 210 ± 55 days and the 38 Resumers 233 ± 80 days after the baseline assessment. All 19 of the Abstainers were again successfully re-contacted in person or via telephone after the initial follow-up assessment, at different intervals, to obtain self-reports on drinking status. At the longest follow-up interval, Abstainers self-reported 917 ± 502 days (min = 165, max = 2184) of continuous sobriety following their baseline assessment.
Data Analyses
In cross-sectional analyses, we compared frontal GM and parietal GM perfusion in 28 nsLD, 19 Abstainers and 38 Resumers at baseline and in 28 nsLD, 16 Abstainers and 25 Resumers at AP2 with the generalized linear model. In all cross-sectional analyses, participant age, and percent GM contribution to perfusion voxels were statistically different between nsLD and alcohol dependent; therefore, these factors were used as individual covariates in group comparisons among nsLD, Abstainers and Resumers (see Table 1). Significant omnibus tests were followed up with pairwise t-tests. In cross-sectional analyses at baseline and AP2, normalized perfusion data were used to control for differences in percent GM contribution in perfusion voxels between the nsLD and alcohol dependent groups. Average number of drinks per month over 1, 3 and 8 year prior to enrollment, lifetime average drinks per month, months of heavy drinking and BDI were statistically different or showed trends for differences between Abstainers and Resumers (see Table 1), and were used as covariates in comparisons among these groups in all cross-sectional and longitudinal analyses. Effect sizes for omnibus models were calculated with eta squared (η2), and effect size for pairwise group comparisons was calculated with Cohen's d (Cohen, 1988). Longitudinal frontal and parietal GM perfusion change in Abstainers (n = 16) and Resumers (n = 25) from baseline to AP2 was assessed with a 2-group (Abstainer, Resumer) × 2-assessment point (baseline, AP2) repeated measures analysis via generalized estimating equations. Abstainers and Resumers did not differ in percent GM contribution to perfusion voxels in cross-sectional or longitudinal comparisons. Therefore, to avoid the introduction of additional noise into analyses comparing Abstainers and Resumers, the non-normalized perfusion data was used.
Alpha levels (.05) for all omnibus tests were adjusted for multiple comparisons by the number of regions assessed (i.e., 2) and the correlation among frontal and parietal GM for all groups combined (r = 0.86; adjusted alpha = .045) (see Sankoh et al., 1997). Alpha levels for follow-up pairwise t-tests were adjusted for multiple comparisons according to the number of group comparisons (i.e., 3 for cross-sectional analyses), the number of regions measured (i.e., 2) and correlation for frontal and parietal GM for all groups combined (r = 0.86; adjusted alpha = .039) (see Sankoh et al., 1997). In exploratory analyses, relationships between frontal and parietal GM perfusion and relapse severity variables for Resumers (e.g., duration of relapse, number of drinks consumed during relapse) were examined with Spearman's rho; alpha levels were not adjusted for multiplicity in these exploratory analyses. All analyses were conducted with SPSS v16.
Results
Demographic, Alcohol, and Cigarette Consumption Variables
Seventy-five percent of the nsLD and 81% of the baseline alcohol dependent participants were Caucasian. Of the 57 alcohol dependent participants studied, 33% (n = 19) were Abstainers and 67% (n = 38) were Resumers. Ninety percent of Resumers met Project MATCH criteria for an alcohol relapse. There were no significant differences in indexes of psychosocial functioning (e.g., education, percent gainfully employed) between Abstainers and Resumers at baseline. Abstainers and Resumers were equivalent on age, predicted premorbid verbal intelligence, frequency of smokers, measures of cigarette consumption and length of abstinence at baseline and AP2 (see Table 1). In the baseline cohort, Resumers had significantly higher baseline BDI (p = .03) and greater average number of drinks per month over 3 years prior to enrollment and over lifetime (p = .05). Resumers tended to consume more drinks per month over 1 year, 8 years prior to enrollment and have a greater number of months of heavy drinking than Abstainers (p = .06 to .10) (see Table 1). Table 2 provides alcohol use characteristics for Resumers over the interval between the baseline assessment and follow-up. The above characteristics showed very high correspondence to the longitudinal cohort of Abstainers and Relapsers (n = 41).
Comorbid Psychiatric, Medical, and Substance Use Disorders
Resumers and Abstainers were equivalent on the frequency of comorbid psychiatric (primarily major depression, or substance-induced mood disorder with depressive features), medical (primarily hypertension and hepatitis C) substance use disorders and number of previous treatments for AUD (see Table 1). Abstainers and Resumers were not different on the frequency of biomedical conditions (i.e., hypertension and type II diabetes) that may specifically compromise cerebrovascular function. Resumers and Abstainers were equivalent on baseline and AP2 STAI (see Table 1). Approximately 30% of participants diagnosed with a unipolar mood disorder took an antidepressant medication and approximately 65% percent of participants with hypertension took antihypertensive medications; there were no differences between Resumers and Abstainers in frequency of use of antidepressant or antihypertensive medications.
Baseline Cross-sectional Comparisons of Perfusion in nsLD, Abstainers and Resumers
At, Baseline, significant group differences were observed for perfusion in the frontal [(χ2(3) = 10.51, p = 0.015, η2 = .12] and parietal [(χ2(3) = 9.62, p = 0.022, η 2 = .11] GM. In the frontal GM, Resumers demonstrated significantly lower perfusion than both nsLD (-20.2%; p = .001; Cohen's d effect size (ES) = 0.88) and Abstainers (-16.7%; p = .007; ES = 0.67). For parietal GM, Resumers showed significantly lower perfusion than nsLD (-17.7%; p = .001; ES = 0.79) and Abstainers (-11.4%; p = .034; ES = 0.52). Abstainers and nsLD were not significantly different on frontal or parietal GM perfusion at baseline (see Figure 1). The lower frontal GM perfusion in Resumers compared to Abstainers remained significant after covarying for all measures of alcohol consumption, BDI, and psychiatric, medical (including hypertension and type II diabetes) and substance use comorbidities. Additionally, none of these covariates were significant predictors in models directly comparing frontal and parietal GM perfusion in Resumers and Abstainers.
Figure 1.
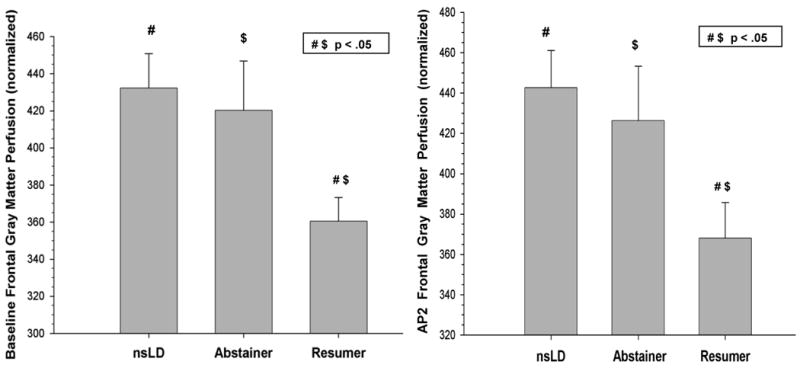
Baseline and AP2 frontal gray matter perfusion for nsLD, Abstainers and Resumers (mean, error bars are standard error)
Longitudinal Perfusion in Abstainers and Resumers
For frontal GM perfusion, a significant main effect was seen for group [(χ2(1) = 5.39, p = 0.020, η2 = .14], where across assessment points Abstainers had higher frontal GM perfusion than Resumers. No significant main effect for assessment point or group-by-assessment point interaction was observed for frontal GM. No significant main effects or interaction was found for parietal GM perfusion. In nsLD, there were no significant longitudinal changes in frontal or parietal GM perfusion over a period of six months (see Mon et al., 2009).
AP2 Cross-sectional Comparisons of Perfusion in nsLD, Abstainers and Resumers
Significant group differences were observed for perfusion in frontal [(χ2(3) = 8.16, p = 0.017, η 2 = .11], with a trend for parietal [(χ2(3) = 6.76, p = 0.080, η2 = .10] GM. For frontal GM, Resumers showed significantly lower perfusion than both nsLD (-20.3%; p = .004; ES = 0.79) and Abstainers (-18.5%; p = .012; ES = 0.71) (see Figure 2). Abstainers and nsLD were not significantly different on frontal GM perfusion at AP2. The lower frontal GM perfusion in Resumers compared to Abstainers at AP2 remained significant after covarying for measures of alcohol consumption, baseline BDI, and psychiatric, medical and substance use comorbidities. Additionally, there was no relationship between length of abstinence and frontal or parietal GM perfusion in the alcohol dependent group as a whole.
Figure 2.
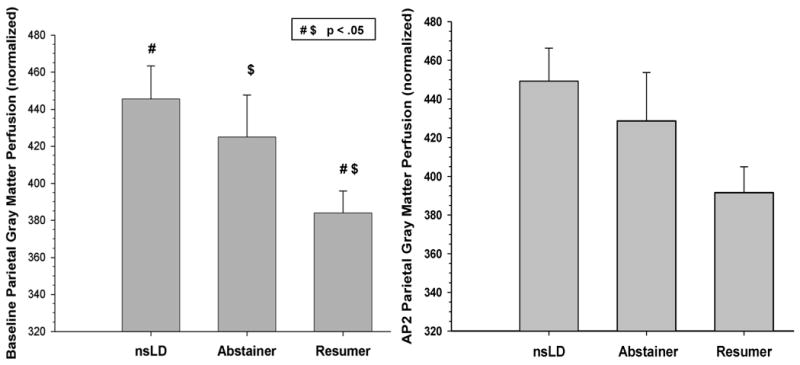
Baseline and AP2 parietal gray matter perfusion for nsLD, Abstainers and Resumers (mean, error bars are standard error)
Associations between Pre-and-Post-Treatment Alcohol Consumption and Perfusion in Resumers
In the alcohol dependent group as a whole, there were no significant correlations between pretreatment/study enrollment measures of drinking severity and perfusion levels at baseline or AP2. In Resumers, a smaller increase of frontal GM perfusion from baseline to AP2 was associated with a greater number of drinks per day during post-treatment relapse (r = -0.56; p = .02). Greater baseline frontal GM perfusion tended to be related to a longer period of sobriety (r = 0.42, p = .08), and greater AP2 frontal GM perfusion tended to be associated with fewer drinks per day during relapse (r = - 0.48, p = .06).
Discussion
To our knowledge, this is the first longitudinal neuroimaging study to investigate the relationships of regional neocortical brain blood flow to resumption of hazardous drinking after early recovery/stabilization treatment for AUD. The primary findings in this sample of predominately Caucasian male alcohol dependent Veterans were as follows: 1) At baseline (7 ± 3 days after last alcohol consumption), Resumers demonstrated lower frontal and parietal GM perfusion than nsLD and Abstainers; 2) No significant changes in frontal and parietal GM perfusion were observed for Abstainers or Resumers over approximately 5-weeks of abstinence; 3) At AP2 (35 ± 11 days after last alcohol consumption), Resumers continued to exhibit lower frontal GM perfusion than both nsLD and Abstainers, while no significant overall group differences were observed for parietal GM perfusion at AP2; 4) Abstainers did not differ from nsLD in frontal and parietal GM perfusion at any assessment point.
The frontal GM perfusion findings from the present study are consistent with Noel and colleagues, (Noel et al., 2002) who reported lower frontal cerebral blood flow (via SPECT) in the middle frontal gyrus at approximately 19 ± 3 days of abstinence in alcohol dependent individuals who relapsed relative to those who remained abstinent for approximately two months following the baseline assessment. Our cross-sectional frontal GM perfusion findings for Resumers are also congruent with our previous report with these participants demonstrating lower frontal GM NAA and Cho and frontal WM NAA in Relapsers versus Abstainers at 5-weeks of abstinence from alcohol (Durazzo et al., 2008).
The significantly lower perfusion levels at baseline and AP2 in Resumers compared to Abstainers were not a function of psychiatric, medical or substance misuse comorbidities and the perfusion differences showed at least moderate effect sizes. Abstainers and Resumers were equivalent on risk factors for peripheral and cerebral vascular disease (e.g., chronic smoking, hypertension, diabetes), and biomedical conditions were not related to perfusion levels. This suggests the significantly lower regional cortical perfusion in Resumers at Baseline and AP2 and the lack of significant change across assessment points for both Abstainers and Resumers were not likely attributable to compromised vascular function. The absence of significant group differences among nsLD, Abstainers and Resumers for parietal GM perfusion at AP2 was likely related to decreased power resultant from the lower number of alcohol dependent participants (n = 41) compared to baseline (n = 57). The effect size for the group factor in the omnibus analysis for AP2 parietal GM was large [see (Cohen, 1988)] and similar to the large group effect sizes observed for TP1 frontal and parietal and AP2 frontal GM perfusion. The follow-up group comparisons for AP2 parietal GM (not reported) also showed the identical pattern as baseline parietal GM perfusion (see Table 2).
Cerebral blood flow and glucose metabolism are tightly coupled (Kuschinsky, 1991; Leybaert, 2005), and, in humans, neuronal metabolism consumes approximately 80% of the total glucose concentration available in the brain under resting conditions (Patel et al., 2004). Therefore, the pattern of significantly decreased frontal GM perfusion in Resumers, compared to both Abstainers and nsLD at baseline and AP2, suggests compromised neuronal viability and/or abnormalities in cellular bioenergetics in Resumers that were persistent in the frontal cortex over approximately 5-weeks of sobriety. For the vast majority of Resumers, the magnitude and duration of their post-treatment drinking was clinically significant, with 90% meeting Project MATCH criteria for relapse. It is noteworthy that Abstainers were not different than nsLD in frontal or parietal GM perfusion at either assessment point, despite long-term hazardous alcohol consumption, and high frequencies of comorbid chronic smoking, unipolar mood disorders and medical conditions. The potential “protective” or mitigating factors associated with the uncompromised perfusion levels in Abstainers were not identified in this study. The lack of differences in perfusion between nsLD and Abstainers along with the absence of longitudinal perfusion changes in Abstainers and Resumers may indicate the abnormalities demonstrated by Resumers predated the onset of their alcohol dependence. Alternately, the perfusion pattern demonstrated by the Resumers may reflect existing premorbid abnormalities in frontal and parietal GM perfusion, which may have been further exacerbated by chronic and excessive alcohol consumption and/or cigarette smoking. However, there were no significant associations with pre-study drinking, smoking variables and perfusion in the alcohol dependent group. Only post-treatment drinking levels in Resumers were related to GM perfusion.
In a preliminary examination of the spatial location of our perfusion voxels, approximately 70% of the frontal GM voxels were distributed across anterior superior mesial (portions of Brodmann areas 8, 9), anterior cingulate cortex (Brodmann areas 24, 35) and dorsolateral (portions of Brodmann areas 8, 9, 46) frontal cortex. These frontal subregions are fundamentally involved in decision-making, problem-solving, impulse control/behavioral inhibition, regulation of mood and affect, craving, evaluation and anticipation of stimulus salience and hedonic valence (Baler and Volkow, 2006; Crews and Boettiger, 2009; Sinha and Li, 2007). The majority of the parietal GM perfusion voxels were spatially located in the superior and inferior parietal lobule. While these association regions of the parietal lobe are not typically considered a part of the brain reward pathway, they are implicated in decision-making and reward processing (Platt and Glimcher, 1999; Paulus et al., 2005; Vincent et al., 2008) as well as in the development and persistence of alcohol/substance use disorders (Verdejo-Garcia and Bechara 2009). Functional neuroimaging studies indicate regions of the posterior parietal lobe are activated during decision-making tasks, such as the Iowa Gambling Task [see (Lin et al., 2008) for review], and performance on this measure is compromised in long-term abstinent alcohol dependent individuals (Fein et al, 2006). Finally, association regions of the parietal lobe are richly interconnected with multiple components of the brain reward system [see (Schmahmann et al., 2007)]. In general, neurobiological abnormalities in both the frontal and parietal lobes are implicated in the persistence of alcohol/substance use disorders and risk of relapse (Baler and Volkow, 2006; Kalivas et al., 2008; Kalivas and O'Brien, 2008; Volkow et al., 2008).
Given that our perfusion sequence was optimized for neocortex, we were unable to assess perfusion levels in white matter and subcortical regions; therefore, it is unknown if the lower perfusion levels observed in Resumers is apparent in other cortical and subcortical regions. Additionally, since the perfusion sequence measured total frontal and parietal GM perfusion, it is unclear if the significantly lower perfusion demonstrated by Resumers showed specific localization within those regions. Other limitations include the reliance on self-report and/or medical records for the determination of drinking status at follow-up in some participants, and the inability to examine sex effects due to the small number of females. It is vital to include comparable number of females in future research to evaluate for the potential influence of sex. Finally, we did not examine the potential influence of coping skills, self-esteem/self-efficacy, social support, neurocognition and personality disorders, which are predictive of relapse after treatment for AUD [e.g., (Miller et al., 1996; Teichner et al., 2001; Bradizza et al., 2006; Krampe et al., 2006; Walter et al., 2006)].
In conclusion, irrespective of the etiology of the perfusion abnormalities observed in Resumers, their baseline abnormalities did not abate with short-term abstinence, which suggest enduring perfusion abnormalities in the frontal cortex over this interval. It is unknown if the Abstainers and Resumers with history of previous treatment showed the same pattern of frontal and parietal GM perfusion at those earlier treatment attempts. It is likely that the magnitude and chronicity of alcohol consumption before and after treatment in this alcohol dependent cohort was influenced by genetic (e.g., Wojnar et al., 2009) and/or other premorbid and environmental factors not assessed in this research. Further longitudinal studies employing additional neuroimaging modalities that examine components of the brain reward pathway (Baler and Volkow, 2006), combined with potential markers of genetic vulnerability (e.g., ApoE genotype, single nucleotide polymorphisms in brain derived neurotrophic factor, dopamine D2 receptors, COMT), and psychosocial variables related to relapse will assist in determining the factors that mitigate against, or increase vulnerability to, the chronic relapse-remit cycle observed in AUD. Study of neurobiological factors associated with the development and maintenance of AUD will provide information that contributes to the improvement of the efficacy of both pharmacological and behavioral interventions for AUD.
Acknowledgments
This material is the result of work supported by NIH AA10788 (DJM) and DA DA24136 (TCD) with resources and the use of facilities at the San Francisco Veterans Administration Medical Center. We thank Mary Rebecca Young, Bill Clift, and Jeanna Eichenbaum of the San Francisco Veterans Administration Substance Abuse Day Hospital and Dr. David Pating, Karen Moise and their colleagues at the San Francisco Kaiser Permanente Chemical Dependency Recovery Program for their valuable assistance in recruiting participants. We also wish to extend our gratitude to the study participants, who made this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, Bergman H. Mr volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol Alcohol. 2003;38:71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11:132–137. doi: 10.1159/000085548. [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev. 2006;26:162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharm Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM. Assessment issues and domains in the prediction of relapse. Addiction. 1996;91(Suppl):S29–36. [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh PH, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol Alcohol. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K. Monitoring the Effects of Chronic Alcohol Consumption and Abstinence on Brain Metabolism: A Longitudinal Proton Magnetic Resonance Spectroscopy Study. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–765. [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clinl Expl Resl. 2006;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision) Biometrics Research Department; New York, NY: 1998. [Google Scholar]
- Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007;3:4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA. Prediction of resumption of drinking in posttreatment alcoholics. Int J Addict. 1991;26:237–254. doi: 10.3109/10826089109053186. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Jahng GH, Zhu XP, Matson GB, Weiner MW, Schuff N. Improved perfusion-weighted MRI by a novel double inversion with proximal labeling of both tagged and control acquisitions. Magn Reson Med. 2003;49:307–314. doi: 10.1002/mrm.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng GH, Song E, Zhu XP, Matson GB, Weiner MW, Schuff N. Human brain: reliability and reproducibility of pulsed arterial spin-labeling perfusion MR imaging. Radiology. 2005;234:909–916. doi: 10.1148/radiol.2343031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kodl MM, Fu SS, Willenbring ML, Gravely A, Nelson DB, Joseph AM. The impact of depressive symptoms on alcohol and cigarette consumption following treatment for alcohol and nicotine dependence. Alcohol Clin Exp Res. 2008;32:92–99. doi: 10.1111/j.1530-0277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Krampe H, Wagner T, Stawicki S, Bartels C, Aust C, Kroener-Herwig B, Kuefner H, Ehrenreich H. Personality disorder and chronicity of addiction as independent outcome predictors in alcoholism treatment. Psychiatr Serv. 2006;57:708–712. doi: 10.1176/ps.2006.57.5.708. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W. Coupling of function, metabolism, and blood flow in the brain. Neurosurg Rev. 1991;14:163–168. doi: 10.1007/BF00310651. [DOI] [PubMed] [Google Scholar]
- Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chiu YC, Cheng CM, Hsieh JC. Brain maps of Iowa gambling task. BMC Neuroscience. 2008 doi: 10.1186/1471-2202-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ. Relapse in the addictive behaviors: integration and future directions. Clin Psychol Rev. 2006;26:229–231. doi: 10.1016/j.cpr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- McKay JR. Studies of factors in relapse to alcohol, drug and nicotine use: a critical review of methodologies and findings. J Stud Alcohol. 1999;60:566–576. doi: 10.15288/jsa.1999.60.566. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Arch Intern Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Miller WR, Westerberg VS, Harris RJ, Tonigan JS. What predicts relapse? Prospective testing of antecedent models. Addiction. 1996;91(Suppl):S155–172. [PubMed] [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ. The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res. 2009;33:1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan SC, Finney JW. Explaining abstinence rates following treatment for alcohol abuse: a quantitative synthesis of patient, research design and treatment effects. Addiction. 1996;91:787–805. doi: 10.1046/j.1360-0443.1996.9167876.x. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Sferrazza R, Van Der Linden M, Paternot J, Verhas M, Hanak C, Pelc I, Verbanck P. Contribution of frontal cerebral blood flow measured by (99m)Tc-Bicisate spect and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002;37:347–354. doi: 10.1093/alcalc/37.4.347. [DOI] [PubMed] [Google Scholar]
- Parekh RS, Klag MJ. Alcohol: role in the development of hypertension and end-stage renal disease. Curr Opin Nephrol Hypertens. 2001;10:385–390. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL, Shulman RG, Behar KL. Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activity. J Cereb Blood Flow Metabol. 2004;24:972–985. doi: 10.1097/01.WCB.0000126234.16188.71. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Neural basis of reward and craving--a homeostatic point of view. Dialogues Clin Neurosci. 2007;9:379–387. doi: 10.31887/DCNS.2007.9.4/mpaulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Ritvo JI, Park C. The psychiatric management of patients with alcohol dependence. Curr Treat Options Neurol. 2007;9:381–392. [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics [published erratum appears in Alcohol Clin Exp Res 1994 Jun;18(3):766] Alcohol Clin Exp Res. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc.; 1992. pp. 41–72. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Self-Evaluation Questionaire. 1977. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. Int J Neurosci. 2001;106:253–263. doi: 10.3109/00207450109149753. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 1:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Duersteler-MacFarland KM, Weijers HG, Boening J, Wiesbeck GA. Social factors but not stress-coping styles predict relapse in detoxified alcoholics. Neuropsychobiology. 2006;54:100–106. doi: 10.1159/000096991. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Sliwerska E, Burmeister M. Association between Val66Met brain-derived neurotrophic factor (BDNF) genepolymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res. 2009;33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, Gazdzinski S, Durazzo TC, Sjostrand K, Meyerhoff DJ. Hierarchical linear modeling (HLM) of longitudinal brain structural and cognitive changes in alcohol-dependent individuals during sobriety. Drug Alcohol Depend. 2007;91:195–204. doi: 10.1016/j.drugalcdep.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Stout RL, Trefry WB, Glasser I, Connors GJ, Maisto SA, Westerberg VS. Alcohol relapse repetition, gender, and predictive validity. J Subst Abuse Treat. 2006;30:349–353. doi: 10.1016/j.jsat.2006.03.004. [DOI] [PubMed] [Google Scholar]


