Abstract
TREK channels produce background currents that regulate cell excitability. These channels are sensitive to a wide variety of stimuli including polyunsaturated fatty acids (PUFAs), phospholipids, mechanical stretch, and intracellular acidification. They are inhibited by neurotransmitters, hormones, and pharmacological agents such as the antidepressant fluoxetine. TREK1 knockout mice have impaired PUFA-mediated neuroprotection to ischemia, reduced sensitivity to volatile anesthetics, altered perception of pain, and a depression-resistant phenotype. Here, we investigate TREK1 regulation by Gq-coupled receptors (GqPCR) and phospholipids. Several reports indicate that the C-terminal domain of TREK1 is a key regulatory domain. We developed a fluorescent-based technique that monitors the plasma membrane association of the C terminus of TREK1 in real time. Our fluorescence and functional experiments link the modulation of TREK1 channel function by internal pH, phospholipid, and GqPCRs to TREK1–C-terminal domain association to the plasma membrane, where increased association results in greater activity. In keeping with this relation, inhibition of TREK1 current by fluoxetine is found to be accompanied by dissociation of the C-terminal domain from the membrane.
Keywords: ion channel; two-pore-domain K+; phosphatidylinositol-4,5-biphosphate; Prozac
TREK1 is a two-pore-domain K+ (K2P) channel that produces a nearly time- and voltage-independent background current. This current drives the membrane potential toward the K+ equilibrium potential and thus affects input resistance. TREK1 displays low basal activity when expressed alone (1) but can be strongly stimulated by temperature (2), mechanical stretch (3), external alkalization (4), intracellular acidification (5), polyunsaturated fatty acids (PUFAs) (6), lysophospholipids (7), phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] (8, 9), and pharmacological agents such as volatile anesthetics (10) and riluzole (11). TREK1 is inhibited by neurotransmitters and hormones that activate Gq and Gs pathways (3, 12–14) and pharmacological agents such as the antidepressant drug fluoxetine (15). TREK1 gene inactivation produces mice with decreased sensitivity to volatile anesthetics, impaired PUFA-mediated neuroprotection (16), and altered perception of pain (17). These mice also display a depression-resistant phenotype (18). This phenotype is in agreement with the sensitivity of TREK1 to fluoxetine, a widely used antidepressant drug.
In the last decade, much effort has been made to elucidate the gating mechanism of the TREK1 channel. The cytosolic carboxyl-terminal domain of TREK1 that follows its fourth transmembrane domain (post-M4) has been implicated in its function and regulation. A glutamate residue, E306, has been shown to be a key element for stimulation by intracellular acidification (19) and a cluster of basic residues in the same region has been shown to be involved in TREK1 regulation by phospholipid (8). However, the mechanism of regulation of the channel by Gq-coupled receptors (GqPCR) and by pharmacological agents such as fluoxetine remains unclear.
In this study, we developed a fluorescence-based technique to address regulation of TREK1 by GqPCRs and fluoxetine by monitoring in real time the association of an EGFP-tagged TREK1 C-terminal domain with the plasma membrane. We investigated the roles of key residues in the TREK1 C terminus and found that mutants with increased or decreased channel activity have, respectively, increased or decreased membrane association. Moreover, GqPCR inhibition of TREK1 channel activity was found to be accompanied by C-terminal domain dissociation from the membrane. This regulatory effect was found to be independent of PKC but to depend on the breakdown of PI(4,5)P2. Strikingly, the inhibitory effect of fluoxetine was also attributed to C-terminal domain dissociation from the membrane, providing both insight into the molecular mechanism of this modulation and a possible route for identifying novel antidepressants.
Results
Monitoring Membrane Association of GFP-Fusion Proteins.
Several classes of ion channels and transporters have been shown to be regulated by lipids, most commonly by PI(4,5)P2, which is located in the inner leaflet of the plasma membrane. Because the modulation by PI(4,5)P2 requires binding, it is expected that the regulatory domain on the channel or transporter will associate with the membrane. To relate PI(4,5)P2 binding to the regulation of function of TREK1 channels, we devised a membrane association assay for the C-terminal domain of the channel, which has been shown to be the key regulatory domain for phospholipid sensing by TREK1.
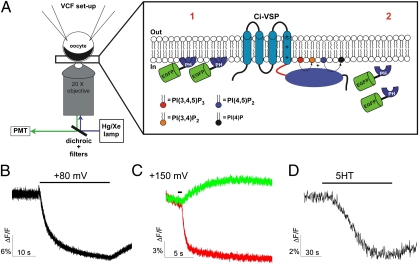
The animal pole of Xenopus laevis oocytes contains a dark pigment layer that separates the yolk from the plasma membrane, thus shielding autofluorescence from the yolk of the oocyte and allowing for direct imaging of fluorescent proteins near the plasma membrane (Fig. 1A). This property makes oocytes an excellent system to monitor fluorescence dynamics at the plasma membrane without optical sectioning or imaging, but with a simple epifluorescence microscope (20). To validate this approach for fluorescently tagged proteins whose membrane association is regulated, we coexpressed an EGFP-tagged pleckstrin homology (PH) domain from phospholipase C-δ1 (EGFP-PHPLC) with the Ciona intestinalis voltage sensing phosphatase (Ci-VSP), a voltage-dependent phosphoinositide phosphatase (21). Fluorescently tagged PHPLC domains have been widely used as optical reporters of PI(4,5)P2 dynamics in mammalian cells (22). The cytosolic EGFP-PHPLC protein could be seen at the plasma membrane when PI(4,5)P2 was at resting levels. (Fig. 1A). Upon membrane depolarization, Ci-VSP hydrolysis of PI(4,5)P2 to PI(4)P (23) triggered loss of EGFP-PHPLC fluorescence (Fig. 1B), consistent with a decrease of membrane PI(4,5)P2 and consequent EGFP-PHPLC dissociation from the membrane. In contrast, the membrane fluorescence increased for an EGFP-tagged PH domain of yeast oxysterol binding protein homolog (EGFP-PHOSH1), a protein that binds PI(4)P (24) (Fig. 1C), consistent with conversion of PI(4,5)P2 to PIP(4) by Ci-VSP. Indeed, PI(4,5)P2 depletion and PI(4)P production could be detected in a single cell coexpressing tagRFP-PHPLC and EGFP-PHOSH1 (Fig. 1C).
Fig. 1.
Development of cell-based assay to study cell membrane binding of GFP-fusion proteins. (A) Measuring membrane binding of GFP protein. Xenopus oocytes expressing the protein of interest are voltage clamped. The fluorescence of the EGFP-fusion protein is recorded by epifluorescence (1). The cortical layer of pigment granules in the animal hemisphere and the opaque cytoplasmic compartment provide a mask for cytoplasmic fluorescence (2). When the fusion proteins lose their interaction with the plasma membrane, the fusion proteins diffuse into the cell, inducing a decrease of fluorescence. (B–D) PH-PLC domain assay. Representative example of EFGP-PHPLC fluorescence decrease induced by Ci-VSP activation (30-s pulse from −80 to +80 mV) (D) and 5HT2cR activation (C) in Xenopus oocyte. (C) Representative example of tagRFP-PHPLC (red) fluorescence decrease and EFGP-PHOSH1 fluorescence increase (green) induced by Ci-VSP activation (200-ms pulse from −80 to +150 mV).
We extended this technique to see whether we can track changes in phospholipids induced by activation of G protein coupled receptors (GPCRs). We coexpressed EGFP-PHPLC with the 5HT2c receptor, a GPCR coupled to the Gq α-subunit of G protein (i.e., a GqPCR), whose receptor activation induces the hydrolysis of PI(4,5)P2 to DAG (25). As expected, activation of the 5HT2c receptor decreased EGFP-PHPLC fluorescence at the plasma membrane (Fig. 1D). Success of this simple translocation assay for known membrane-interacting proteins encouraged us to use the approach to study the membrane association of the regulatory TREK1 C-terminal domain.
TREK Channel Activity Is Correlated with Membrane Association of the C Terminus.
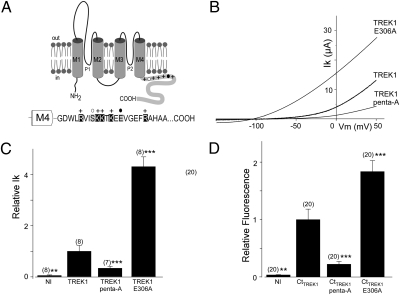
The membrane proximal C-terminal domain (CtTREK1), has been shown to be the key regulatory domain for phospholipid and internal pH sensing (Fig. 2A) (8, 19). We set out to use the methods detailed above to measure the plasma membrane association of the isolated CtTREK1 to relate the membrane interaction to the functional state of the channel. To do this, we engineered an EGFP-CtTREK1 fusion protein in which EGFP was fused at the amino-terminal end of the full-length C-terminal domain, immediately before Gly293. Expression of EGFP-CtTREK1 in oocytes resulted in a strong fluorescence (Fig. 2D), indicating the presence of this protein at the plasma membrane. Mutation of five basic residues in the post-M4 domain (Fig. 2A) has been shown to decrease TREK1 channel activity and to remove phospholipid sensitivity (8). We neutralized these basic residues by substituting each of them with alanine (TREK1-penta-A) in both the full-length channel and in the EGFP-tagged C terminus. In the full-length channel, the five-residue neutralization resulted in a large (3.0 ± 0.2-fold) decrease of current (Fig. 2 B and C). In the C-terminal fragment (EGFP-CtTREK1-penta-A), the five-residue neutralization produced a large (4.0 ± 0.2-fold) decrease in fluorescence at the plasma membrane (Fig. 2D).
Fig. 2.
The current amplitude of TREK1 mutants are correlated with the membrane fluorescence of the corresponding EGFP-CtTREK1 mutants. (A) Membrane topology of TREK1. The sequence of the cytoplasmic C terminus of TREK1 starting at Gly293 is indicated. +, basic residues (R297, K301, K302, K304, and R311); open circles, PKC (S300) phosphorylation site; and dark circle, pH sensor site (E306). (B) Currents were elicited by voltage ramps (from −150 to +50 mV, 1 s in duration). (C) Normalized amplitude of noninjected oocytes (NI), TREK1-WT, TREK1-Penta-A, and TREK1-E306. (D) Normalized membrane fluorescence level of oocytes expressing EGFP-CtTREK1, EGFP-CtTREK1-penta-A, and EGFP-CtTREK1-E306A. Student's t test (**P < 0.01, ***P < 0.001) show the difference between TREK1 and TREK1 mutants (or EGFP-CtTREK1 and EGFP-CtTREK1 mutants). The numbers of cells tested are indicated in parentheses.
We wondered whether the relation between level of current and C-terminus membrane localization was accidental or causal. We therefore turned to another mutant of the channel that is known to have the opposite effect on activity. The mutation we chose was of glutamate 306 to alanine (E306A). E306 is located in the post-M4 region of CtTREK1, embedded in the region containing the five basic residues that we mutated above (Fig. 2A). This mutation has been proposed to mimic internal acidification and has been shown to induce a large increase in the current (19). Indeed, we found that E306A current was 4.3 ± 0.4-fold larger than wild type (Fig. 2 B and C). We found that EGFP-CtTREK1-E306A was higher in basal membrane fluorescence by 1.9 ± 0.2-fold compared with wild-type EGFP-CtTREK1 (Fig. 2D).
The differences in basal membrane fluorescence between EGFP-CtTREK1, EGFP-CtTREK1-penta-A, and EGFP-CtTREK1-E306A could be due to differences in membrane targeting, but they could also be due to differences in protein expression levels. Because the inside of the oocyte is opaque and the yolk highly fluorescent, we could not image the EGFP-tagged TREK1 C-terminal domain in the oocyte interior. We therefore turned to transient expression in HEK cells to distinguish between these possibilities. The cells were fixed with either paraformaldehyde (PFA), to fix all of the proteins throughout the cell, or else they were fixed with ice-cold ethanol, in which case only membrane-associated proteins are fixed to the membrane proteins and unbound proteins leak out of the cells (26). Although the PFA fixation showed that the proteins were expressed at comparable levels (Fig. S1B), the fluorescence in the ethanol fixation was 2.6 ± 0.6-fold lower for EGFP-CtTREK1-penta-A than for the wild-type EGFP-CtTREK1, and EGFP-CtTREK1-E306A was more fluorescent by 1.4 ± 0.1-fold compared with wild-type EGFP-CtTREK1 (Fig. S1 A and B). Thus, the C-terminal domains of the two mutants express at the same level, but associate to different degrees with the membrane in agreement with the basal fluorescence measurements in oocytes.
We next asked whether the effect of the mutations on current amplitude in oocytes could be attributed to differences in membrane targeting of the full-length channel. We quantified the expression levels of the EGFP-tagged full-length channel for the wild type and the two mutations by injecting the same amount of RNA at low enough levels to space channels far enough apart to be counted as individual spots in total internal reflection fluorescence (TIRF) microscopy (27, 28). We found the expression levels of the wild-type and mutant channels to be the same (Fig. S1 C and D).
Thus, the effect of the mutants could be attributed to changes in C-terminus membrane association and channel activity, and an increase in plasma membrane binding (E306A) is associated with increased current, whereas a decrease in membrane binding (penta-A) is associated with decreased current.
GqPCRs Regulate TREK1 Current and C-Terminus Membrane Association.
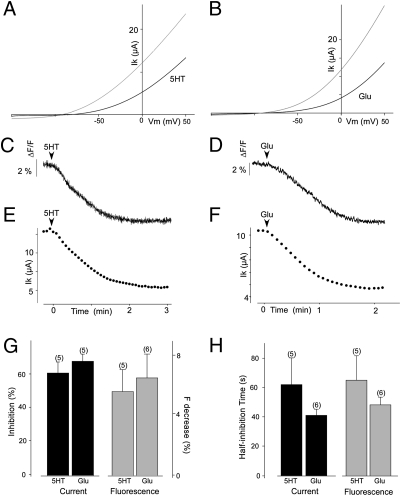
Having observed a correlation between C-terminus membrane interaction and channel activity, we asked whether modulation of the former could underlie the known modulation of TREK1 channel activity by GqPCRs (8, 12). As observed earlier for GqPCRs, we found that activation of either the serotonin 5HT2c receptor (5HT2cR) or of the group I metabotropic glutamate receptor, mGluR1a, inhibits TREK1 current (Fig. 3 A, B, and G). Moreover, we found that activation of 5HT2cR or mGluR1 also induced a decrease of EGFP-CtTREK1 membrane fluorescence (Fig. 3G). The kinetics of GqPCR-induced inhibition of current in the full-length channel were similar to the kinetics of GqPCR-induced loss of EGFP-CtTREK1 membrane fluorescence, as seen in representative traces (Fig. 3 C and E for 5HT2cR and D and F for mGluR1) and in a comparison of half current inhibition times with half fluorescence decrease times from multiple cells (Fig. 3H). These results suggest that the inhibition of TREK1 channel activity by GqPCR activation occurs by uncoupling of CtTREK1 from the membrane.
Fig. 3.
Gq-coupled receptor activation regulates TREK1 currents and membrane association of EGFP-CtTREK1 constructs. (A and B) Effect of activation of 5HTR2bR (A) or mGluRI on TREK1 expressed in Xenopus oocytes. Currents were elicited by voltage ramp (from −150 to +50 mV, 1 s in duration). (C and D) Representative example of EGFP-CtTREK1 fluorescence decrease induced by 5HT2cR (C) and mGluRI (D) in oocytes. (E and F) Representative example of TREK1 current inhibition induced by 5HT2cR (E) and mGluRI (F) in oocytes. Effect of 5HT2cR and mGluRI activation on TREK1 current and on membrane fluorescence of EGFP-CtTREK1. (G) Percentage of inhibition (black) or of fluorescence decrease (gray) induced by 5HT2cR and mGluRI activation. Inhibitions and fluorescence decreases are statistically significant (P < 0.001) for both 5HT2cR and mGluRI. (H) Half current inhibition time (black) or the half decrease time of fluorescence induced by 5HT2cR and mGluRI activation (gray). The numbers of cells tested are indicated in parentheses.
PI(4,5)P2 Depletion Underlies the GqPCR Inhibition of TREK1 Activity.
We next investigated the molecular mechanism underlying GqPCR inhibition of TREK1 channels. GqPCR activation initiates complex signaling pathways, including the activation of phospholipase C, which hydrolyzes PI(4,5)P2 to form the secondary messengers diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3), the latter releasing Ca2+ from internal stores. The mechanism underlying the inhibition of TREK1 by GqPCRs is controversial. TREK1 has been reported to be directly activated by PI(4,5)P2 so that hydrolysis of PI(4,5)P2 would account for channel inhibition (8, 9). Alternatively, activation of protein kinase C (PKC) by elevated Ca2+ following release from internal stores has been proposed to lead to TREK1 inhibition due to phosphorylation (14) of S300, a serine residue located in the post-M4 region (1), although this effect has been questioned (8, 9).
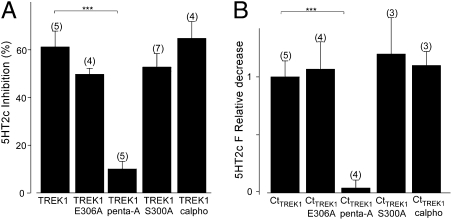
To address this issue, we examined several TREK1 point mutants. First we tested the cluster of five basic residues in the C-terminal domain that was proposed to be involved in PI(4,5)P2 sensitivity (8). Neutralization of these residues to alanine (penta-A), which we showed above to inhibit basal current and reduce basal membrane association of the C terminus (Fig. 2 C and D), was found to suppress both the inhibitory effect of 5HT2cR activation on the current of the full-length channel (Fig. 4A) and the 5HT2cR-induced decrease in membrane fluorescence of the EGFP-tagged C terminus (Fig. 4B). In contrast, mutation of S300 to alanine (S300A) to mimic the unphosphorylated state and prevent phosphorylation did not affect either the 5HT2cR-induced inhibition of channel current or reduction of C-terminal membrane association (Fig. 4 A and B). Moreover, overnight preincubation of oocytes with 5 μM of the PKC inhibitor calphostatin C had no effect on the 5HT2cR-induced inhibition of current or fluorescence decrease of EGFP-CtTREK1. To explore the specificity of the disruption in modulation by 5HT2cR, we also tested the neutralization of glutamate 306 (E306A), the site implicated in acid sensing (19), which we showed above to augment basal channel activity and C-terminus membrane association (Fig. 2 C and D). This mutation did not alter the 5HT2cR-induced inhibition of channel current and reduction of C-terminus membrane association (Fig. 4 A and B).
Fig. 4.
Point mutations in C-terminal domain regulate TREK1 response to GPCR activation. (A) Percentage of current inhibition by 5HT2cR activation for TREK1-WT, TREK1-Penta-A, TREK1-E306, TREK1-S300A, and TREK1-WT preincubated with calphostatin C. (B) Relative fluorescence decrease induced by 5HT2cR activation for EGFP-CtTREK1 WT, EGFP-CtTREK1-Penta-A, EGFP-CtTREK1-E306, EGFP-CtTREK1-S300A, and TREK1-WT preincubated with calphostatin C (overnight 5 μM). The asterisks indicate the significance (P < 0.05) of the difference between TREK1 mutants and TREK1-WT (or EGFP-CtTREK1 mutants and EGFP-CtTREK1). Student's t test: ***P < 0.001. The numbers of cells tested are indicated in parentheses.
Taken together, these results show the inhibition of channel activity by 5HT2cR depends specifically on the post-M4 polybasic motif and not on either PKC activation or on a general sensitivity to mutations in the post-M4 region. This suggests that the key to GqPCR modulation of TREK1 is PI(4,5)P2 breakdown and consequent loss of membrane interaction of the TREK1 C-terminal polybasic motif. We tested this notion further in the next set of experiments.
Is TREK1 Directly Regulated by PI(4,5)P2 in the Cell?
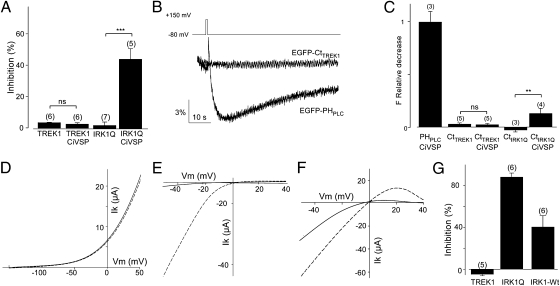
Quenching the charge of membrane phospholipids by polycationic agents such as polylysine reduces TREK1 current (8, 9), and PI(4,5)P2 application to the intracellular surface restores TREK1 channel activity (8). Therefore, it has been proposed that PI(4,5)P2 is a direct and specific regulator of TREK1 channels (8). To test this model, we used two inducible enzymatic systems that specifically deplete PI(4,5)P2, but which, unlike GqPCR activation, do not generate other signaling lipids such as DAG and IP3. First, we used Ci-VSP to hydrolyze PI(4,5)P2 to PI(4)P (Fig. 1 A–C). We found that, although Ci-VSP activation by membrane depolarization inhibited the PI(4,5)P2-dependent IRK1Q current, as shown earlier (29, 30), it did not affect TREK1 current (Fig. 5A). In keeping with the selective inhibition of IRK1Q, the activation of Ci-VSP decreased the membrane fluorescence of the C terminus of IRK1Q, but not of TREK1.
Fig. 5.
TREK1 is not directly regulated by PI(4,5)P2. (A) TREK1 current inhibition induced by the activation of Ci-VSP by a prepulse to +150 mV (200 ms). Currents were measured 15 s after the prepulse. (B) Representative examples of EGFP-CtTREK1 and EFGP-PHPLC fluorescence decrease induced by Ci-VSP activation (pulse at +150 mV from −80 mV, 200-ms duration). (C) Relative fluorescence decrease of EFGP-PHPLC and EGFP-CtTREK1 and EGFP-CtIRK-R228Q induced by Ci-VSP. (D–G) PI(4,5)P2 depletion by rapamycin-inducible 5-phosphatase [PI(4,5)P2→ PI(4)P] failed to regulate TREK current. (D–F) Effect of activation of 5-phosphatase by rapamycin (5 μM) on TREK1 (D), IRK1-R228Q (E), and IRK1-WT (F). Currents before (dashed lines) and after rapamycin (solid lines) were elicited by voltage ramps (from −50 to +50 mV, 1 s in duration). (G) Inhibition induced by PI(4,5)P2 depletion by the rapamycin system on TREK1, IRK1-R228Q, and IRK1-WT. Student's t test: **P < 0.01, ***P < 0.001. The numbers of cells tested are indicated in parentheses.
In a second approach, we used a chemically inducible PI(4,5)P2 depletion system based on the rapamycin-induced translocation of a phosphoinositide 5-phosphatase enzyme to the plasma membrane, to evoke the rapid hydrolysis of (PI(4,5)P2 to PI(4)P) (31, 32). Whereas TREK1 was not inhibited by the application of 5 μM of rapamycin, IRK1Q current was strongly inhibited (Fig. 5 D, E, and G). One possible explanation for the difference in behavior of TREK1 and IRK1Q is that TREK1 might have a higher PI(4,5)P2 affinity than this lower-affinity mutant of IRK1 and, therefore, TREK1 may be insensitive to the degree PI(4,5)P2 depletion induced by rapamycin system. We therefore examined wild-type IRK1 (IRK1-WT), which has a very high PI(4,5)P2 affinity. As shown in Fig. 5 F and G, IRK1-WT current was significantly inhibited by the rapamycin-inducible 5-phosphatase (P < 0.05) (Fig. 5 F and G). The IRK1-WT inhibition was smaller than inhibition seen in the IRK1Q mutant (40 ± 10% versus 87.5 ± 4%, respectively), consistent with a higher affinity of IRK1-WT for PI(4,5)P2 (29). These data show that the rapamycin-inducible 5-phosphatase can reveal specific PI(4,5)P2 interactions even for a high-affinity PI(4,5)P2 interaction and, therefore, suggests that TREK1 is regulated differently from IRK1.
Thus, TREK1 is inhibited by PI(4,5)P2 conversion to DAG and IP3 by GqPCR activation, as shown above, but not by PI(4,5)P2 hydrolysis to PI(4)P. In others words, the modulation of TREK1 activity appears to be compatible with membrane association of the C terminus with either PI(4,5)P2 or PI(4)P, suggesting that it is mediated by a nonspecific electrostatic interaction with the post-M4 polybasic motif of the C terminus. This kind of interaction differs from the stereo-specific binding of PI(4,5)P2 to PHPLC and is more like the nonspecific electrostatic membrane binding of the MARKS peptide (33).
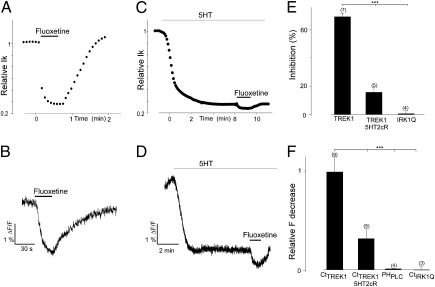
Fluoxetine Inhibition of TREK1 Channel Activity by Membrane Unbinding of the C Terminus.
TREK1 has been implicated in depression (18, 34, 35) and has been proposed to be a therapeutic target of the antidepressant fluoxetine, which has been shown to inhibit channel activity (15). The E306A mutation was found to have reduced fluoxetine inhibition (15), suggesting a possible involvement of the TREK1 C terminus. We wondered, therefore, whether fluoxetine inhibits the channel by promoting dissociation of the TREK1 C terminus from the membrane. As shown earlier, we found that 100 μM of fluoxetine induced a large and reversible inhibition of the TREK1 current (Fig. 6 A and E). The same fluoxetine application induced a large and reversible decrease in the membrane fluorescence of EGFP-CtTREK1 (Fig. 6 B and F). In contrast, fluoxetine had no effect on either the current of the full-length IRK1Q channel or on the membrane fluorescence of the EGFP-tagged IRK1Q C terminus (EFGP-CtIRK1Q) (Fig. 6 E and F). Moreover, fluoxetine has no effect on the membrane fluorescence of EFGP-PHPLC (Fig. 6F). These results indicate that the effects of fluoxetine are specific to TREK1 and, moreover, suggest that the inhibition of TREK1 is due to a fluoxetine-induced C-terminus dissociation from the membrane. In addition, we found that inhibition of TREK1 current and loss of membrane fluorescence of the EGFP-tagged TREK1 C terminus that were triggered by activation of 5HT2cR reduced substantially the effects on current and membrane fluorescence due to subsequent application of fluoxetine (Fig. 6 C–F). The results suggest that fluoxetine and GqPCRs regulate TREK1 by the same C-terminus membrane association mechanism.
Fig. 6.
Fluoxetine reduces TREK1 current and induces EGFP-CtTREK1 dissociation from the membrane. (A and C) Effect of fluoxetine application (100 μM) on TREK1 current and on the membrane fluorescence of EGFP-CtTREK1, respectively. (B and D) Effect of fluoxetine application (100 μM) on TREK1 current and on the membrane fluorescence of EGFP-CtTREK1 after 5HT2cR activation. (E) The histograms represent current inhibition induced by fluoxetine for TREK1 before and after 5HT2cR activation and for IRK1. (F) Relative fluorescence decrease induced by fluoxetine for TREK1 before and after 5HT2cR activation and for EFGP-PHPLC and and EGFP-CtIRK1Q. Student's t test: ***P < 0.001. The numbers of cells tested are indicated in parentheses.
Discussion
Model of Membrane Binding.
It has been proposed that the post-M4 cytosolic C-terminal domain of TREK1 plays key roles in channel function and regulation and that these processes may involve interaction of the TREK1 C terminus with the plasma membrane (8, 19). To study this proposed membrane interaction and its functional relevance, we developed a fluorescence approach to follow the association of an EGFP-tagged TREK1 C terminus with the plasma membrane. We found that the fluorescence of EGFP-CtTREK1 at the plasma membrane is correlated to TREK1 channel activity. First, mutation to alanine of proposed acid sensing E306 (19) increased current in the full-length channel and increased membrane association of the C terminus. Second, mutation of a cluster of five basic residues (located near E306) decreased current and decreased membrane association of the C terminus. The first result is consistent with the known augmentation of current by internal acidification, which is mimicked by E306A (19). The second result is consistent with an earlier proposal that the post-M4 polybasic motif interacts with phospholipid (8).
Having observed a relation between C-terminus membrane association and channel activity, we next asked whether the modulation of the channel by GqPCRs could be accounted for by a change in interaction of the TREK1 C terminus with the membrane. Our results showed that inhibition of channel activity by GqPCRs is, indeed paralleled by, a reduced membrane association of the TREK1 C terminus. Although this loss of membrane interaction was seen with GqPCRs activation, which breaks down PI(4,5)P2 to DAG and IP3, it was not seen when PI(4,5)P2 was hydrolyzed to PI(4)P by either Ci-VSP or the rapamycin system. These results suggest that anchoring of the C terminus to the membrane occurs via a nonspecific electrostatic interaction to phosphoinositide (36) of the post-M4 polybasic motif and not to specific binding of PI(4,5)P2. Such nonspecific binding is reminiscent of the MARKS effector domain (33) and GIRK and KATP channels (37, 38) and differs from the stereo-specific PI(4,5)P2-selective interaction of the IRK1 channel and of the plextrin homology domain of PLC (36). Such nonspecific charge interaction is consistent with TREK1 channel regulation by anionic phospholipids in the inner leaflet lipid and not by cationic lipids (8).
Although the IRK1 channel differs from TREK1 in its selectivity for PI(4,5)P2, it appears to have a common mechanism of channel regulation in that channel activity is enhanced when the IRK1 C terminus associates with the membrane and is reduced when PI(4,5)P2 levels drop and the C terminus dissociates from the membrane. Thus, it appears that dissociation from the membrane of the C terminus of a channel may be a general mechanism of inhibition.
Mechanism of Modulation by Fluoxetine.
TREK1 has been implicated in mood regulation. TREK1 knockout mice (TREK1−/−) display a depression-resistant phenotype (18). Recently, it was reported that spadin, a sortilin-derived peptide, targets TREK1 to generate an antidepressive effect (35). In addition, fluoxetine, the chemical name of the widely used antidepressant Prozac, has been shown to inhibit TREK1 at clinical concentrations (15). Moreover, fluoxetine administration to TREK1−/− mice does not affect their behavior (18). These results suggest that TREK1 is an important target for antidepressive drugs, including fluoxetine. It is important to determine the mechanism of fluoxetine inhibition. Fluoxetine inhibition of TREK1 is not voltage dependent and is less effective on the E306A mutant, which has a higher open probability (15). This argues that the binding site is unlikely to be in the pore or within the span of the membrane, but, rather, is on an internal- or external-facing portion of the channel. Our experiments show that fluoxetine induces dissociation of the TREK1 C terminus from the plasma membrane. This provides a mechanistic explanation for how fluoxetine inhibits TREK1 channel activity.
In summary, we describe a unique technique for monitoring the reversible plasma membrane association of protein domains using epifluorescence without scanning, optical slicing, or imaging. The method can be used to follow changes in lipid composition that result from membrane signaling events or to study the binding of membrane by cytoplasmic regulatory domains of ion channels. We find that dynamic changes in the association of the C-terminal cytoplasmic domain of the TREK1 channel with the plasma membrane that result from activation of GqPCRs are correlated to changes in channel activity, with reduced membrane binding being associated with inhibition of current. The membrane association appears to depend on interaction between a conserved polybasic motif in the C terminus and the most abundant phosphoinositol in the plasma membrane, PI(4,5)P2, although this interaction is not as phospholipid specific as in IRK1 channels. The inhibitory effect of fluoxetine on TREK1 channel activity, which has been proposed to underlie its antidepressant effects, is associated with C-terminus dissociation from the membrane. We propose that this fluorescence assay for C-terminus membrane association could be used to screen for alternative small molecule inhibitors of TREK1, which may be potent and specific antidepressants.
Materials and Methods
Electrophysiology and Fluorometry.
Defolliculated Xenopus oocytes were injected with 50 nL of cRNA at 0.02–0.4 μg/μL and recorded 2–4 d later. For electrophysiology, single oocytes were placed in a 0.3-mL perfusion chamber and impaled with two standard microelectrodes (1–2.5 MΩ resistance) filled with 3 M KCl and voltage clamped with a Dagan CA-1 amplifier, in ND96 solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 2 mM MgCl2, 5 mM Hepes, pH 7.4 with NaOH). Stimulation of the preparation, data acquisition, and analysis were performed using pClamp software (Axon Instruments).
For fluorometry, a Diaphot inverted microscope with a 20× 0.75 NA fluorescence objective (Nikon) was used with illumination by a 150-W xenon lamp. Fluorescence was measured with a Hamamatsu HC120-05 photomultiplier tube. The amplifier, photomultiplier and Uniblitz shutter (Vincent Associates) were controlled by a Digidata-1440 board and pClamp10 software package (Axon Instruments). Filter sets were: tagRFP (HQ535/50ex, HQ610/75em, Q565LP dichroic) and EGFP (HQ480/40ex, HQ535/50em, Q505LP dichroic) (Chroma Technology). Fluorescence signals were low-pass filtered at 2 kHz through an eight-pole Bessel filter (Frequency Devices).
For additional methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Wiese and T. Kim for excellent technical assistance; J. Levitz, R. Arant, J. Patti, and S. Kohout for helpful discussion; and the Fulbright Foundation (G.S.), Philippe Foundation (G.S.), and the National Institutes of Health (R01 NS35549) (to E.Y.I.) for support.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015788108/-/DCSupplemental.
References
- 1.Fink M, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 2.Maingret F, et al. TREK-1 is a heat-activated background K(+) channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel AJ, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci USA. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 6.Fink M, et al. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 8.Chemin J, et al. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes CM, et al. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol. 2005;564:117–129. doi: 10.1113/jphysiol.2004.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AJ, et al. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 11.Duprat F, et al. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- 12.Sandoz G, et al. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K(+) channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandoz G, et al. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J Neurosci. 2008;28:8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murbartián J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- 15.Kennard LE, et al. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heurteaux C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alloui A, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heurteaux C, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 19.Honoré E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannuzzu LM, Moronne MM, Isacoff EY. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- 21.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 22.Várnai P, Balla T. Visualization and manipulation of phosphoinositide dynamics in live cells using engineered protein domains. Pflugers Arch. 2007;455:69–82. doi: 10.1007/s00424-007-0270-y. [DOI] [PubMed] [Google Scholar]
- 23.Halaszovich CR, Schreiber DN, Oliver D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5′-phosphatase. J Biol Chem. 2009;284:2106–2113. doi: 10.1074/jbc.M803543200. [DOI] [PubMed] [Google Scholar]
- 24.Balla T, Varnai P. Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. Curr Protoc Cell Biol. 2009 doi: 10.1002/0471143030.cb2404s42. Chapter 24:Unit 24.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohout SC, et al. Electrochemical coupling in the voltage-dependent phosphatase Ci-VSP. Nat Chem Biol. 2010;6:369–375. doi: 10.1038/nchembio.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalejta RF, Shenk T, Beavis AJ. Use of a membrane-localized green fluorescent protein allows simultaneous identification of transfected cells and cell cycle analysis by flow cytometry. Cytometry. 1997;29:286–291. doi: 10.1002/(sici)1097-0320(19971201)29:4<286::aid-cyto4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Ulbrich MH, Isacoff EY. Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci USA. 2008;105:14163–14168. doi: 10.1073/pnas.0802075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 30.Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol. 2008;15:106–108. doi: 10.1038/nsmb1320. [DOI] [PubMed] [Google Scholar]
- 31.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin S, Hangyás-Mihályné G, Zaitseva I, Golebiewska U. Reversible—through calmodulin—electrostatic interactions between basic residues on proteins and acidic lipids in the plasma membrane. Biochem Soc Symp. 2005;(72):189–198. doi: 10.1042/bss0720189. [DOI] [PubMed] [Google Scholar]
- 34.Perlis RH, et al. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: Association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazella J, et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: A new concept in the antidepressant drug design. PLoS Biol. 2010;8:e1000355. doi: 10.1371/journal.pbio.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci STKE. 2003;2003:re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- 37.Rohács T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K(+) channels for phosphoinositides. J Biol Chem. 1999;274:36065–36072. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- 38.Rohács T, et al. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc Natl Acad Sci USA. 2003;100:745–750. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.