Abstract
Protein aggregation is a hallmark of many diseases, including amyotrophic lateral sclerosis (ALS), where aggregation of Cu/Zn superoxide dismutase (SOD1) is implicated in causing neurodegeneration. Recent studies have suggested that destabilization and aggregation of the most immature form of SOD1, the disulfide-reduced, unmetallated (apo) protein is particularly important in causing ALS. We report herein in depth analyses of the effects of chemically and structurally diverse ALS-associated mutations on the stability and aggregation of reduced apo SOD1. In contrast with previous studies, we find that various reduced apo SOD1 mutants undergo highly reversible thermal denaturation with little aggregation, enabling quantitative thermodynamic stability analyses. In the absence of ALS-associated mutations, reduced apo SOD1 is marginally stable but predominantly folded. Mutations generally result in slight decreases to substantial increases in the fraction of unfolded protein. Calorimetry, ultracentrifugation, and light scattering show that all mutations enhance aggregation propensity, with the effects varying widely, from subtle increases in most cases, to pronounced formation of 40–100 nm soluble aggregates by A4V, a mutation that is associated with particularly short disease duration. Interestingly, although there is a correlation between observed aggregation and stability, there is minimal to no correlation between observed aggregation, predicted aggregation propensity, and disease characteristics. These findings suggest that reduced apo SOD1 does not play a dominant role in modulating disease. Rather, additional and/or multiple forms of SOD1 and additional biophysical and biological factors are needed to account for the toxicity of mutant SOD1 in ALS.
Keywords: aggregation, protein folding, protein misfolding, SOD oligomer
Mutations in Cu,Zn superoxide dismutase (SOD1) cause familial amyotrophic lateral sclerosis (fALS), a devastating and invariably fatal neurodegenerative disease. Although accounting for only a small percentage of all ALS cases, SOD1 mutations represent one of the main known causes of the disease. The similar symptoms and pathology of familial and sporadic ALS suggest common disease mechanisms and the potential for related therapeutic strategies (1–3). The mechanisms by which mutant SOD1 causes ALS are not known; however, extensive evidence supports a toxic gain of function due to increased aggregation of mutant protein. Misfolding and aggregation of diverse proteins are observed in numerous diseases, including other neurodegenerative diseases such as Alzheimer’s, Huntington, and prion diseases (1, 2). Amyloid is a type of aggregate structure formed by many disease-associated proteins, and perhaps by all proteins, often under destabilizing conditions (4). Although there has been some controversy concerning the amyloid-like nature of large insoluble aggregates in mutant SOD1 mice models of ALS, amyloid aggregates are not observed in ALS patients (5–7). Here, we characterize the formation of small, soluble, nonamyloid aggregates by mutant SOD1.
In its mature form, SOD1 is a highly stable, homodimeric protein, with each subunit binding one catalytic copper ion and one structural zinc ion, and containing one intramolecular disulfide bond as well as two nonconserved free cysteines (Fig. S1A). Numerous in vivo and in vitro studies have shown that various immature, destabilized forms of SOD1 are prone to aggregate, and this is often enhanced by disease-associated mutations (8–16). Recently, attention has focused on aggregation of the most immature form of SOD1, in which the disulfide bond is reduced and no metals are bound (reduced apo). Studies of various mutant-SOD1 ALS mice models have shown that small, soluble, misfolded forms of reduced apo SOD1 are enriched in the spinal cord and may be the common cytotoxic species that cause ALS (17, 18). In addition, cell culture studies suggest that ALS-associated mutations can promote disulfide bond reduction and metal loss (19). Relatively little is known, though, about the properties of reduced apo SOD1, and how mutations affect these properties. In vitro studies have shown that agitation and/or oxidation of reduced apo SOD1 results in the formation of large, insoluble, amyloid aggregates (8, 10). However, the relevance of amyloid formation to ALS is questionable, and recent studies of mutant-SOD1 mice models have shown that formation of aberrant intermolecular disulfide bonds and large insoluble aggregates by SOD1 becomes pronounced only in the final, symptomatic stages of disease (13). There is also extensive evidence that smaller, soluble aggregates are particularly neurotoxic (2). Thus, it is of central importance to elucidate the properties of reduced apo SOD1s, and how these may relate to pathogenic mechanisms.
We report here in depth analyses of the effects of chemically and structurally diverse ALS-associated mutations on the stability and aggregation of reduced apo SOD1, under physiologically relevant quiescent, reducing conditions. The mutations are predominantly destabilizing, causing marked changes in the fraction of protein that is unfolded and increasing the propensity of the protein to form soluble aggregates. However, the formation of these aggregates is not well correlated with disease duration. Although the results suggest that aggregation of reduced apo SOD1 may play some role in disease, they do not support increased aggregation of reduced apo mutants as the dominant determinant of ALS severity. Rather, multiple immature or aberrant forms of SOD1 are implicated in playing important roles in modulating disease.
Results
For most experiments herein, we employed a well-established pseudo WT (pWT) construct in order to facilitate measurements of stability and aggregation of reduced apo SOD1s and avoid complications caused by aberrant disulfide bond formation (8, 14–16, 20–22). In pWT, the nonconserved free cysteines at residues 6 and 111 are replaced by alanine and serine, respectively, whereas the highly conserved cysteines at residues 57 and 146 are retained (Fig. S1A). Cysteines 57 and 146 form a disulfide bond in mature forms of SOD1 but are reduced in the current study. pWT is a suitable background because its activity, structure, and stability are extremely similar to wild type, and use of this background formerly enabled thermodynamic stability analyses for disulfide-oxidized holo and apo SOD1s (15, 16, 23, 24). In various in vivo and in vitro studies, the free cysteines are frequently but not always observed to form aberrant disulfide bonds in aggregates, and they have been suggested to also play subtle roles in modulating noncovalent interactions during aggregation (8, 9, 11–14, 25). These effects were controlled for here by analyzing the properties of mutations relative to the pWT background in the absence of disulfide bond formation. In addition, we conducted some experiments using the WT background containing cysteines 6 and 111; the results obtained are consistent with those obtained using pWT.
All experiments on reduced apo SOD1s were conducted under physiologically relevant conditions of pH (20 mM Hepes, pH 7.4) and protein concentration (∼30–60 μM monomer, 0.5–1.0 mg mL-1) (11, 26), under reducing conditions [1 mM Tris(2-carboxyethyl)phosphine (TCEP)], with no agitation and sample incubation under anaerobic conditions. The reduced status of the protein throughout all experiments was confirmed by iodoacetamide modification of the reduced cysteines followed by SDS-PAGE (Fig. S1B) (11).
In the Absence of ALS-Associated Mutations, Reduced apo SOD1 Unfolds with High Reversibility at Well Above Physiological Temperature.
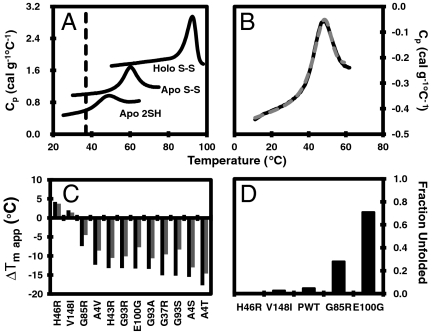
The stability of reduced apo pWT and WT SOD1 were measured by differential scanning calorimetry (DSC) (Figs. 1 A and B, 2D, and Table S1), which shows that the temperature of maximum heat capacity (Cp), tm,app, is ∼48 °C for both constructs. The tm,app for reduced apo SOD1 is markedly lower compared to those for the more mature disulfide-oxidized apo and holo forms (Fig. 1A) (15, 16). However, despite being significantly less stable, reduced apo pWT thermally unfolds with high reversibility, typically ∼95% (Fig. 1B), comparable to the reversibility for disulfide-oxidized forms of SOD1 (15, 23, 24). The reversibility for the WT (Fig. 2D) is somewhat lower, at ∼75%, likely due to the presence of the free thiols that have been shown previously to decrease reversibility due to the formation of aberrant disulfide bonds (27). To minimize inaccuracies due to irreversibility, pWT was used for most of the further analyses.
Fig. 1.
Reversible thermal unfolding of reduced apo pWT SOD1. (A) DSC scans of pWT SOD1 in the reduced apo form in 20 mM Hepes, 1 mM TCEP, pH 7.4 and the disulfide-intact apo and holo forms in 20 mM Hepes, pH 7.8. The dashed black line indicates physiological temperature. (B) Consecutive thermal unfolding traces of disulfide-intact apo pWT SOD1 in which the sample was heated (solid black line), cooled and heated again (dashed gray line). (C) Change in apparent tm of apo SOD1 in the disulfide-oxidized (light shaded bars) and the disulfide-reduced form (dark shaded bars). (D) Fraction of unfolded reduced apo mutant SOD1 at 37 °C, calculated from thermodynamic parameters (Table 1 and SI Text).
Fig. 2.
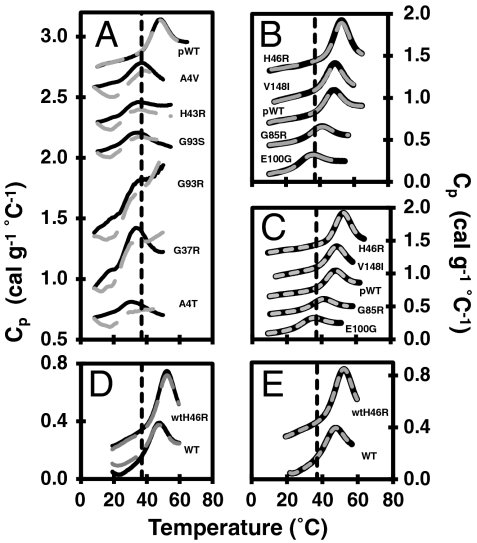
Reversibility and data fitting of reduced apo mutants. The dashed black line indicates physiological temperature. (A) Consecutive thermal unfolding endotherms of reduced apo mutants with low unfolding reversibility and (B) with high unfolding reversibility (scan 1—solid black line; rescan—large dashed gray line). (C) DSC data fitting of the reduced apo mutants and pWT. Typical thermograms (solid black lines) with corresponding two-state monomer fits (small dashed gray lines) are shown. (D) Consecutive thermal unfolding endotherms of WT and wtH46R (scan 1—solid black line; rescan—large dashed gray line). (E) DSC data fitting of WT and wtH46R SOD1. Thermograms (solid black lines) with corresponding two-state monomer fits (small dashed gray lines) are shown. In each panel, the datasets are offset for clarity.
Thermodynamic Analysis Under Physiologically Relevant Conditions Shows Reduced apo SOD1 Undergoes a Monomer Two-State Unfolding Transition and Is Predominantly Folded.
High reversibility of unfolding is a prerequisite for thermodynamic analysis, which has not been reported previously for reduced apo SOD1. In previous studies, we showed that disulfide-oxidized apo and holo pWT SOD1 thermally unfold with high reversibility according to a two-state dimer unfolding mechanism (15); however, reduction or mutation of the disulfide bond in apo SOD1 greatly weakens the dimer interface (26, 28, 29). Measurements of thermal unfolding for reduced apo pWT are consistent with a monomer unfolding transition, showing no systematic shift in tm,app over ∼20-fold range in protein concentration (7–152 μM, 0.1–2.4 mg mL-1) (Fig. S2A). The unfolding data for pWT are well fit using a two-state monomer unfolding model (Table 1 and SI Text), with an average van’t Hoff to calorimetric enthalpy ratio (ΔHvH/ΔHcal) of 1.1 ± 0.2 (Table 1 and Table S1), further confirming the applicability of the two-state monomer model (30). Similar fits are obtained for WT (Fig. 2E, Table 1, and Table S1).
Table 1.
Summary of thermodynamic parameters for reduced apo SOD1s
| apo reduced SOD* | tm, °C | ΔHvH, kcal mol-1 | ΔHvH/ΔHcal | ΔCp, kcal mol-1 °C-1 | ΔG,†,‡, 25 °C, kcal mol-1 | ΔG,† 37 °C, kcal mol-1 | ΔΔG,§37 °C, kcal mol-1 |
| pWT | 47.6 ± 0.5 | 50.5 ± 1.6 | 1.14 ± 0.15 | 0.72 ± 0.57 | 3.5 ± 0.1 4.0 ± 0.2 | 1.8 ± 0.1 | N/A |
| H46R | 52.6 ± 0.5 | 56.1 ± 4.2 | 0.95 ± 0.08 | −0.42 ± 0.84 | 4.9 ± 0.1 5.1 ± 0.1 | 3.1 ± 0.1 | +1.3 |
| V148I | 51.0 ± 1.1 | 58.4 ± 2.6 | 0.93 ± 0.04 | −2.62 ± 1.19 | 3.6 ± 0.1 | 2.2 ± 0.0 | +0.4 |
| G85R | 40.7 ± 0.4 | 46.9 ± 1.8 | 1.06 ± 0.36 | −0.11 ± 0.34 | 2.1 ± 0.1 | 0.6 ± 0.0 | −1.2 |
| E100G | 33.2 ± 1.2 | 47.3 ± 2.0 | 1.27 ± 0.06 | 0.79 ± 0.32 | 1.0 ± 0.1 | −0.6 ± 0.2 | −2.4 |
| WT | 46.8 ± 0.4 | 57.2 ± 1.1 | 1.49 ± 0.27 | 1.01 ± 0.67 | 3.0 ± 0.0 | 1.6 ± 0.0 | N/A |
| wtH46R | 52.7 ± 2.5 | 57.4 ± 5.4 | 0.76 ± 0.22 | −1.59 ± 3.18 | 3.8 ± 0.5 | 2.4 ± 0.4 | +0.8 |
N/A, not applicable.
All values are averages and standard deviations from at least three samples (Table S1), excluding WT and V148I, which are averaged over two samples.
*All mutants are in the pWT background unless otherwise specified.
†Values are calculated using the thermodynamic parameters obtained from the monomer two-state model and a temperature independent ΔCp of 1.1 ± 0.1 kcal mol-1 °C-1 (Fig. 2 B and C).
‡Values in italics are from monomer two-state unfolding fits of equilibrium urea chemical denaturation curves (Fig. S2 D and E).
§ΔΔG = ΔG(mutant)-ΔG(pWT).
Calculation of the temperature dependence of stability requires knowledge of the change in heat capacity upon unfolding, ΔCp (SI Text), which was determined by Kirchoff analysis (31) to be 1.1 ± 0.1 kcal mol-1 °C-1 for reduced apo pWT (Fig. S2 B and C). This value is relatively low compared to that expected for a protein of this size, ∼2 kcal mol-1 °C-1 (32, 33), suggesting that the reduced apo monomer may be less structured than a typical globular protein. The Gibbs free energy of unfolding, ΔG, calculated from the thermodynamic parameters is 3.5 ± 0.1 and 1.8 ± 0.1 kcal mol-1 at 25 °C and 37 °C, respectively (Table 1). Similar values were obtained for WT and are given in Table 1.
An independent measure of ΔG was obtained for pWT using CD-monitored equilibrium urea chemical denaturation and renaturation curves (Fig. S2 D and E and Table 1). These data are also well fit by a two-state monomer unfolding transition, giving a ΔG of 4.0 ± 0.2 kcal mol-1 at 25 °C, which is in reasonable agreement with the value obtained by DSC, and previous chemical denaturation experiments for reduced apo WT at pH 6.3 (29).
Knowledge of ΔG enables calculation of the fraction of protein that is unfolded, fU (SI Text). For pWT at 37 °C, fU is ∼0.05 (Fig. 1D), showing that the protein is predominantly (95%) folded. However, owing to the relatively low value of ΔG, fU is very sensitive to small perturbations in stability caused by mutation, as described below.
DSC Reveals Complex Effects of ALS-Associated Mutations on the Stability and Aggregation Propensity of Reduced apo SOD1.
The effects of chemically and structurally diverse ALS-associated SOD1 mutations on both the disulfide-oxidized and reduced apo forms of the protein were also analyzed by DSC. The mutations include A4V, T and S, and V148I, located in the dimer interface; G37R and H43R, affecting the packing of residues in the beta barrel; metal binding mutants H46R and G85R; G93R, S, A, and D at a mutational hot-spot within a tight turn; and E100G located at the end of strand 6, which eliminates a salt bridge with K30 (Fig. S1A). All of the mutants produced measurable thermograms (Fig. 2 A and B and Table S2), except G93D.
Based on lower tm,app values, the mutants are generally destabilized relative to pWT, except for H46R and V148I, which have slightly increased stabilities (Fig. 1C and Table S2). Furthermore, in the reduced apo form all mutants except H46R, V148I, and G85R have tm,app values at or below 37 °C (Table S2). This is in contrast to the more mature disulfide-oxidized apo form where the mutants all have tm,app values significantly higher than 37 °C (15, 24). Although it is difficult to directly compare the effects of the mutations on the thermodynamic stability of the oxidized to the reduced apo forms due to the change in quaternary structure, it is noteworthy that the changes in melting temperature are larger in the reduced apo form compared to the oxidized apo form (Fig. 1C and Table S2). Similarly, the effects of mutations in nonmetal binding mutants are larger in the oxidized apo forms than in the holo (metallated) forms (15), suggesting that the effects of mutations in SOD1 tend to propagate more as the protein becomes increasingly destabilized and folding becomes less cooperative. Overall, the propensity of most reduced apo SOD1s to misfold/aggregate is evident from the decreased reversibility of thermal unfolding traces (Fig. 2A), which is generally most pronounced in the significantly destabilized mutants.
Nevertheless, the reversibility of thermal unfolding is remarkably high for several mutants: H46R, V148I, G85R, and E100G, enabling thermodynamic analysis using the two-state monomer unfolding model (Fig. 2 B and C, Table 1, and Table S1). Similar results were also obtained for H46R in the WT background (Fig. 2 D and E, Table 1, and Table S1). The stability of pWT and H46R was also measured using chemical denaturation, again giving results consistent with those obtained by DSC (Fig. S2 D and E and Table 1). Using the fitted thermodynamic parameters, fU at 37 °C is calculated to be 0.05, 0.007, 0.03, 0.28, and 0.71 for pWT, H46R, V148I, G85R, and E100G, respectively (Fig. 1D and SI Text). Thus, at physiological temperature the slightly stabilizing H46R and V148I mutants are predominantly folded (in fact, more so than pWT), but the proportion of unfolded protein is markedly increased for the other destabilizing mutants, with E100G being more unfolded than folded. This differs significantly from the effects of the mutations in the disulfide-oxidized apo form where the proteins remain very predominantly folded (Table S2) (24).
Although the thermal unfolding of these reduced apo SOD1s is highly reversible, there is some evidence in the DSC fitted parameters for increased aggregation propensity (15). H46R, G85R, and V148I have relatively low, mostly negative, fitted ΔCps, with average values of -0.42 ± 0.84, -2.62 ± 1.19, and -0.11 ± 0.34 kcal mol-1 °C-1, respectively (Table 1). The negative average ΔCps for H46R and V148I is pronounced and consistent, suggesting the occurrence of exothermic aggregation as these mutants thermally unfold (15). In contrast, E100G does not exhibit unusually low ΔCp values; however, the ΔHvH/ΔHcal ratios tend to be larger than 1 (1.3 ± 0.1 on average), suggesting a larger cooperative unfolding unit, i.e., presence of aggregates (34). Overall, the DSC data are suggestive of subtle increases in aggregation of all mutant SOD1s.
Analytical Ultracentrifugation Shows That Reduced apo SOD1s Are Predominantly Monomeric, and Mutations Slightly Increase Protein–Protein Interactions.
In order to further investigate the tendency of reduced apo SOD1s to aggregate, analytical ultracentrifugation (AUC) sedimentation velocity and equilibrium experiments were performed (SI Text). Sedimentation velocity experiments can assess sample heterogeneity with high sensitivity. Analysis of the velocity data for pWT and H43R revealed species with sedimentation coefficients of 1.5–2 S (Fig. S3), very similar to the values reported previously for reduced apo WT SOD1 (28). The plots of boundary fraction versus sedimentation coefficient show only a modest slope, indicating no significant population of dimers or larger aggregated species for either pWT or H43R in these experiments.
Sedimentation equilibrium experiments at several rotor speeds (20,000, 25,000, 30,000, and 35,000 rpm) were also performed to analyze the molecular weights (MWs) of the species present in solution. Fitting of the equilibrium data for pWT, H43R, A4V, and E100G to a single species model gave highly reproducible results (Table S3). For the pWT protein, the fitted MW at lower rotor speeds is generally close to 15 kDa, just under the calculated mass of ∼15.8 kDa. In contrast to the pWT, the fitted MWs for the A4V and E100G mutants are intermediate between monomer and dimer, whereas fits for the mutant H43R tend to give values closer to what would be expected for a dimer. Additionally, fitted MW values for all mutants markedly decrease with increased rotor speed (Table S3). These results clearly indicate increased intermolecular association of the mutant proteins. Attempts to fit the data to two-state models for monomer/dimer, monomer/trimer, and monomer/tetramer transitions gave poor fits with nonrandom residuals, indicating that the association is likely more complex than a simple two-state process. Overall, the sedimentation equilibrium data indicate that the pWT protein remains predominantly monomeric during the lengthy period required for these studies, but that the mutants have an increased tendency to form small aggregated species. These findings are consistent with the results of the DSC experiments.
Light Scattering Reveals Marked Differences in Aggregation of Mutants upon Prolonged Incubation.
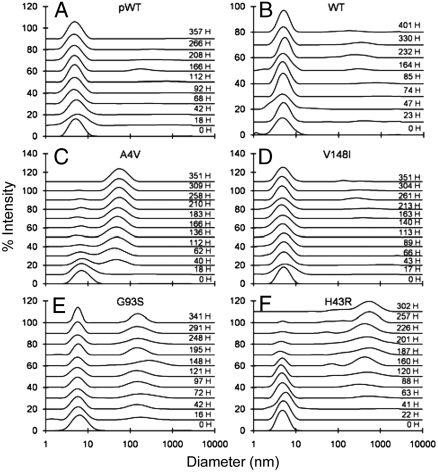
Dynamic light scattering (DLS) was used to monitor the size of particles in solution upon incubating samples at 37 °C (Fig. 3 and Figs. S4 and S5A). It is important to note that light scattering intensity is proportional to the sixth power of the diameter of the scattering particle; thus this technique is extremely sensitive to aggregate formation (35). DLS analyses showed that all reduced apo SOD1 solutions were initially monodisperse, with a single species of hydrodynamic diameter ∼5–6 nm. This diameter is intermediate between those expected for a protein the size of SOD1 in the fully folded and unfolded states (36), consistent with the relatively low ΔCp (see above), and suggesting that the folded reduced apo SOD1 may have an expanded structure.
Fig. 3.
Particle size distributions for reduced apo pWT and mutants at 37 °C, pH 7.4 measured by dynamic light scattering over time, as indicated in hours (H).
Upon prolonged incubation, gradual formation of small amounts of soluble aggregates was observed, with distinct differences between mutants (Fig. 3 and Figs. S4 and S5). At one extreme, H46R and V148I show only very slight evidence for aggregation, with more than 99% of the protein remaining as reduced monomers after ∼300 h of incubation (Fig. 3D and S4D). In contrast, A4V and H43R show the most pronounced evidence of soluble aggregates species (Fig. 3 C and F). A4V forms aggregates with hydrodynamic diameters of 40–60 nm within ∼15 h of incubation, and these approximately double in diameter and increase in abundance over ∼2 weeks. H43R forms 100–1,000 nm species within ∼60 h, which also increase in abundance with time. For both mutants, at long incubation times the larger species dominate the scattering and the soluble monomers can no longer be observed. However, the total intensity of scattered light continues to increase, indicating continued aggregation (Fig. S5B). In general, the extent of aggregation of different mutants, as shown by the prominence of large species in the size distributions (Fig. 3 and Figs. S4 and S5A), is consistent with the extent of aggregation as indicated by total light scattering intensity (Fig. S5 B–D). Both observations give similar indications of the relative aggregation propensities of different mutants. It should be noted, however, that lack of observation of the monomer peak does not indicate a predominantly aggregated sample. If one considers a hypothetical mixture containing only 5- and 50-nm species, due to the dependence of light scattering intensity on the sixth power of the diameter, when 99% of the intensity arises from the 50-nm species, this species will account for only 0.1% by mass of the total protein in solution (35). Therefore, the DLS data indicate only slight to moderate formation of soluble aggregates by reduced apo SOD1 variants, consistent with the DSC and centrifugation data.
Discussion
The biophysical analyses conducted here show that ALS-associated mutations have the most pronounced effects on stability in the reduced apo form of SOD1 and enhance the formation of soluble aggregates. These represent unique in-depth analyses of reduced apo SOD1 stability and aggregation, and they have important implications for understanding mechanisms of SOD1 aggregation that may be involved in ALS, considered further below.
In the Absence of ALS-Associated Mutations, Reduced apo SOD1 Is Predominantly Folded and Has Low Aggregation Propensity Under Physiologically Relevant Conditions.
The unfolding of pWT measured here by DSC and chemical denaturation is well fit by a reversible two-state monomer unfolding transition, based on multiple DSC and chemical denaturation criteria (Fig. 2C, Fig. S2 D and E, and Table 1), and comparable results are obtained by DSC for WT (Fig. 2E and Table 1). These results reveal that the ΔG of unfolding for reduced apo SOD1 at 37 °C, which has not been reported previously, is 1.8 ± 0.1 kcal mol-1 for pWT and 1.6 ± 0.0 kcal mol-1 for WT. Thus, at physiological temperature and pH, the protein is predominantly folded and shows very little tendency to aggregate.
The very minimal aggregation observed here for monomeric reduced apo pWT and WT is particularly noteworthy given that previous studies have reported monomerization and loss of metals greatly enhance, or are required for, aggregation (8, 37). Moreover, several studies have reported observations of amyloid formation by apo SOD1 in which the intramolecular disulfide bond was reduced or removed by mutagenesis (8, 10, 14). A key difference between these and the current studies is their use of agitation rather than quiescent solution conditions. It is well established that agitation promotes the aggregation of many proteins, often as amyloid (8, 38, 39). This is not well understood but likely involves interface effects and perhaps also accelerated oxidation of free thiols.
The relevance of the formation of amyloid aggregates in previous studies of reduced apo SOD1 to ALS disease mechanisms is not clear. Other forms of SOD1 have also been shown previously to form amyloid under destabilizing conditions caused by denaturant, sonication, trifluoroethanol, or low pH (8, 14, 20, 40), and formation of intermolecular disulfide bonds (25). In contrast, other studies under less extreme conditions have also reported evidence for distinct aggregation processes from native-like states (41–43). Protein aggregation is generally strongly dependent on solution conditions, and many destabilizing and often nonphysiological conditions can result in the formation of amyloid. In this regard, it should be noted that the amyloid-specific characteristic of green–gold Congo red birefringence and ThT binding of aggregates is not observed in ALS (5, 7) and the intracellular SOD1-containing aggregates in fALS have a granule-coated rather than the smooth fibrillar structure characteristic of amyloid (6); thus, ALS is not a typical amyloid disease.
A key aspect for in vitro studies of aggregation is to consider their relation to in vivo conditions. Here we have used physiologically relevant conditions of temperature, pH, protein concentration, and quiescence. Importantly, the very minimal aggregation of reduced apo pWT and WT is consistent with cell culture and mice studies where wild-type SOD1 shows very little tendency to aggregate and mice do not develop ALS symptoms (13, 17). This differs from observations for mutant SOD1s, which tend to aggregate more than WT in cell culture and form small aggregated species in mice prior to the onset of symptoms followed by large disulfide-linked aggregates in the final stages of disease (12, 13). In contrast, in previous in vitro studies the comparable wild-type-like constructs not only formed amyloid (8, 10, 14) but in some cases this was more pronounced than for ALS-associated mutants (10). This suggests fundamentally different aggregation processes are being observed under different conditions.
ALS-Associated Mutations Have Complex Effects on Stability and Aggregation.
Under the physiologically relevant conditions used herein, we were able to measure the effects of many chemically and structurally diverse ALS-associated mutations on stability and aggregation propensity. The effects on stability range from slightly stabilizing to slightly or significantly destabilizing (Fig. 1C, Table 1, and Table S2). Consistent with previous studies on apo SOD1 where metal binding mutations had relatively small effects on tm,app (44), the metal binding mutants H46R and G85R are among the most stable mutants studied here. In the disulfide-oxidized apo form, all the mutants have tm,app values well above physiological temperature; however, in the reduced apo form, most have tm,app values close to or lower than 37 °C, indicating that they will be 50% or more unfolded at physiological temperature (Fig. 1D and Table S2). The observation that decreases in melting temperatures tend to be largest in the reduced apo form implies that substantial increases in the population of unfolded conformations will also occur for many other mutants that have been found to have decreased tm,app in the disulfide-oxidized apo form (15, 24, 44). Thus, overall, many but not all ALS-associated mutations are likely to significantly increase the population of reduced apo unfolded monomers.
Regardless of stability, the DSC, AUC, and DLS experiments indicate that the reduced apo mutants generally have increased propensity to misfold/aggregate. In particular, DLS results indicate that distinct sizes of small, soluble aggregates are observed for different mutants (Fig. 3 and Figs. S4 and S5A). Evidence for structural polymorphism of SOD1 aggregates was also reported for agitation-induced aggregation (26). These findings are intriguing as variations in aggregate structures may cause different disease phenotypes.
Limited Correlations Between the Properties of Reduced apo Mutant SOD1s and ALS Characteristics Implicate Multiple Forms of SOD1 in Modulating Disease.
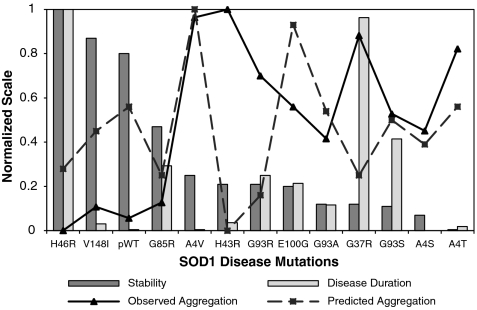
Correlations between the properties of mutant SOD1 and ALS disease characteristics have been sought for many years and are critical for deciphering disease mechanisms. Previous studies have reported evidence for a weak inverse correlation between oxidized apo SOD1 stability and ALS disease duration (45–47), which improves when global or local protein characteristics such as charge (48) or hydrogen bonding (45) are considered. The results for V148I suggest that increased hydrophobicity of the exposed dimer interface may be another significant modulator of aggregation. A weak correlation is observed between reduced apo mutant SOD1 stability and disease duration (Fig. 4 and Fig. S6A), suggesting that the effects of the mutations on the stability of reduced apo SOD1 do not play a more significant role than their effects in oxidized apo in determining disease duration. This implies that factors beyond stability, and multiple forms of SOD1, are important in modulating disease duration.
Fig. 4.
A correlation plot representing the relationship between reduced apo SOD1 mutant stability, fALS disease durations, and observed and predicted aggregation. The stability is determined by a change in apparent tm of mutants compared to pWT and normalized, from 0 (least stable) to 1 (most stable). Observed aggregation is based on DLS measurements as described in Fig. S5A. Predicted aggregation based on the Chiti et al. method (52) was normalized from 0 (lowest propensity) to 1 (highest propensity). Disease duration (47) is normalized from 0 (short) to 1 (long).
There is a significant correlation (r = 0.78, Fig. 4 and Fig. S6B) between observed aggregation and mutant destabilization, consistent with results of general studies of protein aggregation. This has also been observed for more mature forms of SOD1 that tend to aggregate more readily when destabilized (10, 11, 15, 16). The aggregation observed here is poorly correlated with nine different aggregation prediction models (Fig. S6E and SI Text). The lack of correlations may be because most of these prediction algorithms were developed based on datasets of amyloid-forming proteins and peptides. As noted above, amyloid formation may differ significantly from the formation of the soluble, nonamyloid aggregates that are characterized here. There is also no significant correlation between observed aggregation and ALS disease duration (Fig. 4 and Fig. S6C). Furthermore, the aggregation propensities of the SOD1 mutants predicted using the preceding methods are also poorly correlated with disease duration (Fig. S6D).
Consideration of these correlations points to two key conclusions: Neither the association of reduced apo SOD1 mutants into small soluble aggregates nor the predicted aggregation propensities of SOD1 mutants are able to account for fALS disease duration. These key findings have two important implications: (i) Multiple forms of SOD1 are likely to modulate disease characteristics and (ii) amyloid formation is likely not an important factor in SOD1-associated fALS. Further support for the first point is evidence that mutations enhance the population and aggregation of various immature forms of mutant SOD1, and the observation of multiple forms of SOD1 in aggregates in vivo (1, 13, 15, 16, 21, 41, 42, 49). The second point is further supported by evidence that fALS patient data fail to reveal any support for a role of amyloid in disease (7).
In conclusion, the results reported here provide unique and important data on the stability and aggregate formation by reduced apo SOD1s, which should prove useful for further testing of ALS disease hypotheses. The increased aggregation of reduced apo SOD1 upon mutation suggests that this form of the protein may play a role in causing disease. However, the lack of strong correlations between reduced apo SOD1 stability and disease duration and between measured aggregation and disease duration imply that the effects of mutations on reduced apo SOD1 are unlikely to be the dominant factor in modulating disease, and that multiple forms of the protein are involved. Unravelling the complex aggregation processes that are likely to contribute to the syndrome of ALS (50) may ultimately lead to new and urgently needed approaches for treating this devastating disease.
Materials and Methods
Expression and Purification of Mutant SOD1.
Disulfide-oxidized apo SOD1 proteins were prepared as described previously (24, 51). Reduced apo SOD1 was prepared by first unfolding the protein in 2 M GdmCl, 20 mM Hepes, pH 7.8 for 30 min at ambient temperature with degassing. Tris(2-carboxyethyl)phosphine hydrochloride (TCEP.HCl) was then added to a final concentration of 10 mM with reduction occurring in an anaerobic environment for 1 h. Finally, samples were exchanged into buffer containing 1 mM TCEP.HCl, 20 mM Hepes pH 7.4 by successive dilutions and reconcentrations using a 3-kDa cutoff Nanosep centrifugal device (Pall Corporation).
Differential Scanning Calorimetry.
DSC scans of apo SOD1 samples were performed as described elsewhere (15). After subtraction of buffer versus buffer scans from protein versus buffer scans, disulfide-reduced apo SOD1 data were fit to a two-state monomer unfolding model after normalizing for protein concentration (SI Text).
Light Scattering Measurements.
Time average dynamic light scattering measurements were performed using a Zetasizer Nano ZS (Malvern Instruments Ltd.). Particle size was determined from an average of three correlation functions, each being the average of five consecutive 10-s data accumulations. Particle size was analyzed by the CONTIN method using Malvern software. Samples were initially measured daily and then at increasing time intervals.
Supplementary Material
Acknowledgments.
We are grateful to Gian Tartaglia for assistance in using Zyggregator and Joost Schymkowitz for helpful correspondence regarding the Tango and Waltz algorithm. We thank the ALS Society of Canada, Muscular Dystrophy Canada, and Canadian institutes of Health Research for funding this research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913021108/-/DCSupplemental.
References
- 1.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 3.Boillee S, Vande Velde C, Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Fandrich M, et al. Myoglobin forms amyloid fibrils by association of unfolded polypeptide segments. Proc Natl Acad Sci USA. 2003;100:15463–15468. doi: 10.1073/pnas.0303758100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y. New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett. 1991;129:233–236. doi: 10.1016/0304-3940(91)90469-a. [DOI] [PubMed] [Google Scholar]
- 6.Kato S, et al. New consensus research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 (SOD1) gene mutations: Inclusions containing SOD1 in neurons and astrocytes. Amyotroph Lateral Sc. 2000;1:163–184. doi: 10.1080/14660820050515160. [DOI] [PubMed] [Google Scholar]
- 7.Kerman A, et al. Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol. 2010;119:335–344. doi: 10.1007/s00401-010-0646-5. [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay M, et al. Initiation and elongation in fibrillation of ALS-linked superoxide dismutase. Proc Natl Acad Sci USA. 2008;105:18663–18668. doi: 10.1073/pnas.0807058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cozzolino M, et al. Cysteine 111 affects aggregation and cytotoxicity of mutant Cu,Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:866–874. doi: 10.1074/jbc.M705657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa Y, Kaneko K, Yamanaka K, O’Halloran TV, Nukina N. Complete loss of post-translational modifications triggers fibrillar aggregation of SOD1 in the familial form of amyotrophic lateral sclerosis. J Biol Chem. 2008;283:24167–24176. doi: 10.1074/jbc.M802083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furukawa Y, O’Halloran TV. Amyotrophic lateral sclerosis mutations have the greatest destabilizing effect on the apo- and reduced form of SOD1, leading to unfolding and oxidative aggregation. J Biol Chem. 2005;280:17266–17274. doi: 10.1074/jbc.M500482200. [DOI] [PubMed] [Google Scholar]
- 12.Karch CM, Borchelt DR. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:13528–13537. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karch CM, Prudencio M, Winkler DD, Hart PJ, Borchelt DR. Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS. Proc Natl Acad Sci USA. 2009;106:7774–7779. doi: 10.1073/pnas.0902505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oztug Durer ZA, et al. Loss of metal ions, disulfide reduction and mutations related to familial ALS promote formation of amyloid-like aggregates from superoxide dismutase. PLoS ONE. 2009;4:e5004. doi: 10.1371/journal.pone.0005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stathopulos PB, et al. Calorimetric analysis of thermodynamic stability and aggregation for apo and holo amyotrophic lateral sclerosis-associated Gly-93 mutants of superoxide dismutase. J Biol Chem. 2006;281:6184–6193. doi: 10.1074/jbc.M509496200. [DOI] [PubMed] [Google Scholar]
- 16.Stathopulos PB, et al. Cu/Zn superoxide dismutase mutants associated with amyotrophic lateral sclerosis show enhanced formation of aggregates in vitro. Proc Natl Acad Sci USA. 2003;100:7021–7026. doi: 10.1073/pnas.1237797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, et al. Progressive aggregation despite chaperone associations of a mutant SOD1-YFP in transgenic mice that develop ALS. Proc Natl Acad Sci USA. 2009;106:1392–1397. doi: 10.1073/pnas.0813045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zetterstrom P, et al. Soluble misfolded subfractions of mutant superoxide dismutase-1 s are enriched in spinal cords throughout life in murine ALS models. Proc Natl Acad Sci USA. 2007;104:14157–14162. doi: 10.1073/pnas.0700477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari A, Hayward LJ. Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J Biol Chem. 2003;278:5984–5992. doi: 10.1074/jbc.M210419200. [DOI] [PubMed] [Google Scholar]
- 20.DiDonato M, et al. ALS mutants of human superoxide dismutase form fibrous aggregates via framework destabilization. J Mol Biol. 2003;332:601–615. doi: 10.1016/s0022-2836(03)00889-1. [DOI] [PubMed] [Google Scholar]
- 21.Valentine JS, Doucette PA, Potter SZ. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 22.Svensson AK, Bilsel O, Kondrashkina E, Zitzewitz JA, Matthews CR. Mapping the folding free energy surface for metal-free human Cu,Zn superoxide dismutase. J Mol Biol. 2006;364:1084–1102. doi: 10.1016/j.jmb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Rumfeldt JA, Stathopulos PB, Chakrabarrty A, Lepock JR, Meiering EM. Mechanism and thermodynamics of guanidinium chloride-induced denaturation of ALS-associated mutant Cu,Zn superoxide dismutases. J Mol Biol. 2006;355:106–123. doi: 10.1016/j.jmb.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 24.Vassall KA, Stathopulos PB, Rumfeldt JA, Lepock JR, Meiering EM. Equilibrium thermodynamic analysis of amyotrophic lateral sclerosis-associated mutant apo Cu,Zn superoxide dismutases. Biochemistry. 2006;45:7366–7379. doi: 10.1021/bi0600953. [DOI] [PubMed] [Google Scholar]
- 25.Banci L, et al. Metal-free superoxide dismutase forms soluble oligomers under physiological conditions: A possible general mechanism for familial ALS. Proc Natl Acad Sci USA. 2007;104:11263–11267. doi: 10.1073/pnas.0704307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnesano F, et al. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 27.Lepock JR, Frey HE, Hallewell RA. Contribution of conformational stability and reversibility of unfolding to the increased thermostability of human and bovine superoxide dismutase mutated at free cysteines. J Biol Chem. 1990;265:21612–21618. [PubMed] [Google Scholar]
- 28.Doucette PA, et al. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J Biol Chem. 2004;279:54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg MJ, Normark J, Holmgren A, Oliveberg M. Folding of human superoxide dismutase: Disulfide reduction prevents dimerization and produces marginally stable monomers. Proc Natl Acad Sci USA. 2004;101:15893–15898. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturtevant JM. Biochemical applications of differential scanning calorimetry. Annu Rev Phys Chem. 1987;38:463–488. [Google Scholar]
- 31.Privalov PL. Stability of proteins: Small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- 32.Geierhaas CD, Nickson AA, Lindorff-Larsen K, Clarke J, Vendruscolo M. BPPred: A Web-based computational tool for predicting biophysical parameters of proteins. Protein Sci. 2007;16:125–134. doi: 10.1110/ps.062383807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers JK, Pace CN, Scholtz JM. Denaturant m values and heat capacity changes: Relation to changes in accessible surface areas of protein unfolding. Protein Sci. 1995;4:2138–2148. doi: 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Privalov PL, Potekhin SA. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986;131:4–51. doi: 10.1016/0076-6879(86)31033-4. [DOI] [PubMed] [Google Scholar]
- 35.Lomakin A, Benedek GB, Teplow DB. Monitoring protein assembly using quasielastic light scattering spectroscopy. Methods Enzymol. 1999;309:429–459. doi: 10.1016/s0076-6879(99)09029-1. [DOI] [PubMed] [Google Scholar]
- 36.Wilkins DK, et al. Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR techniques. Biochemistry. 1999;38:16424–16431. doi: 10.1021/bi991765q. [DOI] [PubMed] [Google Scholar]
- 37.Khare SD, Caplow M, Dokholyan NV. The rate and equilibrium constants for a multistep reaction sequence for the aggregation of superoxide dismutase in amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2004;101:15094–15099. doi: 10.1073/pnas.0406650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiese S, Papppenberger A, Friess W, Mahler HC. Shaken, not stirred: Mechanical stress testing of an IgG1 antibody. J Pharm Sci. 2008;97:4347–4366. doi: 10.1002/jps.21328. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, et al. Thiol compounds inhibit the formation of amyloid fibrils by beta 2-microglobulin at neutral pH. J Mol Biol. 2008;376:258–268. doi: 10.1016/j.jmb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Stathopulos PB, et al. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2004;13:3017–3027. doi: 10.1110/ps.04831804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elam JS, et al. Amyloid-like filaments and water-filled nanotubes formed by SOD1 mutant proteins linked to familial ALS. Nat Struct Biol. 2003;10:461–467. doi: 10.1038/nsb935. [DOI] [PubMed] [Google Scholar]
- 42.Banci L, et al. Fully metallated S134N Cu,Zn-superoxide dismutase displays abnormal mobility and intermolecular contacts in solution. J Biol Chem. 2005;280:35815–35821. doi: 10.1074/jbc.M506637200. [DOI] [PubMed] [Google Scholar]
- 43.Hwang YM, et al. Non-amyloid aggregates arising from mature Cu/Zn superoxide dismutases resemble those observed in amyotrophic lateral sclerosis. J Biol Chem. 2010;285:41701–41711. doi: 10.1074/jbc.M110.113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez JA, et al. Destabilization of apoprotein is insufficient to explain Cu,Zn-superoxide dismutase-linked ALS pathogenesis. Proc Natl Acad Sci USA. 2005;102:10516–10521. doi: 10.1073/pnas.0502515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bystrom R, Andersen PM, Grobner G, Oliveberg M. SOD1 mutations targeting surface hydrogen bonds promote amyotrophic lateral sclerosis without reducing apo-state stability. J Biol Chem. 2010;285:19544–19552. doi: 10.1074/jbc.M109.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindberg MJ, Bystrom R, Boknas N, Andersen PM, Oliveberg M. Systematically perturbed folding patterns of amyotrophic lateral sclerosis (ALS)-associated SOD1 mutants. Proc Natl Acad Sci USA. 2005;102:9754–9759. doi: 10.1073/pnas.0501957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q, Johnson JL, Agar NY, Agar JN. Protein aggregation and protein instability govern familial amyotrophic lateral sclerosis patient survival. PLoS Biol. 2008;6:e170. doi: 10.1371/journal.pbio.0060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandelin E, Nordlund A, Andersen PM, Marklund SS, Oliveberg M. Amyotrophic lateral sclerosis-associated copper/zinc superoxide dismutase mutations preferentially reduce the repulsive charge of the proteins. J Biol Chem. 2007;282:21230–21236. doi: 10.1074/jbc.M700765200. [DOI] [PubMed] [Google Scholar]
- 49.Hayward LJ, et al. Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2002;277:15923–15931. doi: 10.1074/jbc.M112087200. [DOI] [PubMed] [Google Scholar]
- 50.Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8:94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- 51.Lynch SM, Boswell SA, Colon W. Kinetic stability of Cu/Zn superoxide dismutase is dependent on its metal ligands: Implications for ALS. Biochemistry. 2004;43:16525–16531. doi: 10.1021/bi048831v. [DOI] [PubMed] [Google Scholar]
- 52.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–808. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.