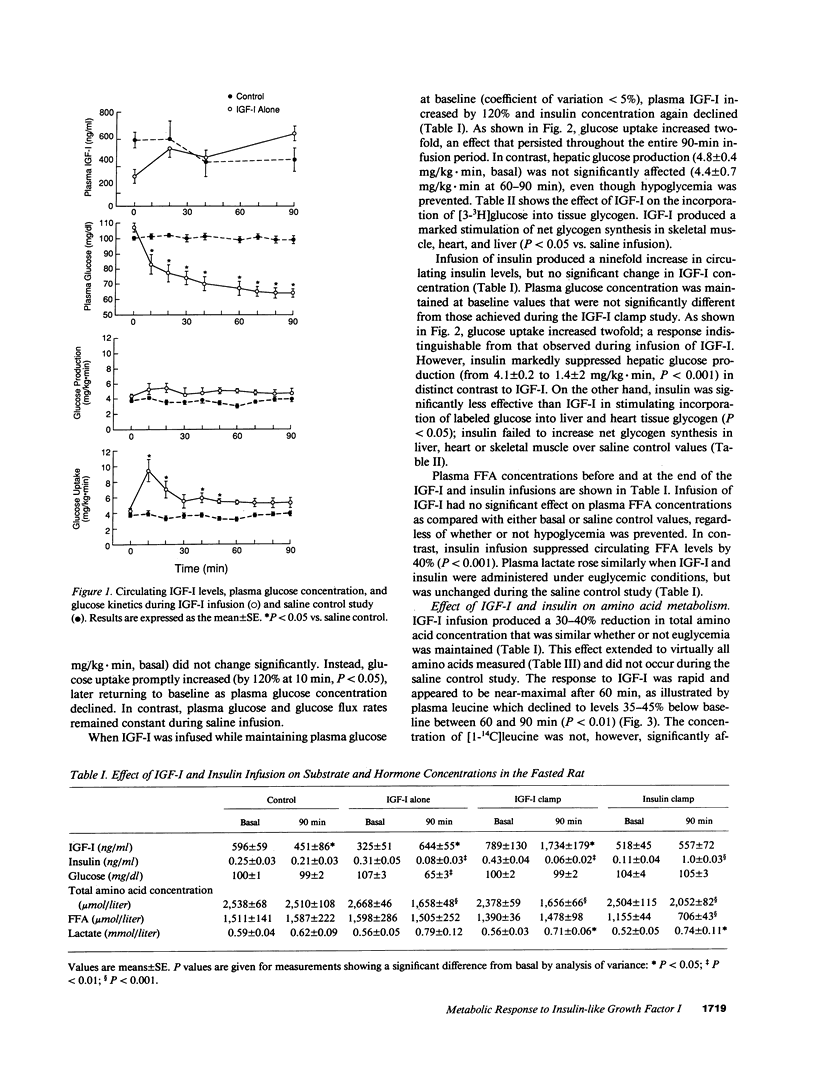
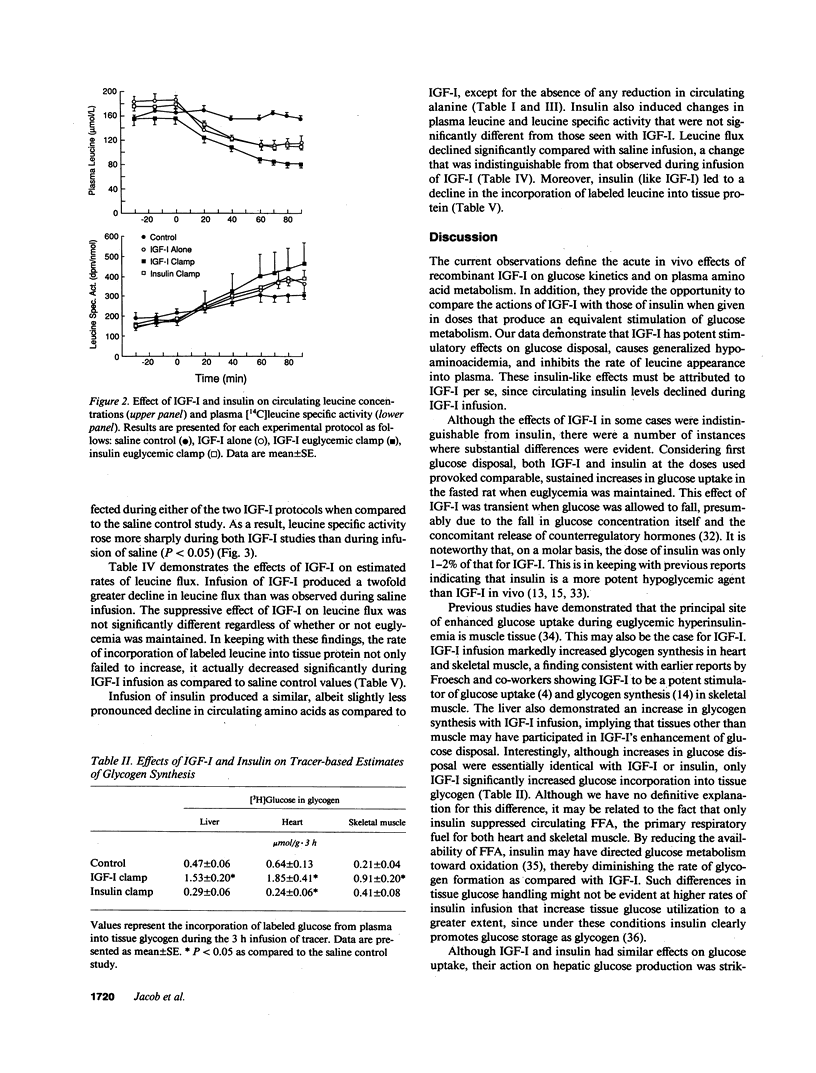
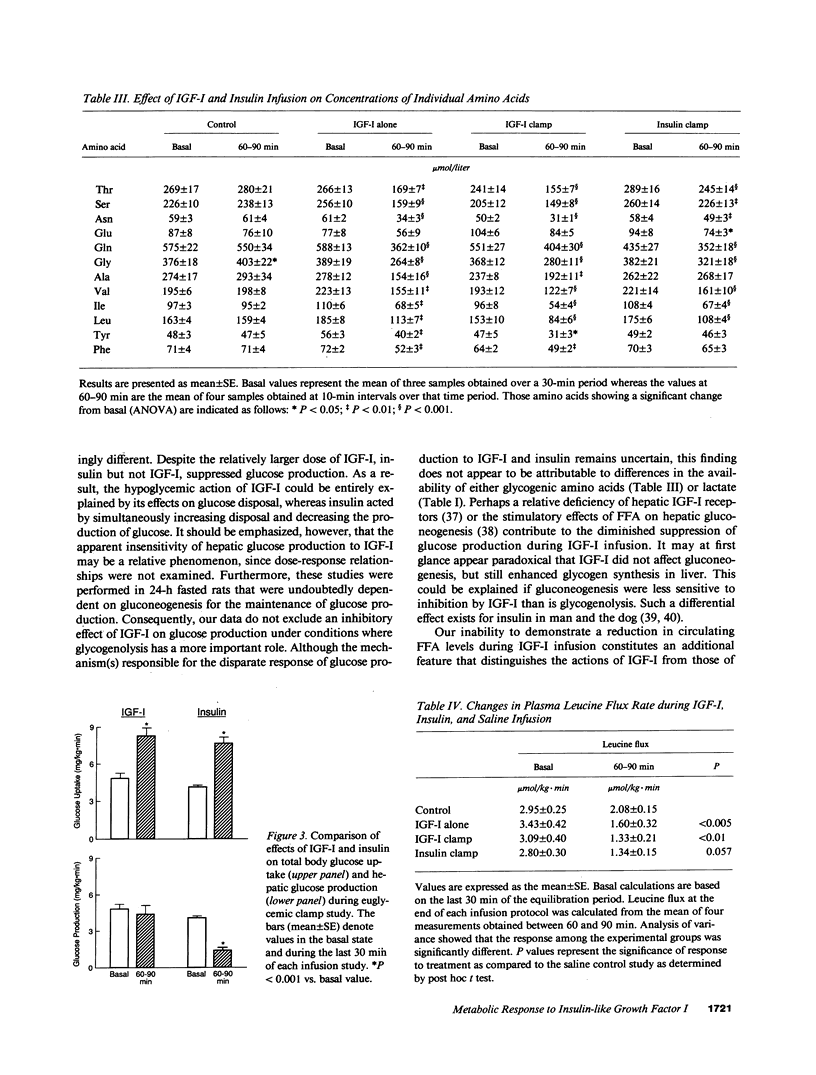
Abstract
To elucidate the acute metabolic actions of insulin-like growth factor I (IGF-I), we administered a primed (250 micrograms/kg), continuous (5 micrograms/kg.min) infusion of human recombinant (Thr 59) IGF-I or saline to awake, chronically catheterized 24-h fasted rats for 90 min. IGF-I was also infused while maintaining euglycemia (glucose clamp technique) and its effects were compared to those of insulin. IGF-I infusion caused a twofold rise in IGF-I levels and a 75-85% decrease in plasma insulin. When IGF-I alone was given, plasma glucose fell by 30-40 mg/dl (P less than 0.005) due to a transient twofold increase (P less than 0.05) in glucose uptake; hepatic glucose production and plasma FFA levels remained unchanged. IGF-I infusion with maintenance of euglycemia produced a sustained rise in glucose uptake and a marked stimulation of [3-3H]glucose incorporation into tissue glycogen, but still failed to suppress glucose production and FFA levels. IGF-I also produced a generalized 30-40% reduction in plasma amino acids, regardless of whether or not hypoglycemia was prevented. This was associated with a decrease in leucine flux and a decline in the incorporation of [1-14C]leucine into muscle and liver protein (P less than 0.05). When insulin was infused in a dosage that mimicked the rise in glucose uptake seen with IGF-I, nearly identical changes in amino acid metabolism occurred. However, insulin suppressed glucose production by 65% and FFA levels by 40% (P less than 0.001). Furthermore, insulin was less effective than IGF-I in promoting glycogen synthesis. We conclude that (a) IGF-I produces hypoglycemia by selectively enhancing glucose uptake; (b) IGF-I is relatively ineffective in suppressing hepatic glucose production or FFA levels; and (c) IGF-I, like insulin, lowers circulating amino acids by reducing protein breakdown rather than by stimulating protein synthesis. Thus, IGF-I's metabolic actions in fasted rats are readily distinguished from insulin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Jefferson L. S., Rannels S. R., Williams P. E., Cherrington A. D., Lacy W. W. Role of insulin in the regulation of leucine kinetics in the conscious dog. J Clin Invest. 1982 Nov;70(5):1031–1041. doi: 10.1172/JCI110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulick L. H., Wilmore D. W. Increased peripheral amino acid release following burn injury. Surgery. 1979 May;85(5):560–565. [PubMed] [Google Scholar]
- Bolinder J., Lindblad A., Engfeldt P., Arner P. Studies of acute effects of insulin-like growth factors I and II in human fat cells. J Clin Endocrinol Metab. 1987 Oct;65(4):732–737. doi: 10.1210/jcem-65-4-732. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Poulos J., Ittoop O., Pories W. J., Flickinger E. G., Sinha M. K. Insulin-like growth factor I binding in hepatocytes from human liver, human hepatoma, and normal, regenerating, and fetal rat liver. J Clin Invest. 1988 Apr;81(4):976–981. doi: 10.1172/JCI113451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino P., Luzi L., Simonson D. C., Haymond M., DeFronzo R. A. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest. 1987 Dec;80(6):1784–1793. doi: 10.1172/JCI113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson J. L., Atkinson R. L., Cherrington A. D., Keller U., Sinclair-Smith B. C., Lacy W. W., Liljenquist J. E. Effects of insulin at two dose levels on gluconeogenesis from alanine in fasting man. Metabolism. 1980 Sep;29(9):810–818. doi: 10.1016/0026-0495(80)90119-5. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Finger F. E., Lacy W. W. Differential sensitivity of glycogenolysis and gluconeogenesis to insulin infusions in dogs. Diabetes. 1976 Apr;25(4):283–291. doi: 10.2337/diab.25.4.283. [DOI] [PubMed] [Google Scholar]
- Copeland K. C., Underwood L. E., Van Wyk J. J. Induction of immunoreactive somatomedin C human serum by growth hormone: dose-response relationships and effect on chromatographic profiles. J Clin Endocrinol Metab. 1980 Apr;50(4):690–697. doi: 10.1210/jcem-50-4-690. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., DeFronzo R. A., Sherwin R. S. Transient hepatic response to glucagon in man: role of insulin and hyperglycemia. Am J Physiol. 1982 Feb;242(2):E73–E81. doi: 10.1152/ajpendo.1982.242.2.E73. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Müller W. A., Bürgi H., Waldvogel M., Labhart A. Non-suppressible insulin-like activity of human serum. II. Biological properties of plasma extracts with non-suppressible insulin-like activity. Biochim Biophys Acta. 1966 Jun 29;121(2):360–374. doi: 10.1016/0304-4165(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Rowe J. W., Goodman M. N., Matthews D. E., Bier D. M., Young V. R. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther H. L., Guenther H. E., Froesch E. R., Fleisch H. Effect of insulin-like growth factor on collagen and glycosaminoglycan synthesis by rabbit articular chondrocytes in culture. Experientia. 1982 Aug 15;38(8):979–981. doi: 10.1007/BF01953688. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Froesch E. R. Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med. 1987 Jul 16;317(3):137–140. doi: 10.1056/NEJM198707163170303. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszynska Y. T., Home P. D., Alberti K. G. In vivo regulation of liver and skeletal muscle glycogen synthase activity by glucose and insulin. Diabetes. 1986 Jun;35(6):662–667. doi: 10.2337/diab.35.6.662. [DOI] [PubMed] [Google Scholar]
- Li J. B., Higgins J. E., Jefferson L. S. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979 Mar;236(3):E222–E228. doi: 10.1152/ajpendo.1979.236.3.E222. [DOI] [PubMed] [Google Scholar]
- Lust W. D., Passonneau J. V., Crites S. K. The measurement of glycogen in tissues by amylo-alpha-1,4-alpha-1,6-glucosidase after the destruction of preexisting glucose. Anal Biochem. 1975 Sep;68(1):328–331. doi: 10.1016/0003-2697(75)90712-5. [DOI] [PubMed] [Google Scholar]
- Martin A. F., Rabinowitz M., Blough R., Prior G., Zak R. Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J Biol Chem. 1977 May 25;252(10):3422–3429. [PubMed] [Google Scholar]
- Meuli C., Froesch E. R. Insulin and nonsuppressible insulin-like activity (NSILA-S) stimulate the same glucose transport system via two separate receptors in rat heart. Biochem Biophys Res Commun. 1977 Apr 11;75(3):689–695. doi: 10.1016/0006-291x(77)91527-3. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Oelz O., Jakob A., Froesch E. R. Nonsuppressible insulin-like activity (NSILA) of human serum. V. Hypoglycaemia and preferential metabolic stimulation of muscle by NSILA-S. Eur J Clin Invest. 1970 Mar;1(1):48–53. doi: 10.1111/j.1365-2362.1970.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Peters M. A., Lau E. P., Snitman D. L., Van Wyk J. J., Underwood L. E., Russell W. E., Svoboda M. E. Expression of a biologically active analogue of somatomedin-C/insulin-like growth factor I. Gene. 1985;35(1-2):83–89. doi: 10.1016/0378-1119(85)90160-x. [DOI] [PubMed] [Google Scholar]
- Poggi C., Le Marchand-Brustel Y., Zapf J., Froesch E. R., Freychet P. Effects and binding of insulin-like growth factor I in the isolated soleus muscle of lean and obese mice: comparison with insulin. Endocrinology. 1979 Sep;105(3):723–730. doi: 10.1210/endo-105-3-723. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P., King G. L., Moses A. C., Van Obberghen-Schilling E. E., Romanus J. A., Knight A. B., Short P. A., White R. M. Multiplication stimulating activity (MSA) from the BRL 3A rat liver cell line: relation to human somatomedins and insulin. J Supramol Struct Cell Biochem. 1981;15(3):253–286. doi: 10.1002/jsscb.1981.380150305. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Hendler R., Felig P. Influence of continuous physiologic hyperinsulinemia on glucose kinetics and counterregulatory hormones in normal and diabetic humans. J Clin Invest. 1979 May;63(5):849–857. doi: 10.1172/JCI109384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiwiller E., Guler H. P., Merryweather J., Scandella C., Maerki W., Zapf J., Froesch E. R. Growth restoration of insulin-deficient diabetic rats by recombinant human insulin-like growth factor I. Nature. 1986 Sep 11;323(6084):169–171. doi: 10.1038/323169a0. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Schwenk W. F., Tsalikian E., Beaufrere B., Haymond M. W. Recycling of an amino acid label with prolonged isotope infusion: implications for kinetic studies. Am J Physiol. 1985 Apr;248(4 Pt 1):E482–E487. doi: 10.1152/ajpendo.1985.248.4.E482. [DOI] [PubMed] [Google Scholar]
- Smith D., Rossetti L., Ferrannini E., Johnson C. M., Cobelli C., Toffolo G., Katz L. D., DeFronzo R. A. In vivo glucose metabolism in the awake rat: tracer and insulin clamp studies. Metabolism. 1987 Dec;36(12):1167–1174. doi: 10.1016/0026-0495(87)90244-7. [DOI] [PubMed] [Google Scholar]
- Van den Brande J. L., van Buul-Offers S. Effect of growth hormone and peptide fractions containing somatomedin activity on growth and cartilage metabolism of Snell dwarfmice. Acta Endocrinol (Copenh) 1979 Oct;92(2):242–257. doi: 10.1530/acta.0.0920242. [DOI] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. The type I insulin-like growth factor receptor mediates the rapid effects of multiplication-stimulating activity on membrane transport systems in rat soleus muscle. J Biol Chem. 1984 Mar 10;259(5):3090–3095. [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Froesch E. R., Humbel R. E. The insulin-like growth factors (IGF) of human serum: chemical and biological characterization and aspects of their possible physiological role. Curr Top Cell Regul. 1981;19:257–309. doi: 10.1016/b978-0-12-152819-5.50024-5. [DOI] [PubMed] [Google Scholar]
- Zapf J., Hauri C., Waldvogel M., Froesch E. R. Acute metabolic effects and half-lives of intravenously administered insulinlike growth factors I and II in normal and hypophysectomized rats. J Clin Invest. 1986 Jun;77(6):1768–1775. doi: 10.1172/JCI112500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Froesch E. R. Insulin-like growth factors I and II: some biological actions and receptor binding characteristics of two purified constituents of nonsuppressible insulin-like activity of human serum. Eur J Biochem. 1978 Jun 15;87(2):285–296. doi: 10.1111/j.1432-1033.1978.tb12377.x. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Waldvogel M., Sand I., Froesch E. R. Effect of trypsin treatment of rat adipocytes on biological effects and binding of insulin and insulin-like growth factors: further evidence for the action of insulin-like growth factors through the insulin receptor. Eur J Biochem. 1981 Jan;113(3):605–609. doi: 10.1111/j.1432-1033.1981.tb05105.x. [DOI] [PubMed] [Google Scholar]


